Abstract
Glutathione was found in several dairy Lactococcus lactis strains grown in M17 medium. None of these strains was able to synthesize glutathione. In chemically defined medium, L. lactis subsp. cremoris strain SK11 was able to accumulate up to ∼60 mM glutathione when this compound was added to the medium. Stationary-phase cells of strain SK11 grown in chemically defined medium supplemented with glutathione showed significantly increased resistance (up to fivefold increased resistance) to treatment with H2O2 compared to the resistance of cells without intracellular glutathione. The resistance to H2O2 treatment was found to be dependent on the accumulation of glutathione in 16 strains of L. lactis tested. We propose that by taking up glutathione, L. lactis might activate a glutathione-glutathione peroxidase-glutathione reductase system in stationary-phase cells, which catalyzes the reduction of H2O2. Glutathione reductase, which reduces oxidized glutathione, was detectable in most strains of L. lactis, but the activities of different strains were very variable. In general, the glutathione reductase activities of L. lactis subsp. lactis are higher than those of L. lactis subsp. cremoris, and the activities were much higher when strains were grown aerobically. In addition, glutathione peroxidase is detectable in strain SK11, and the level was fivefold greater when the organism was grown aerobically than when the organism was grown anaerobically. Therefore, the presence of glutathione in L. lactis could result in greater stability under storage conditions and quicker growth upon inoculation, two important attributes of successful starter cultures.
Glutathione (γ-GluCysGly, reduced form) (GSH) is the major nonprotein thiol compound in living cells, including human, yeast, and bacterial cells. In contrast to the extensive studies of the cellular functions of GSH in eukaryotic cells, relatively little is known about GSH in prokaryotes, except for Escherichia coli. In this organism GSH was found to be involved in the resistance to osmotic stress (24), oxidative stress (4, 25), and toxic electrophiles (9). Fahey et al. (7) reported that GSH was found in many gram-negative bacteria but was not found in most of the gram-positive bacteria examined; two exceptions were Lactococcus lactis and Streptococcus agalactiae. The latter organisms were thought to synthesize GSH, since the total intracellular amount was much greater than the total amount detected in the medium. Subsequently, Newton et al. (18) concluded that streptococci and enterococci must have the capacity to synthesize GSH, because the high GSH levels in these organisms, up to 11 μmol per g (dry weight) of cells, could not be achieved exclusively by import of GSH from the medium. However, these authors did not measure the activities of γ-glutamylcysteine synthetase and GSH synthetase, which would have supplied direct evidence for the existence of a GSH biosynthetic system. On the other hand, by adding [35S]Cys and [35S]GSH to chemically defined medium, Wiederholt and Steele (30) showed that L. lactis subsp. cremoris Z8 takes up GSH efficiently from the medium but is unable to synthesize it, whereas strain C2 could neither import nor synthesize GSH.
In this study our aims were to characterize GSH accumulation in lactic acid bacteria and to assess the physiological functions of GSH (i.e., its role in survival during oxidative stress). Defined lactic acid bacterium starter cultures (e.g., L. lactis) are of great economic importance for the bulk production of cheese. As facultative anaerobes, strains of L. lactis very often encounter a challenge from oxygen, and they have evolved several systems, including an NADH oxidase-NADH peroxidase system, to survive this challenge (6). The GSH-dependent reduction system is responsible for maintaining a reduced environment and plays an important role in survival in the presence of oxidative stress in Escherichia coli and Saccharomyces cerevisiae (4). However, the physiological role of GSH in L. lactis or in gram-positive bacteria remains unknown, except that Sherrill and Fahey (22) found that cellular GSH protected Streptococcus mutans ATCC 33402 against growth inhibition by the thiol oxidizing agent diamide. We therefore examined the presence and role of GSH and GSH-dependent enzymes, including GSH reductase (GR), in different strains of L. lactis. To our knowledge, this is the first study which showed that GSH has a clear physiological role in lactic acid bacteria. In addition, our insights into the presence and protective role of GSH in L. lactis could be applied to the dairy industry for, e.g., screening L. lactis strains that can accumulate GSH as starters for cheese production.
MATERIALS AND METHODS
Chemicals.
GSH, oxidized GSH (GSSG), NADPH, and GR were purchased from Sigma (St. Louis, Mo.), and monobromobimane (mBBr) was purchased from Calbiochem (Darmstadt, Germany). All inorganic compounds were reagent grade or higher quality.
Bacterial strains and culture conditions.
The L. lactis strains used in this study were obtained from the NIZO culture collection. Inocula were transferred from −80°C frozen stock cultures to M17 agar plates and incubated at 30°C for 24 h, and the colonies were then used to inoculate fresh media. Strains were grown anaerobically or aerobically with shaking at 200 rpm at 30°C for 15 to 16 h in M17 broth or chemically defined medium (CDM) (19). To prepare CDM, 3 g of K2HPO4, 3 g of KH2PO4, 1 g of sodium acetate, 0.6 g of ammonium citrate, 0.25 g of tyrosine (predissolved in 400 μl of 10 M NaOH), 0.5 g of ascorbic acid (predissolved in 5 ml of distilled water), and 19 g of sodium β-glycerophosphate were dissolved in distilled water (final volume, 870 ml), and then 100 ml of an amino acid stock solution, 10 ml of a metal ion stock solution, 10 ml of a nucleotide stock solution, and 10 ml of a vitamin stock solution were added. The pH of the final CDM was adjusted to 6.8 by using 5 M NaOH. The CDM was filter sterilized. The 10× amino acid stock solution contained (per liter) 2.40 g of alanine, 1.25 g of arginine, 4.20 g of asparagine, 4.20 g of aspartic acid, 1.30 g of cysteine-HCl, 5.00 g of glutamic acid, 1.75 g of glycine, 1.50 g of histidine, 2.10 g of isoleucine, 4.75 g of leucine, 4.40 g of lysine, 1.25 g of methionine, 2.75 g of phenylalanine, 6.75 g of proline, 3.40 g of serine, 2.25 g of threonine, 0.50 g of tryptophan, and 3.25 g of valine. The 100× metal ion stock solution contained (per liter) 20 g of MgCl2 · 6H2O, 5.0 g of CaCl2 · 2H2O, 1.6 g of MnCl2 · 4H2O, 0.8 g of FeCl3 · 6H2O, 0.7 g of FeSO4 · 7H2O, 0.5 g of ZnSO4 · 7H2O, 0.25 g of CoSO4 · 7H2O, 0.25 g of CuSO4 · 7H2O, and 0.25 g of (NH4)6Mo7O24 · 2H2O; the pH was adjusted to 1.5 with 5 M HCl to dissolve all components. Ten milligrams (each) of adenine, guanine, uracil, and xanthine were dissolved in 10 ml of 0.1 M NaOH to prepare the 100× nucleotide stock solution. The 100× vitamin stock solution contained (per liter) 1,000 mg of p-aminobenzoic acid, 500 mg of inosine, 500 mg of orotic aicd, 500 mg of pyridoxamine-HCl, 500 mg of thymidine, 250 mg of d-biotin, 250 mg of 6,8-thioctic acid, 200 mg of pyridoxine-HCl, 100 mg of folic acid, 100 mg of nicotinic acid, 100 mg of calcium d-(+)-pantothenate, 100 mg of riboflavin, 100 mg of thiamine-HCl, and 100 mg of vitamin B12; the pH was adjusted to 6.8. All stock solutions was prepared by using distilled water, divided into aliquots, and stored at −40°C. The stock solutions were thawed at room temperature prior to use. For most strains lactose (0.5%, wt/vol) was filter sterilized and added to the broth; the exceptions were the media for L. lactis subsp. cremoris MG1363 and L. lactis subsp. lactis IL-1403, to which 0.5% (wt/vol) glucose was added as the carbon source.
Preparation of cell extracts.
A 50-ml overnight culture was harvested, and the cells were washed twice with ice-cold saline (0.85% [wt/vol] NaCl) and resuspended in 1 ml of phosphate buffer B (0.2 M potassium phosphate, 2 mM EDTA; pH 7.0). One milliliter of a cell suspension was added to a vial along with 1 g of glass beads, and the cells were broken with a mini bead beater (FastPrep FP120; Savant, Holbrook, N.Y.) for 30 s at 4°C. The cell debris was pelleted by centrifugation with a microcentrifuge for 10 min (10,000 × g, 4°C), and the supernatant was used for the GSH assay and the GR assay.
GSH assay.
Total GSH (GSH plus GSSG) was assayed by the enzymatic recycling procedure, as modified from the procedures of Tietze (28) and Griffith (14). Seven hundred microliters of 0.3 mM NADPH, 100 μl of 6 mM 5,5′-dithiobis(2-nitrobenzoic acid), and enough of a GSH sample or water to give a final volume of 1.0 ml were mixed in a cuvette with a l-cm light path and equilibrated at 25°C. To the warmed solution 10 μl of a 50-U/ml GR solution was added, and the change in absorbance at 412 nm was monitored continuously. The GSH concentration of the aliquot assayed was determined by comparing the observed reaction rate to a standard curve generated with known amounts of GSH. The working solutions of NADPH, DTNB, and GR were prepared with phosphate buffer A (125 mM potassium phosphate, 6.3 mM EDTA; pH 7.5).
mBBr fluorescent labeling was used to determine the intracellular GSH content as described previously (8). A 90-μl aliquot of a cell suspension was added to 200 μl of 50% aqueous acetonitrile containing 5 mM N,N-bis(2-[bis(carboxymethyl)-amino]ethyl)glycine and 50 mM N-(2-hydroxethyl)piperazine-N′-(2-hydroxypropanesulfonic acid) (pH 7.5), and then 5 μl of mBBr (50 mM in acetonitrile) was added. The mixture was heated for 30 min at 60°C and subsequently acidified by adding 5 μl of 1.2 M methanesulfonic acid. The mixture was centrifuged at 20,000 × g for 5 min prior to injection into a PLRP-S 300 Å column (4.6 by 250 mm) packed with 5-μm reversed-phase material (Polymer Laboratories). A Waters 600E high-performance liquid chromatograph equipped with a Dilutor 401 injector (Gilson) and an FL2000 fluorometer-detector (excitation wavelength, 390 nm; emission wavelength, 480 nm; Spectra Physics) was used. Elution solvent A contained 5% (vol/vol) aqueous acetonitrile and 0.25% (vol/vol) glacial acetic acid (pH 3.5), and solvent B contained 90% acetonitrile and 0.25% glacial acetic acid in water. The following elution profile was used: isocratic conditions (100% solvent A) for 2 min, followed by a linear gradient to 35% solvent B in 35 min and to 100% solvent B in the next 3 min, maintenance of 100% solvent B for 5 min, and then a return to the initial conditions for reequilibration (15 min) before the next sample was loaded. The flow rate was maintained at 1 ml/min.
Measurement of GR and GSH peroxidase activities.
GR (EC 1.6.4.2) activities in L. lactis were measured by using the fact that this enzyme catalyzes the NADPH-dependent reduction of GSSG to GSH (3). To a 1-ml cuvette, 0.5 ml of prewarmed phosphate buffer B, 50 μl of GSSG (20 mM in water), and 350 μl of cell extract were added, while to the blank 50 μl of water was added instead of GSSG. One hundred microliters of NADPH (2 mM in 10 mM Tris-HCl, pH 7.0) was added to the sample and to the blank cuvette to initiate the reaction. Enzyme assays were performed at 30°C, and the decrease in absorbance at 340 nm was monitored. The activity was calculated from the linear slope of decreasing absorption of NADPH by using ɛ = 6,220 M−1 cm−1. Since in all the strains assayed there was a nonspecific NADPH oxidase-dependent background that was responsible for as much as 50% of the NADPH oxidation, the reported GR activity was corrected for the background NADPH oxidation value.
GSH peroxidase (EC 1.11.1.9) activities in L. lactis were measured indirectly by examining a coupled reaction with GR. The principle is that the GSSG produced upon reduction of H2O2 by GSH peroxidase is recycled to GSH by the presence of excess GR and NADPH (11). To a 1-ml cuvette, 250 μl of prewarmed phosphate buffer B, 350 μl of cell extract, 100 μl of GR (2.5 U/ml in phosphate buffer B), and 100 μl of GSH (10 mM in water) were added. After a 5-min preincubation at 30°C, 100 μl of NADPH (2 mM in 10 mM Tris-HCl, pH 7.0) was added, and the H2O2-independent oxidation of NADPH was monitored for 3 min. The reaction was initiated by addition of 100 μl of 1.5 mM H2O2, and the concomitant oxidation of NADPH was monitored at 340 nm at 30°C. The nonenzymatic reduction of H2O2 was measured by replacing the cell extract by phosphate buffer B. The activity was calculated from the linear slope of decreasing absorption of NADPH by using ɛ = 6,220 M−1 cm−1.
Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.).
H2O2 treatment.
L. lactis SK11 cells were grown anaerobically in CDM that did not contain GSH or was supplemented with different concentrations of GSH at 30°C. Portions (10 ml) of the cultures at different growth phases were harvested, washed twice with saline to remove the residual GSH, and resuspended in enough saline to obtain a final optical density at 600 nm of 2.5. One milliliter of each cell suspension was treated with 5 mM H2O2 for 5 min and then pelleted by centrifugation at 20,000 × g for 1 min and washed again with saline to remove the residual H2O2. The cells were resuspended in the same volume of saline and then either spread on M17 plates at different dilutions to measure survival or inoculated into CDM (inoculum size, 1% [vol/vol]) for growth experiments. Colony count results were expressed as means ± standard deviations for three plates incubated at 30°C for 48 h.
Growth experiments.
One milliliter of a mid-stationary-phase (defined as 2 to 3 h after the stationary phase was reached) culture in CDM was washed twice with saline and inoculated into 1 ml of fresh CDM (inoculum size, 1% [vol/vol]). Subsequently, 200 μl of the culture was transferred into a well of a microtiter plate (Corning Incorporated, Corning, N.Y.). Growth was monitored at 600 nm with a kinetic microtiter plate reader (SPECTRAmax PLUS 384; Molecular Devices Corporation, Sunnyvale, Calif.). Each growth experiment was carried out in triplicate. The lag phase was defined as the time needed to reach the optical density that was five times the initial optical density. The results were expressed the means for lag phases calculated from three independent growth curves. Due to different optical geometries the values for the optical densities measured with a microtiter plate reader and with a spectrophotometer were different.
RESULTS
Presence of GSH in L. lactis.
Intracellular GSH contents were determined by using cell extracts of 21 strains of L. lactis grown in M17 broth under anaerobic or aerobic conditions (Table 1). GSH was found in most strains of L. lactis subsp. cremoris but appeared to be absent in most of the L. lactis subsp. lactis and L. lactis subsp. lactis biovar diacetylactis strains. Notably, GSH could not be detected in any of the 21 strains when they were grown in CDM (data not shown). In the strains in which GSH could be detected, GSH accumulation was not always correlated with aeration. Some strains accumulated comparable GSH concentrations under anaerobic and aerobic conditions, whereas other strains (for example, strains SK11, NIZO B30, NIZO B63, and NIZO B74) accumulated larger amounts of GSH when they were grown aerobically than when they were grown in static cultures. Strikingly, strains NIZO B11 and NIZO B20 were not able to accumulate GSH when they were grown anaerobically but did accumulate it when they were grown aerobically.
TABLE 1.
Presence of GSH and GR in L. lactisa
| Strain | Growth in M17 broth
|
Growth in defined medium lacking cysteine (OD600)d
|
||||
|---|---|---|---|---|---|---|
| Intracellular GSH concn (nmol/mg of protein)
|
GR activity (nmol of NADPH/min/mg of protein)
|
|||||
| Anaerobic conditions | Aerobic conditions | Anaerobic conditions | Aerobic conditions | Without GSH | With GSHe | |
| L. lactis subsp. cremoris strains | ||||||
| SK11 | 6.56 ± 0.22 | 10.56 ± 0.19 | 0.25 ± 0.12 | 2.99 ± 0.20 | —c | 0.38 ± 0.03 |
| MG1363 | NDb | ND | 0.76 ± 0.09 | 2.14 ± 0.21 | — | — |
| NIZO B30 | 4.59 ± 0.03 | 7.09 ± 0.20 | ND | 0.59 ± 0.15 | — | 1.43 ± 0.05 |
| NIZO B33 | 9.06 ± 0.09 | 9.50 ± 0.34 | 1.99 ± 0.20 | 2.48 ± 0.19 | 1.93 ± 0.04 | 2.27 ± 0.09 |
| NIZO B42 | 10.21 ± 0.11 | 7.99 ± 0.24 | 0.40 ± 0.10 | 1.40 ± 0.23 | — | — |
| NIZO B63 | 2.73 ± 0.09 | 4.08 ± 0.05 | ND | ND | — | 0.66 ± 0.03 |
| NIZO B66 | 4.17 ± 0.14 | 3.33 ± 0.19 | ND | ND | 1.54 ± 0.05 | 2.43 ± 0.12 |
| NIZO B67 | ND | ND | 0.75 ± 0.09 | 1.83 ± 0.17 | — | 0.23 ± 0.02 |
| NIZO B74 | 5.55 ± 0.07 | 6.53 ± 0.38 | 0.64 ± 0.11 | ND | 0.17 ± 0.01 | 0.23 ± 0.01 |
| L. lactis subsp. lactis strains | ||||||
| IL-1403 | ND | ND | 0.75 ± 0.09 | 5.99 ± 0.28 | — | 0.14 ± 0.02 |
| NIZO B10 | ND | ND | ND | ND | — | 0.46 ± 0.02 |
| NIZO B11 | ND | 6.90 ± 0.17 | ND | 3.23 ± 0.23 | — | — |
| NIZO B13 | ND | ND | ND | ND | 2.64 ± 0.11 | 2.82 ± 0.06 |
| NIZO B14 | ND | ND | 2.86 ± 0.17 | 5.68 ± 0.31 | — | — |
| NIZO B20 | ND | 2.79 ± 0.03 | 2.46 ± 0.15 | 6.06 ± 0.25 | — | 0.12 ± 0.01 |
| NIZO B89 | 4.95 ± 0.27 | 4.75 ± 0.35 | ND | ND | — | 0.92 ± 0.03 |
| L. lactis subsp. lactis biovar diacetylactis strains | ||||||
| NIZO B76 | ND | ND | 0.52 ± 0.09 | 2.25 ± 0.13 | 0.24 ± 0.02 | 2.38 ± 0.05 |
| NIZO B81 | ND | ND | 0.81 ± 0.08 | 1.16 ± 0.19 | 1.51 ± 0.03 | 2.18 ± 0.04 |
| NIZO B86 | ND | ND | ND | 2.40 ± 0.17 | 1.25 ± 0.05 | 1.85 ± 0.03 |
| NIZO B90 | ND | ND | 1.89 ± 0.15 | 3.05 ± 0.29 | — | — |
| NIZO B93 | 9.13 ± 0.04 | 7.35 ± 0.08 | 1.16 ± 0.14 | 5.65 ± 0.28 | — | 0.55 ± 0.04 |
Means ± standard deviations for duplicate determinations for two independent samples. GSH contents were determined by using the enzymatic detection method.
ND, not detected.
—, no growth.
The initial optical density at 600 nm (OD600) (after inoculation) was 0.02.
The GSH concentration was 0.8 mM, which was the original concentration of cysteine (0.13 g of cysteine-HCl, per liters approximately 0.82 mM).
GR activities in L. lactis.
As GSH could not be synthesized by the L. lactis strains tested, further research was focused on GSH metabolism-related enzymes, including GR, which is important in maintaining the equilibrium of the intracellular redox state of GSH in yeast (17). The GR activities of 21 strains of L. lactis grown in M17 broth anaerobically and aerobically were determined (Table 1). On average, L. lactis subsp. lactis strains exhibited 1.8- and 2-fold higher GR activities than L. lactis subsp. cremoris strains exhibited when the organisms were grown anaerobically and aerobically, respectively. However, the GR activities of these strains were not correlated with GSH accumulation. Some strains accumulated GSH but did not exhibit GR activity, whereas other strains did not accumulate GSH but did exhibit GR activity. On the other hand, it interesting that all strains that exhibited GR activity had higher activities aerobically than anaerobically. In particular, it was found that both the GR activity and GSH accumulation of strains SK11, NIZO B11, and NIZO B20 increased dramatically when the organisms were grown aerobically.
GSH can be used as a cysteine source.
A function of GSH in L. lactis could be to serve as a source of cysteine, as suggested previously for S. mutans (22). To investigate this possibility, the strains mentioned above were cultivated in CDM lacking cysteine with or without equivalent concentrations of GSH. Table 1 shows that L. lactis subsp. cremoris NIZO B30, L. lactis subsp. lactis NIZO B89, and L. lactis subsp. lactis biovar diacetylactis NIZO B76 could indeed use GSH as a source of cysteine for growth. For the strains that grew well without cysteine (strains NIZO B33, NIZO B66, NIZO B13, NIZO B81, and NIZO B86), addition of GSH enhanced growth.
Intracellular accumulation of GSH by L. lactis subsp. cremoris SK11.
In contrast to the situation in CDM, the source of GSH in M17 was unknown. Therefore, the GSH contents of the major organic components of M17 broth were determined. Yeast extract had an extremely high GSH content (4,166 ± 37 nmol/g) and accounted for 95% of the GSH in M17 broth. The remaining 5% of the GSH in M17 broth originated from casein peptone and soya peptone.
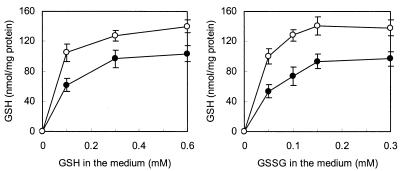
Figure 1 shows the variation in intracellular GSH contents when strain SK11 was cultivated in CDM supplemented with different concentrations of GSH or GSSG. GSSG can be imported and reduced to GSH intracellularly, conceivably by the enzyme GR, as this enzyme activity was found in both anaerobically and aerobically grown cells of strain SK11 (Table 1). The final intracellular GSH contents were similar when GSH and GSSG were supplied at equivalent concentrations, suggesting that both compounds can be taken up by strain SK11. It was also found that GSH-GSSG transport is a highly efficient process, capable of achieving a high intracellular concentration of GSH (up to 140 nmol/mg of protein) (Fig. 1). This is equivalent to approximately 60 mM intracellular GSH, assuming an intracellular volume of 2 to 3 μl per mg of cell protein.
FIG. 1.
Effect of adding different concentrations of GSH and GSSG in defined medium on intracellular GSH accumulation by L. lactis subsp. cremoris SK11 over a 16-h cultivation time. Symbols: ○, aerobic cultures; •, anaerobic cultures. GSH contents were measured by the mBBr fluorescent labeling method. The data are the means for duplicate determinations for two independent samples. The error bars indicate standard deviations.
Intracellular GSH of L. lactis plays a role in protection against oxidative stress.
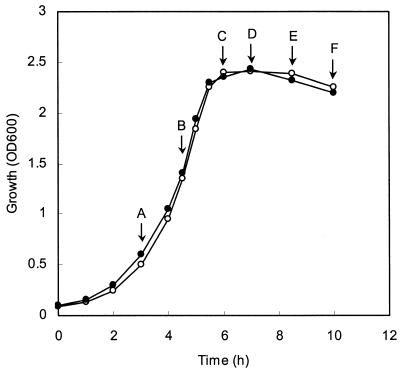
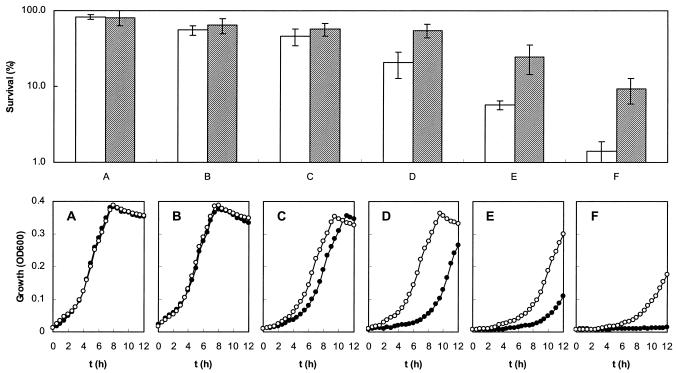
As shown in Fig. 1, aerobically grown cells of L. lactis SK11 always accumulated approximately 30% more GSH than cells grown anaerobically in CDM supplemented with different concentrations of GSH or GSSG accumulated. This suggests that GSH might play a role in protection against oxidative stress. GSH is generally considered to be the predominant low-molecular-weight thiol in many organisms, and it has been proposed that this compound protects cells from oxidative damage induced by H2O2 (15). We therefore investigated whether the presence of GSH in L. lactis affected the ability of the organism to survive H2O2 treatment. As L. lactis SK11 is unable to synthesize GSH but is able to take it up, SK11 cells were grown in CDM lacking GSH or supplemented with 0.2 mM GSH to obtain cells without and with intracellular GSH. These cells were designated GSH− and GSH+ cells, respectively. At different growth phases cells were removed from the cultures, as indicated in Fig. 2. They were washed and subsequently exposed to 5 mM H2O2 for 5 min. The H2O2-treated cells were used to measure the survival and the lag phases upon subcultivation. As Fig. 3 shows, intracellular GSH did play a role in protection against damage caused by H2O2 treatment. Cells at the early exponential (Fig. 3A) and mid-exponential (Fig. 3B) phases exhibited high levels of resistance to H2O2, and there were no obvious differences in susceptibility to H2O2 between GSH+ and GSH− cells in these phases of growth. The killing effect of H2O2 became significant when the cells reached the stationary phase, and the difference in survival between GSH+ and GSH− cells became increasingly larger. When cells were treated with H2O2, there were roughly 0.3-, 1.6-, 3.3-, and 5.6-fold differences between the survival of GSH+ cells and the survival of GSH− cells at the initial stage (Fig. 3C), earlier stage (Fig. 3D), mid-stage (Fig. 3E), and later stage (Fig. 3F) of the stationary phase, respectively. The duration of the lag phase correlated negatively with the capacity to survive after H2O2 treatment (Fig. 3). There were no lag phase differences between GSH+ and GSH− cells at earlier exponential (Fig. 3A) and mid-exponential (Fig. 3B) phases, whereas the lag phases of GSH+ cells were 1.5, 3.6, 4.0, and 9.2 h shorter than the lag phases of GSH− cells at the initial stage (Fig. 3C), earlier stage (Fig. 3D), mid-stage (Fig. 3E), and later stage (Fig. 3F) of the stationary phase, respectively. Considering that the intracellular GSH plays a protective role in oxidative stress, which is growth phase dependent, cells in the mid-stationary phase were chosen for further studies of H2O2-induced stress. Also, the lag phase differences were used to assess the resistance to oxidative stress of different strains of L. lactis in the studies described below.
FIG. 2.
Growth curves for L. lactis SK11 in the absence (○) or in the presence (•) of 0.2 mM GSH. An overnight CDM culture was inoculated into fresh CDM supplemented with GSH or without GSH; the inoculum size was 5% (vol/vol). Arrows A, B, C, D, E, and F indicate the growth phases at which samples were withdrawn for investigating H2O2 resistance (Fig. 3). OD600, optical density at 600 nm.
FIG. 3.
Effect of H2O2 treatment on the survival and subsequent lag phase of L. lactis SK11 in different growth phases. The upper graph shows the survival capacity of cells with (cross-hatched bars) or without (open bars) GSH intracellularly. The lower graphs show the lag phase differences for cells with (○) or without (•) GSH intracellularly. Cells at different growth phases were removed at the times indicated in Fig. 2 (arrows A to F, corresponding to panels A to F, respectively) and were resuspended in enough saline to obtain an initial concentration of 1 × 109 cells/ml. OD600, optical density at 600 nm.
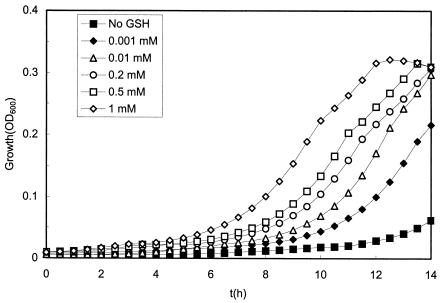
To study whether the H2O2 resistance of GSH+ cells was correlated with the intracellular GSH concentration, SK11 cells were incubated in CDM supplemented with concentrations of GSH ranging from 1 to 1,000 μM in order to obtain GSH+ cells with different GSH contents. Figure 4 shows that the higher the GSH concentration in the preculture medium, the shorter the lag phase of SK11 cells. Notably, the most significant decreases in the length of the lag phase occurred in the low-concentration range (1 and 10 μM) of GSH (Fig. 4), at which the intracellular GSH contents were 0.5 ± 0.1 and 3.8 ± 0.5 nmol/mg of protein, respectively (approximately 0.2 and 1.5 mM intracellularly). This suggests that a very low intracellular GSH concentration protects against damage caused by H2O2 treatment. We also examined the effect of the H2O2 concentration on the resistance of GSH+ and GSH− cells. Compared with the normal growth curve, the lag phase of GSH+ cells was not affected by 1 mM H2O2 but were prolonged from 3.6 to 6.8 h in the presence of 5 mM H2O2, while the lag phase of GSH− cells was prolonged from 4.1 to 7.1 or 12.4 h when the cells were exposed to 1 or 5 mM H2O2. This indicates that GSH+ cells are able to survive in the presence of a concentration of H2O2 (for instance, 1 mM) which is lethal to GSH− cells.
FIG. 4.
Effects of the GSH concentration in preculture on the growth of L. lactis SK11 upon H2O2 treatment. Cells were grown in CDM supplemented with different concentrations of GSH and then treated with 5 mM H2O2 for 5 min and inoculated into fresh medium for growth experiments.
To investigate whether the ability to take up GSH affects the resistance to H2O2, 16 strains of L. lactis were grown anaerobically in CDM supplemented with GSH or lacking GSH. The mid-stationary-phase cells were harvested, washed, and subsequently exposed to 5 mM H2O2 for 5 min. The lag phases upon subcultivation are shown in Table 2. It was found that the lag phases of all strains tested were prolonged after H2O2 treatment. Shortened lag phases of cells which were pregrown in CDM supplemented with 0.2 mM GSH were observed only in the strains that were capable of accumulating GSH from the medium. Interestingly, some of the L. lactis subsp. lactis strains (for instance, NIZO B10, NIZO B11, NIZO B13, and NIZO B90) exhibited high levels of resistance to H2O2, although these strains did not accumulate GSH from the medium. This phenomenon suggests that in these strains there might be alternative mechanisms to survive a lethal H2O2 treatment.
TABLE 2.
Effect of GSH on lag phases upon subcultivation of different strains of L. lactisa
| Strain | Ability to accumulate GSH when grown anaerobically | Lag phase (h) with the following GSH concn in preculture
|
|
|---|---|---|---|
| 0 | 0.2 mM | ||
| L. lactis subsp. cremoris strains | |||
| SK11 | + | 12.60 ± 0.60 | 6.67 ± 0.12 |
| MG1363 | − | 6.17 ± 0.17 | 6.42 ± 0.17 |
| N1ZO B30 | + | 6.79 ± 0.39 | 5.38 ± 0.11 |
| NIZO B33 | + | 11.77 ± 0.34 | 5.55 ± 0.35 |
| NIZO B42 | + | 12.67 ± 0.19 | 6.35 ± 0.23 |
| NIZO B67 | − | 10.45 ± 0.22 | 10.79 ± 0.33 |
| NIZO B74 | + | 5.94 ± 0.28 | 4.15 ± 0.14 |
| L. lactis subsp. lactis strains | |||
| IL-1403 | − | 8.93 ± 0.32 | 9.35 ± 0.34 |
| NIZO B10 | − | 5.56 ± 0.08 | 5.60 ± 0.04 |
| NIZO B11 | − | 3.46 ± 0.11 | 3.79 ± 0.11 |
| NIZO B13 | − | 2.79 ± 0.11 | 2.85 ± 0.04 |
| NIZO B20 | − | 7.99 ± 0.34 | 8.01 ± 0.16 |
| L. lactis subsp. lactis biovar diacetylactis strains | |||
| NIZO B81 | − | 7.15 ± 0.08 | 7.32 ± 0.13 |
| NIZO B86 | − | 9.50 ± 0.30 | 9.67 ± 0.14 |
| NIZO B90 | − | 4.00 ± 0.26 | 4.04 ± 0.09 |
| NIZO B93 | + | 7.31 ± 0.26 | 6.15 ± 0.14 |
Cells were precultured in CDM lacking GSH or supplemented with 0.2 mM GSH and then treated with 5 mM H2O2 for 5 min. The cells were pelleted after the H2O2 treatment and washed once with saline, and then they were resuspended in the same volume of CDM and inoculated for growth experiments as described in Materials and Methods.
DISCUSSION
GSH and GR are considered part of the mechanism of defense against oxidative stresses in eukaryotic cells and in gram-negative bacteria like E. coli (4). Much less is known about the function of this system in gram-positive bacteria, including lactic acid bacteria. Therefore, we examined the occurrence of GSH and GR in L. lactis. We observed striking differences in the concentrations of GSH in different L. lactis strains. Fernandes and Steele reported that GSH was not found in L. lactis subsp. lactis (10). However, in the present study, GSH was found in strains of L. lactis subsp. cremoris, as well as in L. lactis subsp. lactis, which indicates that the accumulation of GSH by L. lactis is not subspecies specific but only strain specific. None of the strains tested in this report was able to synthesize GSH in CDM. This is consistent with the results of Wiederholt and Steele (30), who concluded that L. lactis subsp. cremoris Z8 can import GSH but not synthesize it. Also, the genome sequence of L. lactis subsp. lactis IL-1403 lacks the genes encoding γ-glutamylcysteine synthetase and GSH synthetase (2). Therefore, we believe that L. lactis generally lacks the ability to synthesize GSH. Although the GR activities also exhibited strain-specific characteristics, this enzyme still can be considered a ubiquitous enzyme in L. lactis. The gor gene encoding GR is present in the genome sequence of L. lactis subsp. lactis IL-1403 (2). In other species, like Streptococcus thermophilus and Enterococcus faecalis, it was found that GR activities were increased at high O2 concentrations (21, 20). In L. lactis, we also found that most of the strains that expressed GR activity exhibited higher levels of activity when they were grown under shaking conditions, suggesting that expression of the gene encoding GR is responsive to the oxygen concentration. Surprisingly, strains like MG1363, IL-1403, and NIZO B90 and many other strains that do not accumulate GSH from the medium also exhibited high GR activities. This suggests that the physiological substrate of their GR may be a different compound. Another possibility is that some of these strains do take up GSH from the medium but metabolize it intracellularly, resulting in undetectable low concentrations which, however, are sufficient for catalysis and serve as the substrate for GR.
Although some strains of L. lactis could use GSH as a cysteine source, the growth rates and lag phases of most L. lactis strains were not affected by addition of GSH in CDM (data not shown). This demonstrates that L. lactis has no absolute requirement for GSH for growth. This is also true for E. coli, because the growth of mutants that are unable to synthesize GSH is not affected (1). The GSH taken up by L. lactis SK11 can be used to increase the resistance to H2O2, which is the only physiological role of GSH found in L. lactis so far. Interestingly, the GSH+ cells of L. lactis SK11 exhibited growth phase-dependent H2O2 resistance. Although GSH− cells of L. lactis SK11 are as resistant as GSH+ cells to H2O2 during the exponential growth phase, during the stationary phase they have a mortality rate that is up to 5.6-fold higher than that of GSH+ cells upon H2O2 treatment. This observation is similar to that made when a GSH-deficient mutant and wild-type E. coli were compared (5, 13). The observed growth phase-dependent protection by GSH in L. lactis cannot be explained by differences in levels of accumulation, since these levels were similar in exponential- and stationary-phase cells. Recently, an alternative mechanism for protection of L. lactis against H2O2 was suggested by van Niel et al. (29). These authors found that fast-growing aerobic cultures of L. lactis ATCC 19435 contained significant concentrations of both extracellular and intracellular pyruvate, which could scavenge the H2O2 generated by NADH oxidase nonenzymatically. Since it has been observed that anaerobically growing L. lactis strains have relatively high intracellular concentrations of pyruvate (12), we postulated that the high level of resistance to H2O2 of cells in the exponential growth phase is conferred by the high intracellular pyruvate concentration. As the intracellular pyruvate concentration declines along with the decrease in the growth rate (23), the pyruvate concentration in stationary-phase cells of SK11 may become too low to scavenge the lethal amount of H2O2. The GSH− cells, therefore, become more susceptible to H2O2 during the stationary phase. Cells containing GSH might employ a different mechanism, namely, a GSH-GSH peroxidase-GR system, to protect themselves against damage from H2O2 treatment. This system has been identified in higher eukaryotic cells (16) and yeast (26), in which the reduction of H2O2 to H2O was catalyzed by GSH peroxidase. This enzyme uses GSH as a hydrogen donor, and the GSSG formed is reduced in turn by GR and NADPH. We have demonstrated that GR is present in L. lactis. The genome of L. lactis subsp. lactis IL-1403 also contains a gene encoding the enzyme GSH peroxidase (2). The activity of GSH peroxidase in strain SK11 is detectable and is fivefold greater when the organisms is grown aerobically than when it is grown anaerobically (data not shown), which makes the proposed mechanism conceivable. Strikingly, some L. lactis subsp. lactis strains that do not accumulate GSH still exhibited strong resistance to H2O2, suggesting that there are alternative mechanisms for H2O2 resistance, such as thioredoxin and thioredoxin reductase (31). Notably, the genes encoding these two proteins are also present in the genome of L. lactis subsp. lactis IL-1403 (2).
It seems likely that the increased accumulation of GSH under aerobic conditions observed in several strains is a regulatory mechanism that protects the cells against oxidative damage, since other experiments (Fig. 1 and 4) showed that an increase in the intracellular GSH concentration leads to increased resistance to oxidative stress. Even a very low concentration of extracellularly supplied GSH (1 to 10 μM) led to significant protection of L. lactis against damage by H2O2. In particular, the shortened lag phase upon H2O2 treatment was found to be dependent on the accumulation of GSH in L. lactis. In conclusion, although GSH was thought not to function in bacteria as an antioxidant directed against organic hydroperoxides (27), our results strongly suggest that GSH plays a role in protection against H2O2 in stationary-phase cells of L. lactis. These insights have industrial importance, since increased starter stability or activity could result from accumulation of GSH.
Acknowledgments
We thank Jan van Riel for his assistance in performing high-performance liquid chromatography experiments and Igor Mierau for discussions.
REFERENCES
- 1.Apontoweil, P., and W. Berends. 1975. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim. Biophys. Acta 399:10-22. [DOI] [PubMed] [Google Scholar]
- 2.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlberg, I., and B. Mannervik. 1975. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250:5475-5480. [PubMed] [Google Scholar]
- 4.Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439-461. [DOI] [PubMed] [Google Scholar]
- 5.Chesney, J. A., J. W. Eaton, and J. R. Mahoney, Jr. 1996. Bacterial glutathione: a sacrificial defense against chlorine compounds. J. Bacteriol. 178:2131-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condon, S. 1987. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 46:269-280. [Google Scholar]
- 7.Fahey, R. C., W. C. Brown, W. B. Adams, and M. B. Worsham. 1978. Occurrence of glutathione in bacteria. J. Bacteriol. 133:1126-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahey, R. C., and G. L. Newton. 1987. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid-chromatography. Methods Enzymol. 143:85-96. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, G. P. 1999. Protective mechanisms against toxic electrophiles in Escherischia coli. Trends Microbiol. 7:242-247. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes, L., and J. L. Steele. 1993. Glutathione content of lactic-acid bacteria. J. Dairy Sci. 76:1233-1242. [Google Scholar]
- 11.Flohe, L., and W. A. Gunzler. 1984. Assays of glutathione peroxidase. Methods Enzymol. 105:114-121. [DOI] [PubMed] [Google Scholar]
- 12.Garrigues, C., P. Loubiere, N. D. Lindley, and M. Cocaign-Bousquet. 1997. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J. Bacteriol. 179:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg, J. T., and B. Demple. 1986. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J. Bacteriol. 168:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith, O. W. 1980. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 106:207-212. [DOI] [PubMed] [Google Scholar]
- 15.Meister, A., and M. E. Anderson. 1983. Glutathione. Annu. Rev. Biochem. 52:711-760. [DOI] [PubMed] [Google Scholar]
- 16.Mezzetti, A., C. Di Ilio, A. M. Calafiore, A. Aceto, L. Marzio, G. Frederici, and F. Cuccurullo. 1990. Glutathione peroxidase, glutathione reductase and glutathione transferase activities in the human artery, vein and heart. J. Mol. Cell. Cardiol. 22:935-938. [DOI] [PubMed] [Google Scholar]
- 17.Muller, E. G. 1996. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol. Biol. Cell 7:1805-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton, G. L., K. Arnold, M. S. Price, C. Sherrill, S. B. Delcardayre, Y. Aharonowitz, G. Cohen, J. Davies, R. C. Fahey, and C. Davis. 1996. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto, R., B. ten Brink, H. Veldkamp, and W. N. Konings. 1983. The relationship between growth rate and electrochemical proton gradient of Streptococcus cremoris. FEMS Microbiol. Lett. 16:69-74. [Google Scholar]
- 20.Patel, M. P., J. Marcinkeviciene, and J. S. Blanchard. 1998. Enterococcus faecalis glutathione reductase: purification, characterization and expression under normal and hyperbaric O2 conditions. FEMS Microbiol. Lett. 166:155-163. [DOI] [PubMed] [Google Scholar]
- 21.Pebay, M., A. C. Holl, J. M. Simonet, and B. Decaris. 1995. Characterization of the gor gene of the lactic acid bacterium Streptococcus thermophilus CNRZ368. Res. Microbiol. 146:371-383. [DOI] [PubMed] [Google Scholar]
- 22.Sherrill, C., and R. C. Fahey. 1998. Import and metabolism of glutathione by Streptococcus mutans. J. Bacteriol. 180:1454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjoberg, A., I. Persson, M. Quednau, and B. Hahn-Hagerdal. 1995. The influence of limiting and non-limiting growth conditions on glucose and maltose metabolism in Lactococcus lactis ssp. lactis strains. Appl. Microbiol. Biotechnol. 42:931-938. [Google Scholar]
- 24.Smirnova, G. V., T. A. Krasnykh, and O. N. Oktyabrsky. 2001. Role of glutathione in the response of Escherichia coli to osmotic stress. Biochemistry (Moscow) 66:973-978. [DOI] [PubMed] [Google Scholar]
- 25.Smirnova, G. V., N. G. Muzyka, M. N. Glukhovchenko, and O. N. Oktyabrsky. 2000. Effects of menadione and hydrogen peroxide on glutathione status in growing Escherichia coli. Free Radic. Biol. Med. 28:1009-1016. [DOI] [PubMed] [Google Scholar]
- 26.Stephen, D. W. S., and D. J. Jamieson. 1996. Glutathione is an important antioxidant molecule in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Lett. 141:207-212. [DOI] [PubMed] [Google Scholar]
- 27.Sundquist, A. R., and R. C. Fahey. 1989. Evolution of antioxidant mechanisms: thiol-dependent peroxidases and thioltransferase among procaryotes. J. Mol. Evol. 29:429-435. [DOI] [PubMed] [Google Scholar]
- 28.Tietze, F. 1969. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: application to mammalian blood and other tissues. Anal. Biochem. 27:502-522. [DOI] [PubMed] [Google Scholar]
- 29.van Niel, E. W., K. Hofvendahl, and B. Hahn-Hagerdal. 2002. Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions. Appl. Environ. Microbiol. 68:4350-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiederholt, K. M., and J. L. Steele. 1994. Glutathione accumulation in lactococci. J. Dairy Sci. 77:1183-1188. [Google Scholar]
- 31.Zhang, Z., P. J. Hillas, and P. R. Ortiz de Montellano. 1999. Reduction of peroxides and dinitrobenzenes by Mycobacterium tuberculosis thioredoxin and thioredoxin reductase. Arch. Biochem. Biophys. 363:19-26. [DOI] [PubMed] [Google Scholar]