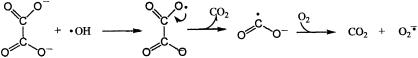Abstract
The redox cycle of 2,5-dimethoxybenzoquinone (2,5-DMBQ) is proposed as a source of reducing equivalent for the regeneration of Fe2+ and H2O2 in brown rot fungal decay of wood. Oxalate has also been proposed to be the physiological iron reductant. We characterized the effect of pH and oxalate on the 2,5-DMBQ-driven Fenton chemistry and on Fe3+ reduction and oxidation. Hydroxyl radical formation was assessed by lipid peroxidation. We found that hydroquinone (2,5-DMHQ) is very stable in the absence of iron at pH 2 to 4, the pH of degraded wood. 2,5-DMHQ readily reduces Fe3+ at a rate constant of 4.5 × 103 M−1s−1 at pH 4.0. Fe2+ is also very stable at a low pH. H2O2 generation results from the autoxidation of the semiquinone radical and was observed only when 2,5-DMHQ was incubated with Fe3+. Consistent with this conclusion, lipid peroxidation occurred only in incubation mixtures containing both 2,5-DMHQ and Fe3+. Catalase and hydroxyl radical scavengers were effective inhibitors of lipid peroxidation, whereas superoxide dismutase caused no inhibition. At a low concentration of oxalate (50 μM), ferric ion reduction and lipid peroxidation are enhanced. Thus, the enhancement of both ferric ion reduction and lipid peroxidation may be due to oxalate increasing the solubility of the ferric ion. Increasing the oxalate concentration such that the oxalate/ferric ion ratio favored formation of the 2:1 and 3:1 complexes resulted in inhibition of iron reduction and lipid peroxidation. Our results confirm that hydroxyl radical formation occurs via the 2,5-DMBQ redox cycle.
A prerequisite to gaining access to the cellulose and hemicellulose components of woody biomass is the circumvention of the lignin barrier. Filamentous fungi, the predominant degraders of wood, have evolved at least two mechanisms to circumvent this barrier. White rot fungi circumvent the lignin barrier by degrading it with extracellular peroxidases (14, 47), with eventual degradation to the level of CO2 (28). In contrast, brown rot fungi cannot degrade the lignin component to CO2. However, these fungi can access the cellulose components with minimal modification of the lignin. These modifications include demethylation of aryl methoxy groups and ring hydroxylation (for a more extensive review, see reference 29).
Due to the limited size of the wood pores and the nonspecific nature of wood degradation, Cowling and Brown (12) suggested that low-molecular-weight oxidants are the initial agents in wood decay. Koenigs (33) showed that a number of wood-decomposing fungi produce H2O2 and noted the similarities between wood treated with the hydroxyl radical and with brown rot fungi (34). Illman et al. (23) subsequently detected the hydroxyl radical in incubations with the brown rot fungus Poria placenta by use of electron spin resonance and spin trapping agents. Further supporting the involvement of the hydroxyl radical is the formation of 3-hydroxy derivatives (the expected products from a hydroxyl radical attack) of phthalic hydrazide in incubations with brown rot fungi (5).
The most likely nonphotochemical source of the hydroxyl radical is Fenton's reagent, defined by the following chemistry: Fe2+ + H2O2 → Fe3+ + •OH + OH−. The key reagents are iron, molecular oxygen, and a reducing agent (35), two of which, iron and O2, are readily available. Three different reducing agents for the iron have been suggested for brown rot fungi. One is an enzyme, cellobiose dehydrogenase (22), and the other two are chemicals, oxalate (42) and 2,5-dimethoxyhydroquinone (2,5-DMHQ) (26, 38). Although Hyde and Wood (22) demonstrated that cellobiose dehydrogenase can reduce iron, this enzyme has not been found in all brown rot fungi. Schmidt et al. (42) proposed that oxalate serves as a chelator and as a reducing agent for iron-dependent hydroxyl radical formation. More recently, three groups have proposed that 2,5-dimethoxybenzoquinone (2,5-DMBQ) and its hydroquinone, discovered in 1955 by Bu'Lock (8) and again isolated in 1976 by Nakajima et al. (37), serve as the extracellular reducing agents (26, 38). Kerem et al. (26) demonstrated the involvement of this quinone, as well as 4,5-dimethoxy-1,2-benzoquinone, in the extracellular cleavage of polyethylene glycol. The quinone undergoes cyclic oxidation-reduction reactions, serving as a shuttle for electrons from intracellular donors to extracellular acceptors. Although a similar mechanism has been proposed for white rot fungi (4, 17, 18) for hydroxyl radical formation, product analysis suggests that hydroxyl radical oxidation is relatively minor in comparison to peroxidase oxidation (30, 32).
The role of oxalate, ubiquitously found in brown rot fungi, as a chelating agent, and the role of pH, which is altered by the fungus, are not clear. Our objective in this study was to use a lipid peroxidation system to characterize 2,5-DMBQ-dependent hydroxyl radical formation and to determine the effect of oxalate and pH on 2,5-DMBQ-dependent production of the hydroxyl radical. Our results indicate that 2,5-DMBQ plays a key role in hydroxyl radical formation, that oxalate acts as a sequestering agent, and that pH plays a central role in these reactions.
MATERIALS AND METHODS
Chemicals.
2,5-DMBQ was purchased from TCI America (New York, N.Y.). Linolenic acid, oxalic acid, ferrozine, Lubrol (polyoxyethylene-9-lauryl ether), p-aminobenzoic acid, protocatechuic acid, deferoxamine mesylate, and superoxide dismutase from bovine erythrocytes were purchased from Sigma Chemical Company (St. Louis, Mo.). 2,2-Dimethyl succinic acid (DMS) was purchased from Aldrich Chemical Company (Milwaukee, Wis.). Catalase from Aspergillus niger was purchased from Calbiochem (La Jolla, Calif.).
2,5-DMBQ was chemically reduced by the procedure of Kerem et al. (26). The hydroquinone (2,5-DMHQ) crystals were stored desiccated under an argon atmosphere and were dissolved in argon-purged acetonitrile prior to dilution in solvents.
Organism.
Liquid cultures of Gloeophyllum trabeum (Mad 617-R) were started with homogenized mycelia. The inoculum was prepared by initial growth in static 250-ml Erlenmeyer flasks containing 50 ml of YMPG medium (46) at 30°C for 10 days. This mycelial preparation was collected by decanting the YMPG medium and washing the mat with 500 ml of distilled water, and then the preparation was decanted. The washed mycelium was added to 50 ml of the high-carbon, low-nitrogen liquid medium described by Kerem et al. (26) in a 2.6-liter Fernbach flask and grown statically at 30°C for 10 days. The mycelium collected from two Fernbach flasks was homogenized and used to inoculate 1 liter of the same medium. Static liquid cultures were grown in 125-ml Erlenmeyer flasks at 30°C with 5 ml of medium containing 1% glucose, 66 mg of asparagine/liter, 4 mg of NH4NO3/liter, BIII trace elements (46), and 1.5 g of DMS (pH 4.5)/liter as described by Kerem et al. (26). Cultures were flushed with water-saturated O2 on days 3, 6, and 9 of growth.
Oxygen consumption.
Oxygen consumption was measured with a YSI model 5300 (Instech Laboratories, Plymouth Meeting, Pa.) oxygen electrode. The concentration of dissolved O2 used in our calculation was assumed to be 230 μM. Reaction mixtures contained 100 μM FeSO4 in different buffers with different pH values: 25 mM Tris-Cl for pH 7 to 9 and 25 mM DMS for pH 2.5 to 6. Incubations were performed at 28°C.
Lipid peroxidation.
Oxidation of linolenic acid was used to assess hydroxyl radical formation. Malondialdehyde, an oxidation product of linolenic acid, was measured with thiobarbituric acid (7). An extinction coefficient of 1.56 × 105 M−1 cm−1 at 535 nm was used (7). Incubation mixtures, unless otherwise stated, contained 0.25 mg of linolenic acid/ml, 50 μM 2,5-DMHQ, and 100 μM FeCl3 in 25 mM DMS buffer (pH 4). Reaction rates were obtained by removing 0.5-ml aliquots which were then added to 1 ml of the thiobarbituric acid reagent (7) containing 25 μl of a solution of 2% butylated hydroxytoluene in ethanol. In incubation mixtures where the effect of hydroxyl radical scavengers was determined, 0.2% (wt/vol) Lubrol was added. In experiments where catalase and superoxide dismutase were added, the DMS-buffered incubation mixtures were adjusted to pH 5 with 10 M sodium hydroxide, the concentration of FeCl3 was either 100 or 200 μM, and Lubrol was at 0.06%. These concentrations were utilized to minimize the inhibiting effect of the detergent on the enzymes.
Reduction of iron.
Experiments where Fe3+ was reduced by 2,5-DMHQ were performed in 25 mM DMS, pH 4.0, and monitored by the formation of the ferrozine-Fe2+ complex. At various times, aliquots were removed and 1/10 volume of 10 mM ferrozine was added (10). An extinction coefficient of 27.9 mM−1 cm−1 at 562 nm was used.
Rapid kinetic measurements of iron reduction.
Reduction of Fe3+ was also measured by rapid kinetic techniques. The stopped-flow apparatus used was purchased from KinTek Instruments (State College, Pa.) and contained a 2.6-cm light path. The rates were determined by averaging kinetic traces from three shots. The reduction of Fe3+ was determined by direct reduction of the ferrozine-Fe3+ complexes. Typical experiments contained 3 mM ferrozine-15 μM FeCl3 in one syringe and various concentrations of 2,5-DMHQ in the second syringe. Ferrous ion formation was monitored at 562 nm (10). All reactions were performed at 28°C, and the mixtures were buffered with 25 mM DMS, pH 4.0.
Effect of oxalate on the hydroxylation of p-hydroxybenzoic acid.
Static liquid cultures (5 ml in 125-ml Erlenmeyer flasks) were grown for 9 days. Gently, 0.5 ml of 10 mM p-hydroxybenzoate (in water) was added to the cultures, yielding a final concentration of 0.91 mM. In some of these incubation mixtures, oxalate was also included in the liquid media at final concentrations of 100, 200, 400, and 800 μM. After an 8-h incubation, the extracellular medium was separated from the mycelia by filtration with cheesecloth and then analyzed for protocatechuic acid by high-performance liquid chromatography (HPLC) with a Supelcosil LC-18 column (Supelco, Bellefonte, Pa.). Protocatechuic acid was monitored at 254 nm. The product was identified and quantitated based on a comparison of the retention time to known standards. The column chromatography was operated at 1 ml/min and eluted with a linear gradient of 0 to 30% methanol in 10 mM phosphoric acid.
Calculation of concentration of iron complexes.
The relative contribution of each iron form to the total iron concentration is given by equation 1, where [Fe], [Fe(C)],…, [Fe(C)n] correspond to the concentrations of free iron ions, a 1:1 iron-chelator complex,…, and a 1:n iron-chelator complex, respectively.
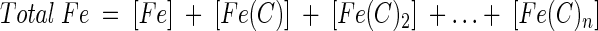 |
(1) |
This relationship can be rewritten with binding constants, as in equation 2, where K1, K2,…, Kn are the binding constants for the first, second, to the nth molecule of chelator to the iron ion.
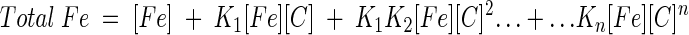 |
(2) |
The fractions of iron ions as free ions and as the iron complex are calculated with equations 3 to 6, where αFe, αFe(C), and αFe(C)n designate the fractions of iron ions in the form of free iron ions, a 1:1 iron-chelator complex, and a 1:n iron-chelator complex, respectively.
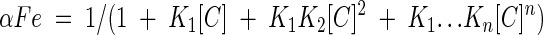 |
(3) |
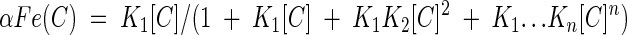 |
(4) |
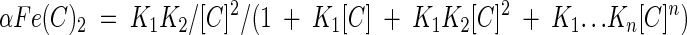 |
(5) |
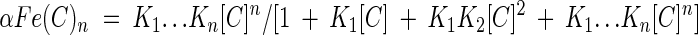 |
(6) |
Binding constants K1 of 2.5 × 109 M−1, K2 of 6.3 × 106 M−1, and K3 of 1 × 104 M−1 were used for the Fe3+ complexes with oxalate (36, 44). A K1 of 5.01 × 104 M−1, a K2 of 7.07 × 102 M−1, and a K3 of 1 × 10 M−1 were used for the Fe2+ complexes with oxalate (36, 44).
RESULTS
Reduction of iron by 2,5-DMHQ.
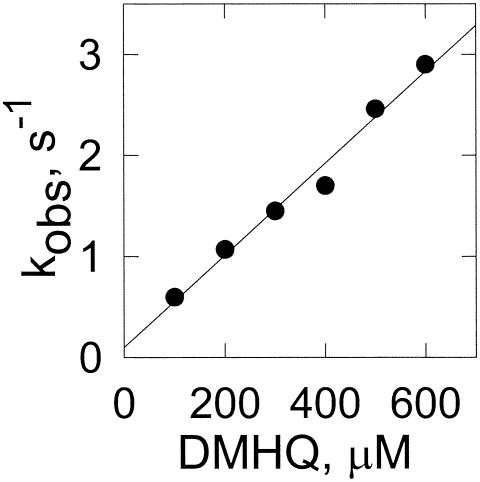
Fenton's reagent is composed of the ferrous ion and H2O2. Both can be formed from Fe3+ and O2 in the presence of a reducing agent. 2,5-DMHQ has been shown to be the reducing agent formed by brown rot fungi (27, 38). To determine the rate constant for the reduction of iron, we monitored the formation of the ferrous ion-ferrozine complex. However, the rate is too high to monitor by conventional techniques. Thus, we monitored iron reduction in a stopped-flow apparatus with one syringe containing various concentrations of 2,5-DMHQ and another syringe containing the ferric ion-ferrozine complex. Reduction of the ferric complex to the ferrous complex results in an increase in absorbance at 562 nm. The rate of reduction is dependent on the concentration of 2,5-DMHQ (Fig. 1). The second-order rate constant is 4.5 × 103 M−1 s−1.
FIG. 1.
Reduction of iron by 2,5-DMHQ based on stopped-flow experiments. The preformed ferrozine-Fe3+ complex was reduced by 2,5-DMHQ. Data points are the mean results for three stopped-flow shots with standard deviations of less than 10% of the means. The linear fit of the data drawn is a linear fit with r2 being 0.982. The observed rate (kobs) is plotted.
Autoxidation of the ferrous ion and 2,5-DMHQ.
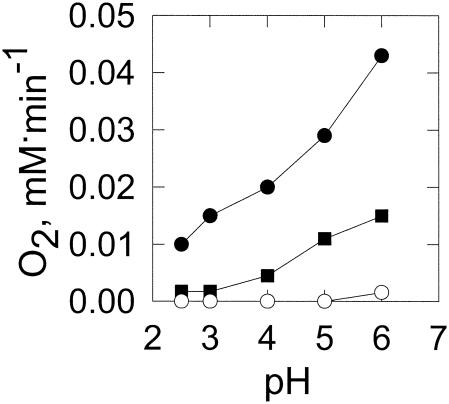
Solutions of ferrous ions alone can form H2O2 (6). This autoxidation is a second-order process with respect to the ferrous ion concentration (19) and can be measured by the rate of oxygen consumption. At a low pH, this reaction is slow as the ferrous ion does not readily autoxidize (Fig. 2). Thus, at the acidic pH values observed for fungal growth, the ferrous ion is relatively stable and very little of the reduced oxygen species is formed by this mechanism.
FIG. 2.
Effect of pH on autoxidation of the ferrous ion and 2,5-DMHQ. Autoxidation of ferrous ions (○) and 2,5-DMHQ was measured by oxygen consumption. 2,5-DMHQ-containing incubation mixtures contained 50 μM 2,5-DMHQ with (•) or without (▪) 100 μM FeSO4 in 25 mM DMS buffer. Data points are the means of results from three experiments where the standard deviations are less than 10% of the means.
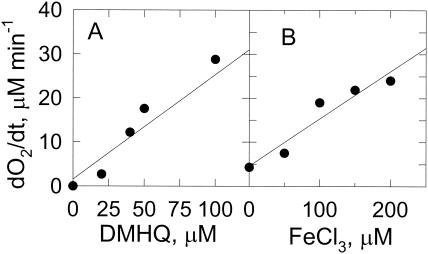
At low pH, ferric ions are readily reduced by 2,5-DMHQ (Fig. 1), but molecular oxygen is not (Fig. 2). In the absence of iron, the rate of 2,5-DMHQ autoxidation increases gradually from pH 3 to 6. In the presence of iron, the rate of 2,5-DMHQ-dependent oxygen consumption is much higher. The rate of oxygen consumption increased linearly with the increases in 2,5-DMHQ concentration and ferric ion concentration (Fig. 3). These results indicate that the predominant route for O2 reduction by 2,5-DMHQ is through an iron-dependent mechanism rather than a direct reaction of 2,5-DMHQ with O2. The formation of H2O2 during this autoxidation process was demonstrated by an increase in O2 concentration following the addition of catalase (data not shown).
FIG. 3.
Dependence of 2,5-DMHQ autoxidation on iron. Autoxidation was measured by oxygen consumption. (A) 2,5-DMHQ concentration dependence of iron-dependent 2,5-DMHQ oxidation. Reaction mixtures contained 100 μM FeCl3 and various 2,5-DMHQ concentrations in 25 mM DMS buffer, pH 4.0. The data drawn are a linear fit with r2 being 0.937. (B) Effect of FeCl3 concentration on 2,5-DMHQ oxidation. Reaction mixtures contained 50 μM 2,5-DMHQ in 25 mM DMS, pH 4.0. Data points are the means of results from three experiments where the standard deviations are less than 10% of the means. The data drawn are a linear fit with r2 being 0.919. The change in O2 concentration over time (dO2/dt) is plotted.
2,5-DMHQ-dependent hydroxyl radical formation.
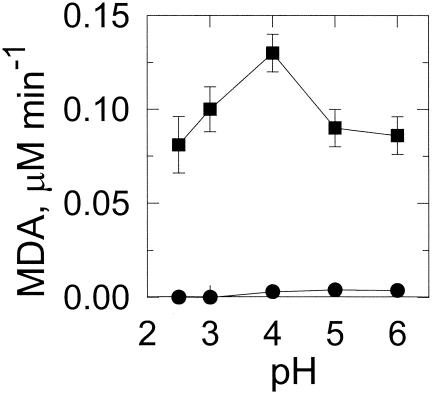
A lipid peroxidation system containing linolenic acid, which forms malondialdehyde and is readily detected using thiobarbituric acid (7), was used to assess hydroxyl radical formation. Malondialdehyde was formed readily in 2,5-DMHQ-containing reaction mixtures (Table 1). In the absence of 2,5-DMHQ or iron, little or no peroxidation was detected. The dependence on iron is consistent with the increased rates of oxygen consumption resulting from the addition of iron. The rate of lipid peroxidation increased with increased pH up to pH 4 (Fig. 4). Above pH 4, the rate of peroxidation decreased, possibly due to the higher rate of ferrous autoxidation.
TABLE 1.
DMHQ-dependent lipid peroxidation
| Reaction mixture | Malondialdehyde produced (nmol/min/ml)a |
|---|---|
| Complete reaction mixture | 1.20 ± 0.02 |
| Minus 2,5-DMHQ | 0.04 ± 0.01 |
| Minus Fe3+ | 0.01 ± 0.00 |
| Complete reaction mixture plus hydroxyl radical scavenger | |
| Mannitol (100 mM) | 1.11 ± 0.10 |
| Ethanol (100 mM) | 1.50 ± 0.40 |
| Lubrol (0.2%, wt/vol) | 1.02 ± 0.20 |
| Lubrol (0.2%, wt/vol), mannitol (100 mM) | 0.46 ± 0.05 |
| Lubrol (0.2%, wt/vol), ethanol (100 mM) | 0.50 ± 0.05 |
| Complete reaction mixture plus chelator or enzyme | |
| Deferoxamine mesylate (0.2 mM) | 0.16 ± 0.05 |
| EDTA (1 mM) | 0.13 ± 0.05 |
| Catalase (1 U) | 0.91 ± 0.08 |
| Boiled catalase | 1.32 ± 0.08 |
| Superoxide dismutase (1 U) | 1.26 ± 0.08 |
| Boiled superoxide dismutase | 1.34 ± 0.04 |
Results are the means and standard deviations of results from three incubations.
FIG. 4.
Effect of pH on 2,5-DMHQ-dependent lipid peroxidation. Reaction mixtures were with (▪) or without (•) 100 μM FeCl3 in 25 mM DMS at various pH values. Lipid peroxidation was measured by malondialdehyde (MDA) formation. Data points and error bars are the means and standard deviations of results from three experiments.
Inhibition of 2,5-DMHQ-dependent lipid peroxidation.
The addition of the hydroxyl radical scavengers mannitol and ethanol did not inhibit lipid peroxidation (Table 1). However, when the micelles were dispersed with the detergent Lubrol, mannitol and ethanol could inhibit this one-phase system. This result is consistent with previous observations of hydroxyl radical-dependent oxidation of phospholipid liposomes in which hydroxyl radical scavengers inhibited the reaction only when detergent was added (48). The addition of EDTA or deferoxamine inhibited malondialdehyde formation (Table 1), but the addition of superoxide dismutase did not (Table 1). The addition of catalase resulted in significant, but not complete, inhibition (Table 1). Neither boiled catalase nor boiled superoxide dismutase had a significant effect.
Effect of oxalate on lipid peroxidation and reactions with iron.
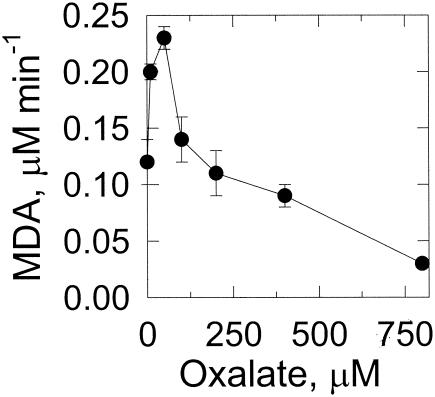
At concentrations up to 50 μM, oxalate stimulates lipid peroxidation (Fig. 5), but further increases in oxalate concentration decrease the rate of peroxidation. Due to the possible photochemical reactions of oxalate and iron (15, 20, 22), experiments were also performed in the absence of light, with identical results (data not shown).
FIG. 5.
Effect of oxalate on lipid peroxidation. Reaction mixtures are described in Materials and Methods and contained the specified oxalate concentrations. Lipid peroxidation was measured by malondialdehyde (MDA) formation. Data points and error bars are the means and standard deviations of results from three experiments.
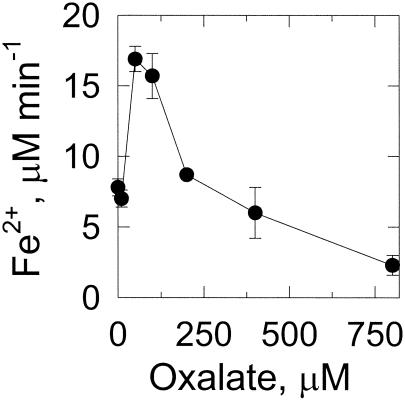
Under anaerobic conditions and at low concentrations, oxalate stimulates iron reduction by 2,5-DMHQ, but at concentrations above 50 μM, the reduction of iron gradually decreases (Fig. 6). Thus, at a high oxalate concentration, the oxalate appears to sequester the iron and to prevent its reaction with 2,5-DMHQ. Again, these experiments were repeated in the dark, with identical results (data not shown).
FIG. 6.
Effect of oxalate on reduction of the ferric ion by 2,5-DMHQ. Reaction mixtures contained 50 μM 2,5-DMHQ and 100 μM FeCl3 in 25 mM DMS, pH 4.0, and various concentrations of oxalate. At 10 min, the ferrous ion was quantitated by removing 0.25-ml aliquots and adding them to 0.25 ml of water and 0.05 ml of 10 mM ferrozine (final concentration of ferrozine, 0.91 mM). Data points and error bars are the means and standard deviations of results from three experiments.
Hydroxylation of p-hydroxybenzoic acid and effect of oxalate.
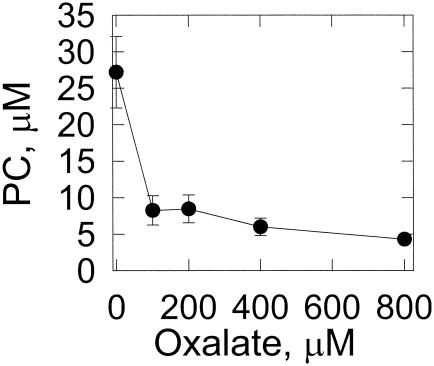
The effect of oxalate on hydroxyl radical formation in cultures was measured by hydroxylation of p-hydroxybenzoic acid to form protocatechuic acid (41). Increasing the concentration of oxalate added to high-carbon, low-nitrogen cultures resulted in decreased protocatechuic acid formation (Fig. 7).
FIG. 7.
Effect of oxalate on the hydroxylation of p-hydroxybenzoic acid. The p-hydroxybenzoic acid was added to liquid cultures of G. trabeum, as described in Materials and Methods, containing various concentrations of oxalate. After an 8-h incubation, protocatechuic acid (PC) was quantitated by HPLC. Data points and error bars are the means and standard deviations of results from three experiments.
DISCUSSION
The production of extracellular hydroxyl radicals enables brown rot fungi to oxidize a large number of seemingly unrelated chemicals, such as dimethyl sulfoxide (21), phthalic hydrazide (5), lignin (25), and cellulose (11, 31). The substrate of the hydroxyl radical is hypothesized to be cellulose and hemicellulose. Cleavage of these polymers into small, diffusible fragments allows the fungus to circumvent the lignin barrier and the crystalline structure of cellulose, which are formidable problems for large, bulky enzymes. The formation of a nonphotochemically generated hydroxyl radical requires a metal (typically ferric ions), molecular oxygen, and a reducing agent. In biological systems (wood), free iron and molecular oxygen are readily available. Thus, for brown rot fungi, secretion of a reducing agent can result in extracellular hydroxyl radical formation. The delivery of extracellular electrons by 2,5-DMBQ and 2,5-DMHQ and the subsequent reactions with the ferric ion and molecular oxygen may be summarized as follows (26, 38):
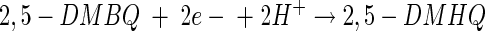 |
(7) |
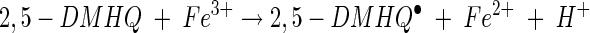 |
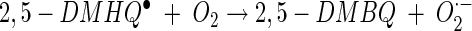 |
(9) |
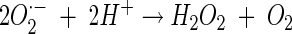 |
(10) |
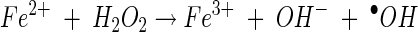 |
(11) |
In reaction 7, 2,5-DMBQ is reduced by a mycelial reductase to yield the hydroquinone 2,5-DMHQ (24). At a low pH, secreted 2,5-DMHQ is stable for autoxidation but is a good reductant for the ferric ion. Reduction by one electron yields the ferrous ion and the semiquinone radical (reaction 8). The semiquinone radical is further oxidized to the quinone by molecular oxygen to yield superoxide (reaction 9). The predominant source of H2O2 is probably the dismutation of superoxide rather than the autoxidation of the ferrous ion (reaction 10). At a low pH, the oxidation of Fe2+ is relatively slow. Thus, the components of Fenton's reagent (reaction 11) are formed with the ferric ion, 2,5-DMHQ, and molecular oxygen. In the present study, we (i) determined the rate constant for reaction 8, (ii) determined the effect of pH on the sequence of reactions 8 to 11, and (iii) determined the effect of oxalate on reactions 8 to 11. Reactions 7 to 11 are also consistent with our data on inhibition with superoxide dismutase, catalase, and hydroxyl radical scavengers. Although we obtained only 24% inhibition with 1 U of catalase/ml, this level of inhibition is comparable to or better than that previously observed. For example, in a study by Chen and Schopfer (9), 26 U of catalase/ml caused only 3% inhibition of hydroxyl radical-dependent oxidation of RNA.
The pH is important for 2,5-DMBQ-driven Fenton chemistry since protons (or hydroxide ions) are reactants or products in all five reactions. The pH also affects the chelation (and, thus, the reactivity) of iron by organic acids such as oxalate (22). Fungi often lower the pH of the extracellular medium through secretion of organic acids and thus establish a pH gradient around the mycelium (22). By lowering the pH of the medium, 2,5-DMHQ and ferrous ions (Fig. 2) are effectively stabilized and do not autoxidize. Yet, in this pH range, 2,5-DMHQ can reduce ferric ions, and the 2,5-DMHQ semiquinone can reduce molecular oxygen to form superoxide. As the pH increases toward neutrality, both 2,5-DMHQ and ferrous ions are destabilized and more readily autoxidize. These properties could explain the pH profile observed for 2,5-DMHQ-dependent lipid peroxidation (Fig. 4), where the highest rates occur at pH 4. As pH increases up to pH 4, the rate of iron-dependent oxidation of 2,5-DMHQ increases (Fig. 2), resulting in increased Fe2+ and H2O2 concentrations and increasing the rate of hydroxyl radical formation. Above pH 4, the enhanced rate of 2,5-DMHQ autoxidation decreases the steady-state level of Fe2+, thereby reducing the rate of hydroxyl radical formation.
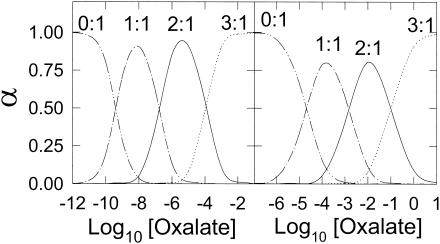
The pH also impacts the speciation of organic acids with ferric ions. The organic acid oxalate is produced by most, if not all, wood-degrading fungi (43). Hyde and Wood (22) calculated that in a solution of 10 mM oxalate, increasing the pH from 1.5 to 3.5 changes a 50:50 mixture of 2:1 and 3:1 oxalate-Fe3+ complexes to 100% of the 3:1 complex. Complexation of Fe3+ with oxalate is also affected by changes in oxalate concentration. A concentration range of 100 (2) to 500 (27) μM oxalate has been reported for G. trabeum. Within this range, the dominant ferric ion species is a mixture of the 2:1 and 3:1 complexes (Fig. 8). Whether Fe3+ is complexed by two oxalates or three oxalates greatly affects the reactivity of the iron. For example, the reduction potential is 771 mV for free Fe3+, 468 mV for the 1:1 complex, 181 mV for the 2:1 complex, and −120 mV for the 3:1 complex (22). Thus, changes in reduction potential caused by speciation changes may preclude certain reductants from reducing the Fe3+ (22).
FIG. 8.
Relative fraction (α) of oxalate-iron species. The left panel plots the oxalate-ferric ion species by using a K1 of 2.5 × 109 M−1, a K2 of 6.3 × 106 M−1, and a K3 of 1 × 104 M−1. The right panel plots the relative fraction of the oxalate-ferrous species by using a K1 of 5.01 × 104 M−1, a K2 of 7.07 × 102 M−1, and a K3 of 1 × 10 M−1. The complex ratios are labeled.
To study the effect of oxalate on 2,5-DMBQ-dependent reactions, we maintained the pH at 4, the approximate pH of fungal cultures, and varied the oxalate concentration within the physiologically reported range. At physiological concentrations, oxalate inhibits iron reduction, hydroxyl radical formation (lipid peroxidation), and hydroxylation of p-hydroxybenzoic acid. Our inhibition studies of iron reduction and lipid peroxidation were performed with 100 μM Fe3+. This Fe3+ level is much higher than that found in wood; however, this concentration facilitated our in vitro studies. When concentrations of oxalate are less than 100 μM, Fe3+ reduction and lipid peroxidation are both enhanced and a mixture of 2:1 and 3:1 complexes is expected (Fig. 8). However, in our experiments, the 1:1 oxalate-iron complex probably dominates because of the high iron concentration used. Thus, enhanced lipid peroxidation and ferric ion reduction may result from oxalate increasing the solubility of ferric ions. Inhibition of 2,5-DMHQ-dependent reactions occurs at oxalate concentrations above 100 μM (where the 2:1 or 3:1 complexes are favored under our experimentally high iron concentrations). The reduction potential for 2,5-DMBQ is −590 mV (3), so this inhibition cannot be explained by unfavorable reduction potentials. We cannot explain the inhibition of Fe3+ reduction by oxalate. Oxalate may prevent the formation of the ferrozine-Fe2+ complex, thereby preventing its detection, but the addition of increasing concentrations of oxalate to ferrozine-Fe2+ did not decrease the absorbance (data not shown).
The inhibition of lipid peroxidation by oxalate may also result from the inhibition of iron reduction or the radical scavenging activity of oxalate. The one-electron oxidation of oxalate yields the formate radical. This radical reduces molecular oxygen to form superoxide at a rate limited by diffusion (1) (Fig. 9).
FIG. 9.
Oxalate yields the formate radical which reduces molecular oxygen to form superoxide.
This concentration dependence of Fenton chemistry on oxalate was also reported by Tanaka et al. (45). They studied the change in viscosity of cellulose following oxidation by Fenton's reagent. A similar effect was also found by Schmidt et al. (42) and Shimada et al. (43). The addition of increasing concentrations of oxalate to fungal cultures also inhibited hydroxyl radical-dependent hydroxylation of p-hydroxybenzoic acid.
In conclusion, our results indicate that at low concentrations, oxalate facilitates hydroxyl radical formation, but at higher concentrations, oxalate inhibits hydroxyl radical formation by brown rot fungi. The ability of oxalate to inhibit hydroxyl radical formation can be attributed to the speciation of iron by oxalate, which is greatly affected by both the pH and the oxalate concentration (22). Because our experiments were also performed in the dark with similar results, none of the effects observed are due to photochemistry (15, 20). Due to the sensitivity of oxalate-dependent reactions to oxalate concentration and to pH, and in light of the physiological variation in pH (from pH 2 to 6.7) (2, 13) and in oxalate concentration (2, 27), it is not possible to determine an exact role for oxalate in hydroxyl radical formation. Although oxalate is reported as a reductant of Fe3+ for brown rot fungi (42), this role has since been questioned due to the photochemical dependence of this process (15, 20). The ability of oxalate to sequester ferric ions may protect brown rot fungi from hydroxyl radicals. Further support for this hypothesis is that white rot fungi, which do not utilize the hydroxyl radical as the major oxidant (30, 32), also produce oxalate (39, 40, 42, 49). Despite reports on how the hydroxyl radical may be formed in white rot fungi (4, 17, 18), it is unlikely that oxalate would have a role in its formation. The chemical signatures of wood affected by white rot fungi and brown rot fungi are different (30). If oxalate has a common role in both fungi, then the hypothesis of Green et al. (16), that oxalate's role in wood decay is to chelate calcium, resulting in a weakening of the wood structure and increasing the pore size, should be more critically evaluated.
Acknowledgments
This work was supported in part by U.S. Department of Energy grant DE-FG02-87ER13690.
We thank Andrew Zimmerman for assistance with the HPLC analysis of 2,5-DMBQ.
REFERENCES
- 1.Adams, G. E., and R. L. Willson. 1969. Pulse radiolysis studies on the oxidation of organic radicals in aqueous solution. Faraday Soc. Trans. 65:2981-2987. [Google Scholar]
- 2.Akamatsu, Y., M. Takahashi, and M. Shimada. 1994. Production of oxalic acid by wood-rotting basidiomycetes grown on low and high-nitrogen culture media. Mater. Org. 28:251-264. [Google Scholar]
- 3.Antelman, M. S. 1982. The encyclopedia of chemical electrode potentials. Plenum Press, New York, N.Y.
- 4.Backa, S., J. Gierer, T. Reitberger, and T. Nilsson. 1993. Hydroxyl radical activity associated with the growth of white-rot fungi. Holzforschung 47:181-187. [Google Scholar]
- 5.Backa, S., J. Gierer, T. Reitberger, and T. Nilsson. 1992. Hydroxyl radical activity in brown-rot fungi studied by a new chemiluminescence method. Holzforschung 46:61-67. [Google Scholar]
- 6.Barb, W. G., J. H. Baxendale, P. George, and K. R. Hargrave. 1951. Reactions of ferrous and ferric ions with hydrogen peroxide. Part I. The ferrous ion reaction. Faraday Soc. Trans. 47:462-500. [Google Scholar]
- 7.Buege, J. A., and S. D. Aust. 1978. Microsomal lipid peroxidation. Methods Enzymol. 52:302-310. [DOI] [PubMed] [Google Scholar]
- 8.Bu'Lock, J. D. 1955. Constituents of the higher fungi. Part IV. A quinone from Polyporus fumosus. J. Chem. Soc. 1955:575-576. [Google Scholar]
- 9.Chen, S. X., and P. Schopfer. 1999. Hydroxyl radical production in physiological reactions. A novel function of peroxidase. Eur. J. Biochem. 260:726-735. [DOI] [PubMed] [Google Scholar]
- 10.Cowart, R. E., F. L. Singleton, and J. S. Hind. 1993. A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Anal. Biochem. 211:151-155. [DOI] [PubMed] [Google Scholar]
- 11.Cowling, E. B. 1961. Comparative biochemistry of sweetgum sapwood by white rot and brown rot fungi, p. 79. USDA Technical Bulletin 1258. U.S. Department of Agriculture, Washington, D.C.
- 12.Cowling, E. B., and W. Brown. 1969. Structural features of cellulosic materials in relation to enzymatic hydrolysis. ACS Adv. Chem. Ser. 95:152-187. [Google Scholar]
- 13.Espejo, E., and E. Agosin. 1991. Production and degradation of oxalic-acid by brown rot fungi. Appl. Environ. Microbiol. 57:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glenn, J. K., M. A. Morgan, M. B. Mayfield, M. Kuwahara, and M. H. Gold. 1983. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun. 114:1077-1083. [DOI] [PubMed] [Google Scholar]
- 15.Grayson, M. 1982. Kirk-Othmer encyclopedia of chemical technology, vol. 20. John Wiley, New York, N.Y.
- 16.Green, F., C. A. Clausen, T. A. Kuster, and T. L. Highley. 1995. Induction of polygalacturonase and the formation of oxalic-acid by pectin in brown-rot fungi. World J. Microbiol. Biotechnol. 11:519-524. [DOI] [PubMed] [Google Scholar]
- 17.Guillen, F., V. Gomez-Toribio, M. J. Martinez, and A. T. Martinez. 2000. Production of hydroxyl radical by the synergistic action of fungal laccase and aryl alcohol oxidase. Arch. Biochem. Biophys. 383:142-147. [DOI] [PubMed] [Google Scholar]
- 18.Guillén, F., C. Muñoz, V. Gómez-Toribio, A. T. Martínez, and M. J. Martínez. 2000. Oxygen activation during oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii. Appl. Environ. Microbiol. 66:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond, G. S., and C.-H. S. Wu. 1968. Oxidation of iron(II) chloride in nonaqueous solvents. ACS Adv. Chem. Ser. 77:186-206. [Google Scholar]
- 20.Hatchard, C. G., and C. A. Parker. 1956. A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. Lond. A 235:518-536. [Google Scholar]
- 21.Hirano, T., H. Tanaka, and A. Enoki. 1995. Extracellular substance from the brown rot basidiomycete Tyromycetes palustris that reduces molecular oxygen to hydroxyl radicals and ferric iron to ferrous iron. Mokuzai Gakkaishi 41:334-341. [Google Scholar]
- 22.Hyde, S. M., and P. M. Wood. 1997. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe(III) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology 143:259-266. [DOI] [PubMed] [Google Scholar]
- 23.Illman, B. L., D. C. Meinholtz, and T. L. Highley. 1989. Oxygen free radical detection in wood colonized by the brown rot fungus Postia placenta. Biodeterioration. Res. 2:497-509. [Google Scholar]
- 24.Jensen, K. A., Jr., Z. C. Ryan, A. Vanden Wymelenberg, D. Cullen, and K. E. Hammel. 2002. An NADH:quinone oxidoreductase active during biodegradation by the brown-rot basidiomycete Gloeophyllum trabeum. Appl. Environ. Microbiol. 68:2699-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, L., T. P. Schultz, and D. D. Nicholas. 1990. Structural characterization of brown-rotted lignin. Holzforschung 44:133-138. [Google Scholar]
- 26.Kerem, Z., W. Bao, and K. E. Hammel. 1998. Rapid polyether cleavage via extracellular one-electron oxidation by a brown-rot basidiomycete. Proc. Natl. Acad. Sci. USA 95:10373-10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerem, Z., and K. E. Hammel. 1999. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven Fenton reaction. FEBS Lett. 446:49-54. [DOI] [PubMed] [Google Scholar]
- 28.Keyser, P., T. K. Kirk, and J. G. Zeikus. 1978. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J. Bacteriol. 135:790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk, T. K. 1983. Degradation and conversion of lignocelluloses, p. 266-295. In J. E. Smith, D. R. Berry, and B. Kristiansen (ed.), The filamentous fungi, vol. 4. Edward Arnold, London, United Kingdom.
- 30.Kirk, T. K., and R. L. Farrell. 1987. Enzymatic “combustion”: the microbial degradation of lignin. Annu. Rev. Microbiol. 41:465-505. [DOI] [PubMed] [Google Scholar]
- 31.Kirk, T. K., R. Ibach, M. D. Mozuch, A. H. Conner, and T. L. Highley. 1991. Characteristics of cotton cellulose depolymerized by a brown-rot fungus, by acid, or by chemical oxidants. Holzforschung 45:239-244. [Google Scholar]
- 32.Kirk, T. K., M. D. Mozuch, and M. Tien. 1985. Free hydroxyl radical is not involved in an important reaction of lignin degradation by Phanerochaete chrysosporium Burds. Biochem. J. 226:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenigs, J. W. 1972. Production of extracellular hydrogen peroxide and peroxidase by wood-rotting fungi. Phytopathology 62:100-110. [Google Scholar]
- 34.Koenigs, J. W. 1974. Hydrogen peroxide and iron: a proposed system for decomposition of wood by brown-rot basidiomycetes. Wood Fibre 6:66-80. [Google Scholar]
- 35.Koppenol, W. H. 2001. The Haber-Weiss cycle-70 years later. Redox Rep. 6:229-234. [DOI] [PubMed] [Google Scholar]
- 36.Martell, A. E., and R. M. Smith (ed.). 1977. Critical stability constants, vol. 3, p. 29-97. Plenum Press, New York, N.Y.
- 37.Nakajima, S., K. I. Kawai, S. Yamada, and Y. Sawai. 1976. Isolation of oospo lactone as antifungal principle of Gloeophyllum sepiarium. Agric. Biol. Chem. 40:811-812. [Google Scholar]
- 38.Paszczynski, A., R. Crawford, D. Funk, and B. Goodell. 1999. De novo synthesis of 4,5-dimethoxycatechol and 2,5-dimethoxyhydroquinone by the brown rot fungus Gloeophyllum trabeum. Appl. Environ. Microbiol. 65:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popp, J. L., B. Kalyanaraman, and T. K. Kirk. 1990. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry 29:10475-10480. [DOI] [PubMed] [Google Scholar]
- 40.Punja, Z. K., and S. F. Jenkins. 1984. Influence of medium composition on mycelial growth and oxalic acid production in Sclerotium rolfsii. Mycologia 76:947-950. [Google Scholar]
- 41.Rivas, F. J., F. J. Beltran, J. Frades, and P. Buxeda. 2001. Oxidation of p-hydroxybenzoic acid by Fenton's reagent. Water Res. 35:387-396. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, C. J., B. K. Whitten, and D. D. Nicholas. 1981. A proposed role for oxalic acid in nonenzymatic wood decay by brown rot fungi. Proc. Am. Wood Preservers Assoc. 77:157-164. [Google Scholar]
- 43.Shimada, M., Y. Akamatsu, T. Tokimatsu, K. Mii, and T. Hattori. 1997. Possible biochemical roles of oxalic acid as a low molecular weight compound involved in brown-rot and white-rot wood decays. J. Biotechnol. 53:103-113. [Google Scholar]
- 44.Sillen, L. G., and A. E. Martell. 1964. Stability constants of metal-ion complexes, vol. 17. The Chemical Society, London, United Kingdom.
- 45.Tanaka, N., Y. Akamatsu, T. Hattori, and M. Shimada. 1994. Effect of oxalic acid on the oxidative breakdown of cellulose by the Fenton reaction. Wood Res. 81:8-10. [Google Scholar]
- 46.Tien, M., and T. K. Kirk. 1988. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 161:238-249. [Google Scholar]
- 47.Tien, M., and T. K. Kirk. 1983. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science 221:661-663. [DOI] [PubMed] [Google Scholar]
- 48.Tien, M., B. A. Svingen, and S. D. Aust. 1982. An investigation into the role of hydroxyl radical in xanthine oxidase-dependent lipid peroxidation. Arch. Biochem. Biophys. 216:142-151. [DOI] [PubMed] [Google Scholar]
- 49.Traquair, J. A. 1987. Oxalic acid and calcium oxalate produced by Leucostoma cincta and L. persoonii in culture and in peach bark tissue. Can. J. Bot. 65:1952-1956. [Google Scholar]