Abstract
Fumonisin B1 (FB1) is a mycotoxin that commonly occurs in maize. FB1 causes a variety of toxic effects in different animal species and has been implicated as a contributing factor of esophageal cancers in humans. In the present study, we examined the effect of dietary exposure to FB1 on intestinal colonization by pathogenic Escherichia coli associated with extraintestinal infection. Three-week-old weaned pigs were given FB1 by gavage as a crude extract or as a purified toxin at a dose of 0.5 mg/kg of body weight daily for 6 days. On the last day of the toxin treatment, the pigs were orally inoculated with an extraintestinal pathogenic E. coli strain. All animals were euthanized 24 h later, necropsies were performed, and tissues were taken for bacterial counts and light microscopic examination. Ingestion of FB1 had only a minimal effect on animal weight gain, did not cause any macroscopic or microscopic lesions, and did not change the plasma biochemical profile. However, colonization of the small and large intestines by an extraintestinal pathogenic E. coli strain was significantly increased. Our results show that FB1 is a predisposing factor to infectious disease and that the pig can be used as a model for the study of the consequences of ingesting mycotoxin-contaminated food.
Mycotoxins are secondary metabolites of fungi which may contaminate animal and human feeds at all stages of the food chain.Their global occurrence is considered an important risk factor for human and animal health, as up to 25% of the world crop production may be contaminated with mycotoxins (16, 31).
Fumonisin B1 (FB1) belongs to the fumonisin family of toxins (4) which are produced by Fusarium verticillioides and Fusarium proliferatum, fungi that commonly contaminate maize. Recent surveys of fumonisins in food and feed throughout the world, including the United States and most European countries, raised concerns about the extent of FB1 contamination of maize and its implications for food safety (13, 52, 53). FB1 was found in up to 50% of maize samples collected between 1988 and 1991 from the midwestern United States (41). In this survey, up to 10% of the samples had toxin levels between 10 and 50 ppm (41). Similarly, another survey of fumonisins in maize gluten and other maize products in the United Kingdom found these mycotoxins in almost every sample at concentrations of up to 32 ppm (52).
At high concentrations (50 to 500 ppm), FB1 causes a variety of species-specific toxicological effects in domestic and laboratory animals. It induces leukoencephalomalacia in horses, pulmonary edema in pigs, and nephrotoxicity in rats, rabbits, lambs, and calves (3, 14, 21, 22, 32, 54). In all species studied, both acute and chronic exposure to FB1 are associated with alteration of sphingolipid metabolism and hepatotoxicity (9, 20, 21, 26, 44, 46, 48). FB1 also has been implicated as a contributing factor in human esophageal cancers (45) and is a renal and hepatic carcinogen in male and female rats, respectively (22). The mechanism(s) of toxicity for fumonisins is complex and may involve several molecular sites (47). The primary biochemical effect of fumonisin is to inhibit ceramide synthase activity, leading to the accumulation of sphingoid bases and sphingoid base metabolites and the depletion of more complex sphingolipids (36).
Although Escherichia coli is a normal inhabitant of the intestinal flora, it is frequently associated with both intestinal and extraintestinal infections. Extraintestinal pathogenic E. coli (ExPEC) strains usually possess virulence determinants that allow them to persist in the intestine, cross epithelial barriers, resist nonspecific host defense mechanisms, establish specifically in extraintestinal tissues, and potentially cause damage at these sites (50, 55). For instance, ExPEC strains with similar virulence determinants have been associated with urinary tract diseases in humans and septicemia in pigs (7, 15, 23). We have established a septicemia model involving oral inoculation of porcine ExPEC strains in newborn, colostrum-deprived, germfree pigs to study the pathogenic mechanisms of these bacteria in the natural host when it is highly susceptible to bacterial infection (17). These bacteria are also opportunistic pathogens, as they have been found in the intestines of healthy older pigs (19), dogs (23), and humans (7). Host conditions, therefore, are of critical importance in the ability of bacteria to infect and colonize the host and cause disease (34, 38, 42, 58).
The intestinal tract is the first barrier to ingested mycotoxins but is also the first line of defense against intestinal infection. Ingestion of some mycotoxins increases susceptibility to experimental or natural mucosal infections (18, 56, 57), but no data are available concerning the effect of fumonisin as a predisposing factor to intestinal infections. The objective of the present study was to determine the effect of dietary exposure to low doses of FB1 on intestinal colonization by the pathogenic bacterium E. coli.
MATERIALS AND METHODS
Animals.
Thirty-five 3-week-old weaned healthy male Yorkshire hybrid pigs were used for the experiments. They were acquired locally at 2 weeks of age, just after weaning, and acclimatized for 1 week in the isolation rooms of the animal care facilities of the Faculté de Médécine Vétérinaire, Université de Montréal, at an ambient temperature of 24°C. The pigs were weighed daily. They had free access to water and were fed a commercial starter diet, free of FB1, throughout the experiment. Animals were cared for in accordance with guidelines of the Canadian Council for Animal Care.
Toxin.
In a first experiment, the mycotoxin was administered as a crude extract obtained after in vitro culture. Briefly, sterilized crushed maize (50% water content) was inoculated with the high FB1-producing F. verticillioides strain NRRL 34281 (deposited in the ARS Culture Collection, Peoria, Ill.). The fungal strain was incubated for 4 weeks at 25°C. This culture was extracted with acetonitrile-water (1:1), filtered, and concentrated with a rotary evaporator. The culture extract contained 1.4 g of FB1/liter.
The FB1 concentration was measured by quantitative planar chromatography (28). Briefly, after a two-step thin-layer chromatography development, the plates were visualized with p-anisaldehyde. This planar chromatography technique has a detection limit of 50 ng (28). The culture extract used in this preparation did not produce detectable amounts of the following fusariotoxins: zearalenone, deoxynivalenol, fusarochromanone, and trichothecene (12, 39). Purified FB1 obtained from PROMEC (Tygerberg, South Africa) was used for the second experiment. This toxin (purity, >95%) was extracted and purified according to the method of Cawood (8).
Bacterial isolate.
Piglets were inoculated with an ExPEC strain designated 28CNalr (O75:K95). This strain possesses pap, sfa, hly, cnf-1, and aerobactin genomic sequences, as determined by colony hybridization and produces CNF1, alpha-hemolysin, and P and F1C fimbriae (10). It is a Nalr variant of strain 28C (10) that was obtained by serial passage of 28C following growth in Luria broth containing concentrations of nalidixic acid from 0 to 50 μg/ml at 37°C for 24 h. This strain was deposited in the Pasteur Institute Collection under the designation CIP 107983.
General experimental protocol.
Two experiments were performed with the same protocol. For 6 days, treated pigs were given by gavage 0.5 mg of FB1/kg of body weight/day as a crude extract (experiment 1) or as a purified toxin (experiment 2). Based on average feed consumption for piglets of this age, the dose used (0.5 mg/kg/day) corresponds to feed contaminated with 5 to 8 ppm of FB1. The crude extract was given undiluted to the piglets. The purified toxin was diluted in sterile water to a final concentration of 1 mg/ml and given in a volume of 3 to 5 ml according to the animal weight. Control animals received 4 ml of sterile water. On the last day of toxin treatment, half of the pigs within each group were orally inoculated with 1 × 109 to 1.1 × 109 CFU of strain 28CNalr. Pigs received 10 ml of 1.2% NaHCO3 through an intragastric tube to neutralize gastric acid, followed by 20 ml of tryptic soy broth (Difco Laboratories, Detroit, Mich.) containing the bacteria. Control, noninfected animals were treated similarly, receiving NaHCO3 plus tryptic soy broth.
Twenty-four hours after bacterial inoculation, the pigs were euthanized with an intracardiac injection of sodium pentobarbital (Euthanyl Forte; Biomedia-MTC, Cambridge, Ontario, Canada; 540 mg/ml diluted in 0.20 ml of propylene glycol). Following exsanguination, a complete necropsy was performed and standard samples of the lung, liver, spleen, kidney, duodenum, jejunum, ileum, cecum, colon, and mesenteric lymph nodes (at the level of the ileum) were collected. These samples were consistently taken from the same areas of the respective organs in all animals.
A portion of each tissue was placed on ice and used immediately for bacteriological examination (17). A second portion was immersed in 10% neutral buffered formalin for histopathology.
Bacteriological counts.
Tissues were evaluated quantitatively for the presence of E. coli. Samples were weighed and suspended in 2 ml of phosphate-buffered saline, homogenized at 5,000 rpm with a Cat homogenizer model X120 (PolyScience, Niles, Ill.), and serially diluted 10-fold in sterile phosphate-buffered saline. Dilutions were plated on tryptic soy agar (Difco) supplemented with 0.2% nalidixic acid with a spiral plater system model C (Meyer Service and Supply, Ltd., Long Sault, Canada) as recommended by the manufacturer. After overnight incubation at 37°C, bacterial colonies were counted with a minimum of 1 colony per plate. Several colonies from each individual were positively identified as the infecting strain by PCR and agglutination tests.
Histopathology.
Tissue samples, fixed in 10% neutral buffered formalin, were embedded in paraffin, sectioned at approximately 5-μm intervals, and stained with hematoxylin and phloxine saffron for examination by light microscopy.Bacterial localization in intestinal and extraintestinal tissues was determined by immunohistochemistry. Sections were stained with Vector red (Vector Laboratories, Burlington, Canada) and examined by light microscopy as previously described (17) by using rabbit polyclonal anti-O75 serogroup serum.
Biochemical analysis.
At the time of necropsy, blood was collected on EDTA for plasma biochemical analysis (Biochemistry Laboratory, Rangueil Hospital, Toulouse, France). The analysis included determinations of creatinine, urea nitrogen, total protein, calcium, phosphorus, sodium, potassium, chloride, glucose, cholesterol, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotranferase, lactate dehydrogenase, and gamma-glutamyl transferase.
Statistical analysis.
Student's t test and analysis of variance (ANOVA) were used to analyze weight gain and bacterial counts. P values of <0.05 were considered significant.
RESULTS
Effect of FB1 on weight gain.
Separate experiments were performed using FB1 as either a crude extract or purified toxin. We first examined the effect of 0.5 mg of FB1/kg of body weight on clinical signs and animal performance. Pigs receiving FB1, either as a crude extract or as the purified toxin, appeared clinically normal throughout the study, and no deaths occurred. Pigs in the FB1-treated groups did not gain as much weight as those in the control group, but the difference was not statistically significant (Table 1). At necropsy, no gross changes were considered to be related to the administration of FB1. Microscopic lesions not usually associated with FB1 toxicity, including nonspecific superficial colitis and a mostly mild interstitial pneumonia with a mononuclear cell infiltration, were sometimes observed, although to similar extents in pigs of both groups. No microscopic lesions considered to be compatible with FB1 toxicity, such as apoptosis, were observed during examination of liver and other tissues following routine hematoxylin and phloxine saffron staining in FB1-treated pigs. Plasma biochemical analysis did not reveal any effect of FB1 (data not shown).
TABLE 1.
Effect of FB1 on piglet weighta
| Expt and treatment | No. of animals | Initial wt (kg) | Wt gain (kg)b |
|---|---|---|---|
| Expt 1 | |||
| Control | 9 | 4.43 ± 0.26 | 1.32 ± 0.12 |
| FB1 extract | 9 | 4.66 ± 0.19 | 1.17 ± 0.12 |
| Expt 2b | |||
| Control | 4 | 6.55 ± 0.07 | 2.41 ± 0.29 |
| Purified FB1 | 4 | 6.23 ± 0.17 | 2.05 ± 0.10 |
| Expt 2 | |||
| Control | 4 | 5.95 ± 0.05 | 1.98 ± 0.26 |
| Purified FB1 | 5 | 5.42 ± 0.15 | 1.78 ± 0.20 |
Results are expressed as means ± standard errors of the means of the indicated number of animals.
Two-way ANOVA did not reveal any effect of FB1 administration (FB1 versus control) on weight gain of the animals.
In experiment 2, piglet weight was not homogenous, and, thus, the animals were divided into two groups according to their initial weight. In each group, half of the piglets received purified FB1 and the other half were kept as controls.
Effect of FB1 on bacterial colonization.
In control pigs from experiment 1 (Table 2, experiment 1), strain 28CNalr was recovered in low numbers from the intestine, colonizing mostly the cecum and the colon. Very few bacteria were translocated to the mesenteric lymph nodes or disseminated to extraintestinal organs. Of the five inoculated pigs, strain 28CNalr was recovered from an extraintestinal organ of only one pig, this being the lung. When pigs were treated with FB1 administered as a crude extract, 28CNalr colonized the examined tissues to a greater extent than it did in untreated animals (Table 2, experiment 1). We recovered 400- to 700-fold more CFU of the inoculated strain per gram of tissue from the intestines of treated animals than from animals that had received no fumonisin. In three of the four FB1-treated pigs, bacteria were translocated to the mesenteric lymph nodes and disseminated to the lungs. In one pig, bacteria of strain 28CNalr were also found in the liver and the spleen.
TABLE 2.
Effect of oral administration of FB1 on bacterial colonization of piglet intestines by E. coli strain 28CNalr
| Expt and treatment | Bacterial colonization of
sections (log10 [CFU/g])
ofc:
|
||
|---|---|---|---|
| Ileum | Cecum | Colon | |
| Expt 1a | |||
| Control | 1.66 ± 0.14b | 2.99 ± 0.32 | 3.32 ± 0.77 |
| FB1 extract | 4.26 ± 0.42 | 5.85 ± 0.40 | 6.03 ± 0.37 |
| Expt 2 | |||
| Control | 2.74 ± 0.34 | 3.72 ± 0.42 | 3.73 ± 0.38 |
| Purified FB1 | 3.67 ± 0.64 | 5.07 ± 0.58 | 5.62 ± 0.63 |
Pigs were dosed for 7 days with 0.5 mg of FB1/kg of body weight, administered as a crude extract (experiment 1) or as a purified toxin (experiment 2).
Results are expressed as geometric mean bacterial counts ± standard errors of the means for a group of four to five pigs.
Two-way ANOVA did not reveal any effect of the experiment but indicated a significant effect of FB1 treatment on the bacterial count in the different parts of the intestine. For the results for the ileum, cecum, and colon, the effects of the FB1 treatment in both experiments were significant at a P value of 0.0009, 0.0003, and 0.0001, respectively.
To confirm that the increase in susceptibility of the pigs to E. coli infection was due to FB1, the experiment was repeated with purified mycotoxin (Table 2, experiment 2). As expected, greater intestinal colonization was observed in FB1-treated pigs than in the untreated animals. However, in this experiment, the bacteria translocated poorly to extraintestinal organs, and E. coli 28C was recovered only from the mesenteric lymph nodes of two out of five FB1-treated pigs.
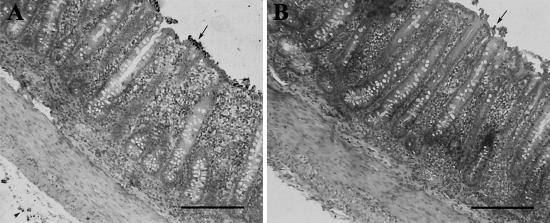
Based on immunohistochemistry with Vector red, red-stained bacteria were often observed in the lumen and in close contact with the intestinal mucosal surface and in the serosa, mostly of the cecum and colon, of FB1-treated pigs (Fig. 1A). Similar red-stained bacteria were occasionally observed in the lumen, but only rarely were they in contact with the mucosa or in the serosa of the intestines of untreated animals (Fig. 1B).
FIG. 1.
In situ visualization of bacteria in colon tissue by immunohistochemistry using an anti-O75 serum and Vector red staining. Piglets were treated with FB1 (A), or left untreated (B), inoculated with E. coli strain 28CNalr, and euthanized 24 h postinoculation. (A) Bacteria, stained red on direct microscopic observation, were found in aggregates closely associated with the colon surface epithelium (arrow) and in the serosa (arrowhead). (B) Similar bacteria were occasionally found individually associated with the colon surface epithelium (arrow) but not in the serosa. Bar size, 100 μm.
DISCUSSION
Ingestion of FB1 increased intestinal colonization by E. coli strain 28CNalr. This bacterial strain can persist in the large intestine of pigs under normal conditions and can colonize the gut and translocate to internal organs when the immune system is compromised, e.g., in the absence of colostrum in conventional or germfree newborn pigs (J. M. Fairbrother, unpublished results). It is possible that a similar effect occurs in older animals when other agents, e.g., mycotoxins, affect the intestinal tract and/or the immune system. The low pathogenicity of this strain is also reflected by its poor ability to elicit an inflammatory response in the intestines, as demonstrated by the absence of an inflammatory cell infiltrate (Fig. 1), and of the induction of RNA encoding inflammatory cytokines (data not shown). Since strains of this pathotype are also recovered from patients with urinary tract infections, strain 28CNalr appears to typify opportunistic ExPEC organisms.
The dose of FB1 administered to pigs in our experiments (0.5 mg of FB1/kg of body weight, equivalent to 5 to 8 ppm in the feed) significantly increased the bacterial colonization of the intestine (Table 2); however, this dose did not induce clinical or pathological changes and had no significant impact on weight gain. Using the same concentration of FB1, but for a longer period of time (8 weeks), Rotter et al. (49) reported an 11% decrease in daily weight gain of pigs, and a 31% decrease in weight was observed in pigs fed a high dose (20 mg/kg of body weight) of FB1 for 7 days (54). The toxic dose of FB1 depends upon the animal species and parameters investigated. In pigs, changes in serum sphingolipids are detected at 5 ppm of FB1 (46), liver damage occurs at 23 ppm (40), and pulmonary edema occurs at 175 ppm (40). Clinical chemistry profiles indicate that alkaline phosphatase is the most sensitive measure of fumonisin toxicity in pigs (20, 40, 46). The dose and the time exposure used in our study did not induce any change in serum biochemical parameters (data not shown) but did significantly increase bacterial colonization by pathogenic E. coli. Several researchers have described an alteration of biochemical values in pig serum, but these were obtained with higher doses of toxin (54), longer exposure (49, 60), or both (40, 59).
We found that FB1 increases bacterial colonization in the intestines of piglets. Interestingly, the difference in bacterial colonization between the FB1-treated and the control (untreated) pigs was greater in the first experiment than in the second one (Table 2). This result may be due to an unidentified compound present in the culture material that was acting synergistically with FB1. Alternatively, the difference in the initial weights of the pigs might have had an impact. The administration of similar bacterial loads to pigs in the first experiment, who had lower body weights, and consequently smaller intestines than pigs in the second experiment, may have resulted in the presence in the intestinal lumen of a greater number of bacteria relative to the lumen size and thereby may have exacerbated the effect of FB1 on intestinal colonization.
Several mycotoxins can alter the immune response and increase susceptibility to infectious disease (33, 42, 57), and sublethal concentrations of FB1 decrease bacterial clearance after intravenous infections (29, 54). However, a recent paper (11) indicates that diets contaminated with 50 or 150 ppm of FB1 enhance the resistance of mice to parasitic infection. There are a few reports on the influence of mycotoxins on intestinal colonization by pathogenic bacteria; however, none of these reports evaluated fumonisin. Fukata et al. (18) reported increased intestinal colonization by Salmonella enterica serovar Typhimurium in 11-day-old chickens fed high doses of ochratoxin A, although Kubena et al. (27), using the same model, observed no effects attributable to either aflatoxin or T-2 toxin.
FB1 specifically inhibits ceramide synthase activity, resulting in the disruption of sphingolipid metabolism (35, 48).Sphingolipids and sphingoglycolipids are essential components of eukaryotic cell membranes, and these molecules may act as membrane receptors for bacteria (2, 5, 24) and bacterial toxins (30, 51). Thus, ingestion of FB1 may induce sphingolipid changes in the gastrointestinal tract and modify bacterial receptors on the surfaces of epithelial cells. These changes may contribute to the increased colonization of the intestinal tract by pathogenic bacteria.
We used pigs in this study for at least three reasons. First, due to their maize-rich diet, pigs are potentially exposed to high levels of fumonisins. From a public health perspective, increased colonization of the pig intestine by potentially pathogenic E. coli following the ingestion of fumonisin may increase animal-to-human transmission of pathogens and/or increased antibiotic concentrations in meat as a consequence of animal treatment. Second, rodents are very resistant to most mycotoxins (25, 37) and are not available as models. Finally, pigs and humans have many biological similarities, especially with regard to the intestinal tract (1, 6, 43), which makes the pig a good model for the study of the consequences of ingestion of mycotoxin-contaminated food.
In conclusion, we found that exposure to FB1 is a predisposing factor to infectious disease. Considering the high levels of FB1 that may be present in animal feeds and human food preparations (41, 52, 53), further studies are needed to identify the mechanism(s) by which this mycotoxin acts on the intestinal tract to modulate colonization by opportunistic pathogens. Epidemiological studies are also needed to assess the extent to which fumonisins are involved in the development of infectious diseases in humans and animals.
Acknowledgments
Sylvie Fournout and Sylvie Pérès were supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada, grant GPG0181728, and by the Ministère de l'Éducation du Québec, respectively. Marielle Odin was supported by NSERC grant 2294.
We thank Francis Girard for assistance in preparation of photographs. This work was supported in part by the Programme Environnement et Santé EN98-27 of the Ministère de l'Aménagement du Territoire et de l'Environnement, Paris, France, by the région Midi-Pyrénées (DAER-Rech/99008345), and by the Transversalité INRA (Mycotoxines-P00263).
REFERENCES
- 1.Almond, G. W. 1996. Research applications using pigs. Vet. Clin. N. Am. Food Anim. Pract. 12:707-716. [DOI] [PubMed] [Google Scholar]
- 2.Backhed, F., B. Alsen, N. Roche, J. Angstrom, A. von Euler, M. E. Breimer, B. Westerlund-Wikstrom, S. Teneberg, and A. Richter-Dahlfors. 2002. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277:18198-18205. [DOI] [PubMed] [Google Scholar]
- 3.Bane, D. P., E. J. Neumann, W. F. Hall, K. S. Harlin, and R. L. Slife. 1992. Relationship between fumonisin contamination of feed and mystery swine disease. A case-control study. Mycopathologia 117:121-124. [DOI] [PubMed] [Google Scholar]
- 4.Bezuidenhout, S. C., W. C. A. Gelderblom, C. P. Gorst-Alleman, R. M. Horak, W. F. O. Marasas, G. Spiteller, and R. Vleggaar. 1988. Structure elucidation of the fumonisin, mycotoxins from Fusarium moniliforme. J. Soc. Chem. Commun. 1988:743-745. [Google Scholar]
- 5.Bibel, D. J., R. Aly, and H. R. Shinefield. 1992. Inhibition of microbial adherence by sphinganine. Can. J. Microbiol. 38:983-985. [DOI] [PubMed] [Google Scholar]
- 6.Bondy, G. S., and J. J. Pestka. 2000. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B 3:109-143. [DOI] [PubMed] [Google Scholar]
- 7.Brauner, A., M. Katouli, K. Tullus, and S. H. Jacobson. 1990. Production of cytotoxic necrotizing factor, verocytotoxin and haemolysin by pyelonephritogenic Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 9:762-767. [DOI] [PubMed] [Google Scholar]
- 8.Cawood, M. E. 1991. Isolation of the fumonisin mycotoxins: a quantitative approach. J. Agric. Food Chem. 39:1958-1962. [Google Scholar]
- 9.Colvin, B. M., and L. R. Harrison. 1992. Fumonisin-induced pulmonary edema and hydrothorax in swine. Mycopathologia 117:79-82. [DOI] [PubMed] [Google Scholar]
- 10.Dozois, C. M., S. Clément, C. Desautels, E. Oswald, and J. M. Fairbrother. 1997. Expression of P, S, and F1C adhesins by cytotoxic necrotizing factor 1-producing Escherichia coli from septicemic and diarrheic pigs. FEMS Microbiol. Lett. 152:307-312. [DOI] [PubMed] [Google Scholar]
- 11.Dresden-Osborne, C., G. Pittman-Noblet, E. N. Enongene, C. W. Bacon, R. T. Riley, and K. A. Voss. 2002. Host resistance to Trypanosoma cruzi infection is enhanced in mice fed Fusarium verticillioides (=F. moniliforme) culture material containing fumonisins. Food Chem. Toxicol. 40:1789-1798. [DOI] [PubMed] [Google Scholar]
- 12.Dupuy, J., J. Le Bars, and P. Le Bars. 1992. Mycotoxinogénèse de souches de Fusarium: contrainte en vue de leur utilisation dans la lutte biologique. Cryptogam. Mycol. 13:159-169. [Google Scholar]
- 13.Dutton, M. F. 1996. Fumonisins, mycotoxins of increasing importance: their nature and their effects. Pharmacol. Ther. 70:137-161. [DOI] [PubMed] [Google Scholar]
- 14.Edrington, T. S., C. A. Kamps-Holtzapple, R. B. Harvey, L. F. Kubena, M. H. Elissalde, and G. E. Rottinghaus. 1995. Acute hepatic and renal toxicity in lambs dosed with fumonisin-containing culture material. J. Anim. Sci. 73:508-515. [DOI] [PubMed] [Google Scholar]
- 15.Fairbrother, J. M., and M. Ngeleka. 1994. Extraintestinal E. coli infections in pigs, p. 221-236. In G. Gyles (ed.), E. coli disease in animals. CAB International, Wallingford, Oxon, United Kingdom.
- 16.Finks-Gremmels, J. 1999. Mycotoxins: their implications for human and animal health. Vet. Q. 21:115-120. [DOI] [PubMed] [Google Scholar]
- 17.Fournout, S., C. M. Dozois, M. Odin, C. Desautels, S. Peres, F. Herault, F. Daigle, C. Segafredo, J. Laffitte, E. Oswald, J. M. Fairbrother, and I. P. Oswald. 2000. Lack of a role of cytotoxic necrotizing factor 1 toxin from Escherichia coli in bacterial pathogenicity and host cytokine response in infected germfree piglets. Infect. Immun. 68:839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukata, T., K. Sasai, E. Baba, and A. Arakawa. 1996. Effect of ochratoxin A on Salmonella typhimurium-challenged layer chickens. Avian Dis. 40:924-926. [PubMed] [Google Scholar]
- 19.Garabal, J. I., E. A. González, F. Vazquez, J. Blanco, and M. Blanco. 1995. Toxigenic Escherichia coli in Spanish piggeries from 1986 to 1991. Vet. Microbiol. 47:17-25. [DOI] [PubMed] [Google Scholar]
- 20.Gumprecht, L. A., V. R. Beasley, R. M. Weigel, H. M. Parker, M. E. Tumbleson, C. W. Bacon, F. I. Meredith, and W. M. Haschek. 1998. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine: morphological and biochemical alterations. Toxicol. Pathol. 26:777-788. [DOI] [PubMed] [Google Scholar]
- 21.Haschek W. M., L. A. Gumprecht, G. Smith, M. E. Tumbleson, and P. D. Constable. 2001. Fumonisin toxicosis in swine: an overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 109(Suppl. 2):251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard, P. C., R. M. Eppley, M. E. Stack, A. Warbritton, K. A. Voss, R. J. Lorentzen, R. M. Kovach, and T. J. Bucci. 2001. Fumonisin B1 carcinogenicity in a two-year feeding study using F344 rats and B6C3F1 mice. Environ. Health Perspect. 109(Suppl. 2):277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, J. R., A. L. Stell, and P. Delavari. 2001. Canine feces as a reservoir of extraintestinal pathogenic Escherichia coli. Infect. Immun. 69:1306-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, A. S., B. Kniep, T. A. Oelschlaeger, I. Van Die, T. Korhonen, and J. Hacker. 2000. Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect. Immun. 68:3541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan, M. A. 1984. Minipig: advantages and disadvantages as model in toxicity testing. J. Am. Coll. Toxicol. 3:337-342. [Google Scholar]
- 26.Kriek, N. P. J., T. S. Kellerman, and W. F. O. Marasas. 1981. A comparative study of the toxicity of Fusarium moniliforme to horses, primates, pigs, sheep and rats. Onderstepoort J. Vet. Res. 48:129-131. [PubMed] [Google Scholar]
- 27.Kubena, L. F., R. H. Bailey, J. A. Byrd, C. R. Young, D. E. Corrier, L. H. Stanker, and G. E. Rottinghaus. 2001. Cecal volatile fatty acids and broiler chick susceptibility to Salmonella typhimurium colonization as affected by aflatoxins and T-2 toxin. Poult. Sci. 80:411-417. [DOI] [PubMed] [Google Scholar]
- 28.Le Bars, J., P. Le Bars, J. Dupuy, H. Boudra, and R. Cassini. 1994. Biotic and abiotic factors in fumonisin B1 production and stability. J. AOAC Int. 77:517-521. [Google Scholar]
- 29.Li, Y. C., D. R. Ledoux, A. J. Bermudez, K. L. Fritsche, and G. E. Rottinghaus. 1999. Effects of fumonisin B1 on selected immune responses in broiler chicks. Poult. Sci. 78:1275-1282. [DOI] [PubMed] [Google Scholar]
- 30.Lingwood, C. A. 1999. Glycolipid receptors for verotoxin and Helicobacter pylori: role in pathology. Biochim. Biophys. Acta 1455:375-386. [DOI] [PubMed] [Google Scholar]
- 31.Mannon, J., and E. Johnson. 1985. Fungi down the farm. New Sci. 105:12-16. [Google Scholar]
- 32.Marasas, W. F., T. S. Kellerman, W. C. Gelderblom, J. A. Coetzer, P. G. Thiel, and J. J. van der Lugt. 1988. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 55:197-203. [PubMed] [Google Scholar]
- 33.Marin, D. E., I. Taranu, R. P. Bunaciu, F. Pascale, D. S. Tudor, N. Avram, M. Sarca, I. Cureu, R. D. Criste, V. Suta, and I. P. Oswald. 2002. Influence of low-level exposure to aflatoxin on performance, blood parameters, humoral and cellular responses in weanling pigs. J. Anim. Sci. 80:1250-1257. [DOI] [PubMed] [Google Scholar]
- 34.Maxson, R. T., R. J. Jackson, and S. D. Smith. 1995. The protective role of enteral IgA supplementation in neonatal gut origin sepsis. J. Pediatr. Surg. 30:231-234. [DOI] [PubMed] [Google Scholar]
- 35.Meivar-Levy, I., and A. H. Futerman. 1999. Up-regulation of neutral glycosphingolipid synthesis upon long term inhibition of ceramide synthesis by fumonisin B1. J. Biol. Chem. 274:4607-4612. [DOI] [PubMed] [Google Scholar]
- 36.Merrill, A. H., Jr., E. Wang, T. R. Vales, E. R. Smith, J. J. Schroeder, D. S. Menaldino, C. Alexander, H. M. Crane, J. Xia, D. C. Liotta, F. I. Meredith, and R. T. Riley. 1996. Fumonisin toxicity and sphingolipid biosynthesis. Adv. Exp. Med. Biol. 392:297-306. [DOI] [PubMed] [Google Scholar]
- 37.Miller, E. R., and D. E. Ullrey. 1987. The pig as a model for human nutrition. Annu. Rev. Nutr. 7:361-382. [DOI] [PubMed] [Google Scholar]
- 38.Mims, C. A. 1987. The pathogenesis of infectious disease, 3rd ed. Academic Press, London, United Kingdom.
- 39.Mirocha, C. J., H. K. Abbas, and R. F. Vesonder. 1990. Absence of trichothecenes in toxigenic isolates of Fusarium moniliforme. Appl. Environ. Microbiol. 56:520-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motelin, G. K., W. M. Haschek, D. K. Ness, W. F. Hall, K. S. Harlin, D. J. Schaeffer, and V. R. Beasley. 1994. Temporal and dose-response features in swine fed corn screenings contaminated with fumonisin mycotoxins. Mycopathologia 126:27-40. [DOI] [PubMed] [Google Scholar]
- 41.Murphy, P. A., L. G. Rice, and P. F. Ross. 1993. Fumonisin B1, B2 and B3 content of Iowa, Wisconsin and Illinois corn and corn screening. J. Agric. Food Chem. 41:263-266. [Google Scholar]
- 42.Oswald, I. P., and C. Comera. 1998. Immunotoxicity of mycotoxins. Rev. Med. Vet. 149:585-590. [Google Scholar]
- 43.Osweiler, G. D. 2000. Mycotoxins. Contemporary issues of food animal health and productivity. Vet. Clin. N. Am. Food Anim. Pract. 16:511-530. [DOI] [PubMed] [Google Scholar]
- 44.Osweiler, G. D., P. F. Ross, T. M. Wilson, P. E. Nelson, S. T. Witte, T. L. Carson, L. G. Rice, and H. A. Nelson. 1992. Characterization of an epizootic of pulmonary edema in swine associated with fumonisin in corn screenings. J. Vet. Diagn. Investig. 4:53-59. [DOI] [PubMed] [Google Scholar]
- 45.Rheeder, J. P., W. F. O. Marasas, P. G. Thiel, E. W. Sydenham, G. S. Shepard, and D. J. van Schalkwyk. 1992. Fusarium moniliforme and fumonisins in corn in relation to human esophagal cancer in Transkei. Phytopathology 82:353-357. [Google Scholar]
- 46.Riley, R. T., N. H. An, J. L. Showker, H. S. Yoo, W. P. Norred, W. J. Chamberlain, E. Wang, A. H. Merrill, Jr., G. Motelin, V. R. Beasley, and W. M. Haschek. 1993. Alteration of tissue and serum sphinganine to sphingosine ratio: an early biomarker of exposure to fumonisin-containing feeds in pigs. Toxicol. Appl. Pharmacol. 118:105-112. [DOI] [PubMed] [Google Scholar]
- 47.Riley, R. T., K. A. Voss, W. P. Norred, R. P. Sharma, E. Wang, and A. H. Merrill. 1998. Fumonisins: mechanism of mycotoxicity. Rev. Med. Vet. 149:617-626. [Google Scholar]
- 48.Riley, R. T., E. Enongene, K. A. Voss, W. P. Norred, F. I. Meredith, R. P. Sharma, J. Spitsbergen, D. E. Williams, D. B. Carlson, and A. H. Merrill. 2001. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 109(Suppl. 2):301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rotter, B. A., B. K. Thompson, D. B. Prelusky, H. L. Treeholm, B. Stewaer, J. D. Miller, and M. E. Savard. 1996. Response of growing swine to dietary exposure to pure fumonisin B1 during an eight-week period: growth and clinical parameters. Nat. Toxins 4:42-50. [DOI] [PubMed] [Google Scholar]
- 50.Russo, T. A., and J. R. Johnson. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. [DOI] [PubMed] [Google Scholar]
- 51.Sandvig, K., O. Garred, A. van Helvoort, G. van Meer, and B. van Deurs. 1996. Importance of glycolipid synthesis for butyric acid-induced sensitization to shiga toxin and intracellular sorting of toxin in A431 cells. Mol. Biol. Cell 7:1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scudamore, K. A., S. Nawaz, and M. T. Hetmanski. 1990. Mycotoxins in ingredients of animal feeding stuffs: II. Determination of mycotoxins in maize and maize products. Food Addit. Contam. 15:30-55. [DOI] [PubMed] [Google Scholar]
- 53.Shephard, G. S., P. G. Thiel, S. Stockenstrom, and E. W. Sydenham. 1996. Worldwide survey of fumonisin contamination of corn and corn-based products. J. AOAC Int. 79:671-687. [PubMed] [Google Scholar]
- 54.Smith, G. W., P. D. Constable, A. R. Smith, C. W. Bacon, F. I. Meredith, G. K. Wollenberg, and W. M. Haschek. 1996. Effects of fumonisin-containing culture material on pulmonary clearance in swine. Am. J. Vet. Res. 57:1233-1248. [PubMed] [Google Scholar]
- 55.Smith, H. 1992. Virulence determinants of Escherichia coli: present knowledge and questions. Can. J. Microbiol. 38:747-752. [DOI] [PubMed] [Google Scholar]
- 56.Stoev, S. D., D. Goundasheva, T. Mirtcheva, and P. G. Mantle. 2000. Susceptibility to secondary bacterial infections in growing pigs as an early response in ochratoxicosis. Exp. Toxicol. Pathol. 52:287-296. [DOI] [PubMed] [Google Scholar]
- 57.Tai, J. H., and J. J. Pestka. 1988. Impaired murine resistance to Salmonella typhimurium following oral exposure to the trichothecene T-2 toxin. Food Chem. Toxicol. 26:691-698. [DOI] [PubMed] [Google Scholar]
- 58.Tannock, G. W. 1983. Effects of dietary and environmental stress on the gastrointestinal microbiota, p. 517-539. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, New York, N.Y.
- 59.Zomborszky, M. K., F. Vetesi, I. Repa, F. Kovacs, A. Bata, P. Horn, A. Toth, and R. Romvari. 2000. Experiment to determine limits of tolerance for fumonisin B1 in weaned piglets. J. Vet. Med. Ser. B 47:277-286. [DOI] [PubMed] [Google Scholar]
- 60.Zomborszky-Kovacs, M., F. Vetesi, P. Horn, I. Repa, and F. Kovacs. 2002. Effects of prolonged exposure to low-dose fumonisin B1 in pigs. J. Vet. Med. Ser. B 49:197-201. [DOI] [PubMed] [Google Scholar]