Abstract
The influence of substrate composition on the yield, nature, and composition of exopolysaccharides (EPS) produced by the food-grade strain Gluconacetobacter xylinus I-2281 was investigated during controlled cultivations on mixed substrates containing acetate and either glucose, sucrose, or fructose. Enzymatic activity analysis and acid hydrolysis revealed that two EPS, gluconacetan and levan, were produced by G. xylinus. In contrast to other acetic acid strains, no exocellulose formation has been measured. Considerable differences in metabolite yields have been observed with regard to the carbohydrate source. It was shown that glucose was inadequate for EPS production since most of this substrate (0.84 C-mol/C-mol) was oxidized into gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid. In contrast, sucrose and fructose supported a 0.35 C-mol/C-mol gluconacetan yield. In addition, growing G. xylinus on sucrose produced a 0.07 C-mol/C-mol levan yield. The composition of EPS remained unchanged during the course of the fermentations. Levan sucrase activity was found to be mainly membrane associated. In addition to levan production, an analysis of levan sucrase's activity also explained the formation of glucose oxides during fermentation on sucrose through the release of glucose. The biosynthetic pathway of gluconacetan synthesis has also been explored. Although the activity of key enzymes showed large differences to be a function of the carbon source, the ratio of their activities remained similar from one carbon source to another and corresponded to the ratio of precursor needs as deduced from the gluconacetan composition.
Bacterial exopolysaccharides (EPS) generally have unique rheological properties because of their high purity and regular structure (26). Therefore, the food industry frequently uses EPS as thickening, gelling, or stabilizing agents (10, 32). The genus Acetobacter is known mainly for its capacity to synthesize cellulose, which is used for some fermented food products (3, 5, 17). Recently, the strain Gluconacetobacter xylinus I-2281 (originally classified as Acetobacter xylinus [8] but renamed by Yamada et al. [39]) has been isolated from vinegar fermentations. It has been shown that G. xylinus produces a soluble EPS composed of rhamnose, glucose, mannose, and glucuronic acid (P. Duboc, M. Fischer, and S. Vincent, submitted for publication). Its composition is related to acetan, which is produced by some Acetobacter xylinus strains (16). This new EPS, named gluconacetan by Duboc et al. (submitted), may be a promising candidate as a thickening agent in food formulation.
In acetic acid bacteria, contradictory results have been reported concerning the influence of carbon source on EPS yields. Fructose (9, 35, 41), sucrose (18), and glucose (21, 38) have been reported to provide the highest bacterial cellulose yield. There is, however, no information about the influence of the carbon source on the chemical composition of heteropolysaccharides, such as acetan or gluconacetan, produced by Acetobacter strains. In contrast, some reports suggest that the nature of the substrate affects the EPS composition produced by lactic acid bacteria (6, 13).
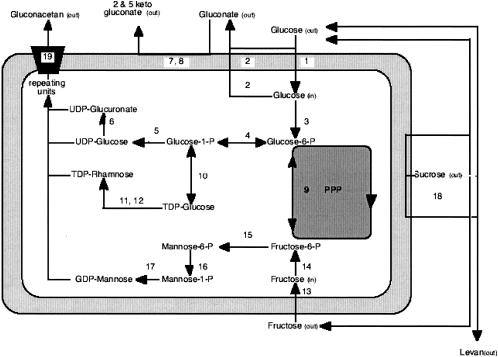
As for many other EPS (32), the building blocks of gluconacetan should be energy-rich forms of monosaccharides. Activated sugar nucleotides are sequentially added to a lipid carrier to form repeating units of the polysaccharide. The last step involves transport of the repeating units across the cell membrane to the outer layer and polymerization to form the EPS (Fig. 1). In acetic acid or lactic acid bacteria, the activities of enzymes involved in EPS biosynthesis could be correlated with product yield and EPS structure. For example, high cellulose yield has been correlated to high UDP-glucose pyrophosphorylase activities in A. xylinum (35, 36), while the amount of galactose in EPS produced by Lactobacillus delbrueckii subsp. bulgaricus is in agreement with an increase of UDP-galactose 4-epimerase activity (13).
FIG. 1.
Proposed pathway for the biosynthesis of sugar nucleotides and the production of gluconacetan in G. xylinus I 2281 with glucose and fructose used as the substrate. Activated sugar nucleotides are sequentially added to a lipid carrier to form repeating units of the polysaccharide. The last step involves transport of the repeating units across the cell membrane to the outer layer and polymerization to form the EPS. 1, glucose permease; 2, glucose dehydrogenase; 3, glucose hexokinase; 4, phosphoglucomutase; 5, UDP-glucose pyrophosphorylase; 6, UDP-glucose dehydrogenase; 7, d-gluconate dehydrogenase (flavin adenine dinucleotide dependent); 8, d-gluconate dehydrogenase (pyrroloquinoline quinone dependent); 9, phosphoglucoisomerase; 10, glucose-1-phosphate thymidylyl transferase; 11, glucose dehydratase; 12, rhamnose synthetase; 13, fructose permease; 14, fructose hexokinase; 15, mannose isomerase; 16, mannose mutase; 17, mannose-1-phosphate guanylyl transferase; 18, levansucrase; 19, polymerase. PPP, penthose phosphate pathway.
In the present study, we investigated the influence of nutritional factors on the nature, yield, and composition of EPS produced by G. xylinus I-2281. Batch cultures were run on three different sources of carbohydrate (glucose, sucrose, and fructose), and acid hydrolysis was applied to the recovered EPS. We assumed that a biosynthetic pathway similar to that of acetan was operating (7) (Fig. 1); thus, the results were correlated with key enzyme activities involved in the synthesis of EPS precursors.
MATERIALS AND METHODS
Cultivation conditions.
Bacterial strain G. xylinus I-2281 was provided by the Nestlé Research Center (Lausanne, Switzerland). Stock cultures were stored at −80°C in a 15% (wt/vol) skim milk powder and 15% (wt/vol) malt extract solution. Cells were reactivated in a 1-liter shake flask containing 100 ml of defined medium at 30°C for 60 h. The defined medium contained 35 g of fructose per liter; 5 g of acetate and 0.163 g of MgCl2 · 6H2O per liter; 5 g of KH2PO4 per liter; 2 g of NH4Cl per liter; 1.060 g of Na2CO3 per liter; 0.114 g of Na2SO4 per liter; and 15 ml of trace element solution per liter. The trace element solution contained 1.47 g of CaCl2 · 2H2O per liter, 0.27 g of FeCl3 · 6H2O per liter, 0.085 g of MnSO4 · H2O per liter, 0.024 g of Na2MoO4 · 2H2O per liter, 0.016 g of CuSO4 · 5H2O per liter, 0.024 g of CoCl2 · 6H2O per liter, 0.024 g of NiCl2 · 2H2O per liter, 0.144 g of ZnSO4 · 7H2O per liter, and 4.1 g of HCl (25% [wt/vol]) per liter. The pH was adjusted to 4.0 by using 2 N KOH or 1 N HCl. The medium was sterilized by filtration (Minisart 0.2-μm-pore-size filter; Sartorius AG, Göttingen, Germany).
Cultivation experiments were undertaken by using a 15:l bioreactor (Fermenteur 15 LP; LSL Biolafitte SA, Saint-Germain-en-Laye, France) with a working volume of 10:l. The bioreactor inoculum (500 ml) was incubated in shake flasks for 24 h by using the same defined medium as that used for the bioreactor cultures. The media for cultivations in bioreactors contained 6 g of acetate per liter and a source of carbohydrate (glucose, fructose, or sucrose) at the concentrations specified in the text. Carbonate was omitted. The temperature was maintained at 30°C, and the pH was kept at 4.0 by the automatic addition of 2 N KOH or 1 N HCl. In order to avoid foaming, a level probe activated the addition of 10 g of antifoam solution (Structol J673; Schill and Seilacher, Hamburg, Germany) per liter. The aeration rate was maintained at 1 vvm (volume of liquid per volume of gas per min) by using a thermal mass flow controller (model number 5850E; Brooks Instrument, Hatfield, Pa.) and a stirring rate of 800 rpm. A polarographic pO2 probe (model Infit 765-50; Mettler Toledo, Greifensee, Switzerland) was used to monitor the level of dissolved oxygen.
Substrate and metabolite analysis.
Culture samples (approximately 15 ml each) were collected by using a purpose-built autosampler and kept at 2°C for up to 8 h before handling. For cell dry weight measurements, biomass was recovered from 10 ml of culture sample by centrifugation (15 min at 20,000 × g at 2°C), resuspended in water, and filtered with preweighed membranes (HT-200; Pall Corporation, Ann Arbor, Mich.). Filters were dried for 15 min in a microwave (power at 150 W) and reweighed (28). A correlation was established between dry cell weight and optical density at 600 nm. This correlation was used to estimate biomass concentrations below 0.4 g per liter.
Glucose, fructose, sucrose, acetate, gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid concentrations were determined by high-performance liquid chromatography analysis (1100 series; Agilent Technologies, Palo Alto, Calif.). An ion-exchange chromatography column (Supelcogel H 300 mm; Supelco, Bellefonte, Pa.) with a guard column (Superlguard C610H; Supelco) was used at 30°C. A 5 mM sulfuric acid solution in ultrapure water was applied at a constant eluent flow rate of 0.5 ml/min. Glucose, acetate, and ethanol were measured by using a refractive index detector. Gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid were measured by using a diode array detector at 360 nm.
Soluble exopolysaccharides were recovered after precipitation of a 1-volume sample with 2 volumes of industrial ethanol followed by centrifugation (10 min at 4,500 × g at 4°C). The pellets were washed twice with a 70% ethanol solution and were then freeze-dried and weighed (20).
The carbon dioxide evolution rate in the bioreactor off-gas was determined by using an infrared gas analyzer (model 2500; Servomex, Crowborough, United Kingdom).
Nature and composition of EPS.
After recovery by ethanol precipitation, the content of cellulose in the EPS pellet was measured by treatment with 1 N NaOH at 80°C for 20 min according to the methods of Masaoka et al. (21). Composition of gluconacetan was analyzed by acid hydrolysis of a solution of 2 g of EPS per liter under harsh conditions in 2 M trifluoroacetic acid (TFA) at 100°C for 8 h in sealed glass tubes that had been previously purged with nitrogen. Levan identification was performed by acid hydrolysis of a solution of 2 g of EPS per liter under mild conditions in 0.5 M TFA at 50°C for 15 min. After hydrolysis, harsh and mild hydrolysis samples were dried in a vacuum centrifuge and redissolved in 500 μl of 50 mM sodium phosphate buffer at pH 7.0. An identical treatment was applied to pure standard solutions of monosaccharides in order to assess degradation during hydrolysis. Concentrations of monosaccharides were determined by high-performance anion-exchange chromatography (Carbopac PA10 column; Dionnex, Salt Lake City, Utah) with an eluent of 18 mM NaOH. After 30 min of elution, the NaOH concentration was linearly increased to 150 mM over the course of 30 min. The eluent flow was maintained at 1 ml/min. Monosaccharides were measured by using a pulsed amperometric detector.
Cell extracts and enzyme activity analysis.
Fermentation broth samples were centrifuged for 10 min at 7,500 × g at 2°C. The pellet of biomass was washed twice with cold buffer supplemented with a cocktail of protease inhibitors (Complete; Roche Diagnostics, Manheim, Germany). The buffer contained 50 mM sodium phosphate (pH 7.0) for glucose dehydrogenase analysis, 50 mM Tris HCl (pH 8.5) for phosphoglucose isomerase analysis, 100 mM triethanolamine HCl (pH 7.6) for phosphomannose isomerase analysis, 10 mM Tris HCl with 10 mM MgCl2 (pH 7.6) for uridine-5-diphosphoglucose pyrophosphorylase analysis, 10 mM Tris HCl with 10 mM MgCl2 (pH 8.0) for glucose-1-phosphate thymidylyltransferase analysis, and 25 mM sodium acetate (pH 5.4) for levansucrase analysis. Washed biomass samples were stored at −40°C until needed further. Cells were disrupted by ultrasonication at 20 kHz for 3 min. Cell debris and supernatant were separated by centrifugation for 10 min at 10,000 × g at 2°C. Enzyme activities in both fractions were determined immediately after cell disruption. The Bradford method (Protein Assay; Bio-Rad, München, Germany) was used to determine total protein concentration in the supernatant. Bovine serum albumin served as the standard. The dry weight of cell debris was measured with preweighed filters (HT-200; Pall Corporation). Filters were dried for 15 min in a microwave (power at 150 W) and reweighed (28). The mass ratio of total protein to dry weight was assumed to be 0.52 (31).
Glucose dehydrogenase activity was measured by monitoring the formation of β-NADPH at 340 nm. Cell extract (20 μl) was added to 180 μl of reaction mix containing 40 mM sodium phosphate, 50 mM glucose, and 0.4 mM β-NADP according to the method of Smith et al. (30). One unit of enzyme activity is defined as the oxidation of 1.0 μmol of glucose to glucono-1,4-lactone per minute at pH 7.0 and 37°C.
Phosphoglucose isomerase activity was determined in the glucose-6-phosphate-forming direction. Cell extract (10 μl) was added to 590 μl of reaction solution containing 93 mM Tris HCl, 2.9 mM d-fructose-6-phosphate, 0.44 mM NADP, 6 U of glucose-6-phosphate dehydrogenase; the solution's pH was adjusted to 9.0. Formation of NADPH was monitored at 340 nm. One unit corresponds to the conversion of 1.0 μmol of d-fructose-6-phosphate to d-glucose-6-phosphate per minute at pH 9.0 and 30°C.
Phosphomannose isomerase activity was assayed in the fructose-6-phosphate-forming direction according to a modified method previously used by Gracy and Noltman (11). In a solution (300 μl) containing 87 mM triethanolamine, 5.5 mM d-mannose-6-phosphate, 1 U of glucose-6-phosphate dehydrogenase, and 0.45 mM NADP, the addition of 10 μl of cell extract produced NADPH, which can be monitored at 340 nm. One unit corresponds to the conversion of 1.0 μmol of d-mannose-6-phosphate to d-fructose-6-phosphate per minute at pH 7.6 and 30°C.
Uridine-5-diphosphoglucose pyrophosphorylase activity was measured according to a method adapted from Bergmeyer et al. (2). Cell extract (10 μl) was added to 300 μl of reaction mixture containing 50 mM Tris HCl, 16 mM MgCl2, 10 mM l-cysteine, 0.01 mM glucose-1.6-diphosphate, 1.7 mM sodium pyrophosphate, 0.67 mM uridine 5′-diphosphoglucose, 0.67 mM NADP, 0.75 U of phosphoglucomutase, and 0.75 U of glucose-6-phosphate dehydrogenase. Formation of glucose-1-phosphate and the subsequent formation of 6-phosphogluconate and NADPH were measured at 340 nm. One unit of enzyme activity corresponds to the conversion of 1.0 μmol of uridine 5′-diphosphoglucose to glucose-1-phosphate per minute at pH 7.6 and 30°C.
Levan synthase activity and invertase activity were assayed by the incubation of 100 mM sucrose at pH 5.4 at 30°C in a buffer containing 25 mM sodium acetate and 1 mM MgCl2. Samples were collected periodically and diluted 1:10 in 1 M NaOH to stop the reaction. The amounts of glucose and fructose released were measured enzymatically by using a standard assay procedure (d-Glucose/d-Fructose; R-Biopharm, Darmstadt, Germany). Levan synthase activity results in conversion of sucrose to glucose and levan. Invertase activity results in the equimolar release of glucose and fructose. Thus, the fructose production rate was used to calculate invertase activity, and the difference between glucose and fructose production rates was used to determine levan synthase activity. One unit of enzyme activity corresponds to the conversion of 1.0 μmol of sucrose per minute at pH 5.4 and 30°C.
All chemicals used in the enzyme assays were supplied by Sigma-Aldrich, Steinheim, Germany. Enzyme activity measurements were performed in triplicate and are expressed as mean values. All enzymatic assays were controlled without the added substrate and tested for linearity by using commercial pure enzymes.
RESULTS
Concentration profiles during batch cultivation.
Three batch fermentations were performed on mixed substrate containing 6 g of acetate and 28 g of glucose per liter, 6 g of acetate and 15 g of sucrose per liter, and 6 g of acetate and 15 g of fructose per liter. During growth on acetate and sucrose, the concentration profiles can be divided into two main phases with respect to substrate consumption (Fig. 2). The first phase corresponds to the oxidation of acetate. During this phase, 0.7 g of biomass per liter was produced (Fig. 2A). In parallel, sucrose starts to be consumed and soluble exopolysaccharides are produced, but no cellulose could be detected. Glucose and fructose concentrations below 1 g/liter were detected, and gluconic acid was produced. Gluconic acid was further oxidized to 2- and 5-ketogluconic acid (Fig. 2B). The concentrations of these three metabolites were added and expressed as (keto)-gluconate in Fig. 2B. The second phase corresponds to the consumption of sucrose only (Fig. 2C). During this phase, bacterial growth was significantly reduced, whereas EPS continued to be secreted and finally reached a concentration of 6.0 g/liter. In parallel, the production of gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid continued and reached a total concentration of 3.3 g/liter.
FIG. 2.
Batch fermentation of G. xylinus on 6 g of acetate per liter and 15 g of sucrose per liter. Concentration profiles can be divided into two main phases with respect to substrate consumption. Phase 1 corresponds to the uptake of acetate; phase 2 corresponds to consumption of sucrose. (A) Δ, acetate; −, carbon dioxide evolution rate; X, biomass. (B) ▴, (keto)-gluconate, which represents the sum of 5-ketogluconate, 2-ketogluconate, and gluconate; ♦, 5-ketogluconic acid; ○, gluconic acid; ⋄, 2-ketogluconic acid. (C) ▪, sucrose; •, gluconacetan.
Growth on acetate with glucose or fructose could also be divided into two phases (data not shown). During the first phase, in which acetate is consumed, a similar amount of biomass (0.7 g/liter) was formed regardless of the source of carbohydrate. Gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid were produced from glucose to a level of 25.2 g/liter. Neither metabolite was detected during growth on fructose. Soluble EPS were produced over the two phases of fermentation to a final concentration of 1.5 g/liter on glucose and 3.7 g/liter on fructose. Similarly to growth on sucrose, no cellulose was detected either from glucose or from fructose.
The yields of metabolites produced during the second phase of growth are shown in Table 1. Considerable differences can be observed with regard to the carbohydrate source (Table 1). During growth on glucose, most of the substrate (84%) was oxidized to gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid, whereas the quantity of EPS formed was minor. By contrast, during growth on sucrose, only 21% of the sucrose was metabolized to glucose oxides, while 46% of the carbon ended up in EPS. During growth on fructose, no oxidation to (keto)-gluconate occurred. The EPS yield amounted to 35%, the rest being oxidized into carbon dioxide.
TABLE 1.
Yields of metabolites produced by G. xylinus on different carbohydrate sources
| Carbohydrate source | Yield of metabolitea
|
C balance | ||
|---|---|---|---|---|
| (Keto)-Gluconatesb | EPSc | CO2 | ||
| Glucose | 0.84 ± 0.03 | 0.09 ± 0.01 | 0.12 ± 0.02 | 1.05 ± 0.06 |
| Sucrose | 0.21 ± 0.01 | 0.46 ± 0.02 | 0.36 ± 0.02 | 1.03 ± 0.07 |
| Fructose | 0.00 ± 0.00 | 0.35 ± 0.01 | 0.65 ± 0.01 | 1.00 ± 0.02 |
Values for yields are given as C-mol/C-mol of total substrates.
(Keto)-Gluconate represents the sum of the yields of gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid.
EPS represents the total amount of soluble EPS (gluconacetan and levan) harvested from fermentation broth. During growth on sucrose, it represents 75% gluconacetan and 25% levan. No levan was measured during growth on glucose or fructose. Regardless of the source of carbohydrate, no cellulose production was measured.
Nature and composition of EPS.
The composition of exopolysaccharides was investigated by using partial and total hydrolysis. Samples were taken at three different points during the fermentation: at the beginning, middle, and end of the second phase (Fig. 2). Standard solutions of monosaccharides were hydrolyzed in the same way as that for EPS samples, and the degradation of free monosaccharides due to the TFA was quantified (77, 83, 77, 62, and 54% recovery for rhamnose, glucose, mannose, glucuronic acid, and fructose, respectively).
Regardless of the source of carbohydrate, glucose, rhamnose, mannose, and glucuronic acid were the only species measured after harsh hydrolysis of EPS pellets (Fig. 3a through c). This result can be correlated with the production of gluconacetan as described by Duboc et al. (submitted). Gluconacetan was then produced either from glucose, fructose, or sucrose. The results presented in Fig. 3a through c also suggest that the gluconacetan composition remains constant during the fermentation. In contrast, no cellulose was detected in any of the growth experiments.
FIG. 3.
Composition of EPS resulting from harsh (panels a, b, and c) and mild (panel d) hydrolysis of samples collected at the beginning (white columns), middle (grey columns), and end (black columns) of the second phase (Fig. 2) during growth on glucose (a), fructose (b), and sucrose (c and d). Monosaccharide concentrations were normalized with respect to rhamnose concentration (panels a, b, and c).
A large amount of fructose was released by mild hydrolysis of EPS pellets collected during growth on sucrose (Fig. 3d). No detectable monosaccharides were measured after mild hydrolysis of EPS pellets collected during growth on glucose and fructose. This result is attributed to the production of levan, a β-2,6 fructose-linked EPS during growth on sucrose. Considering the molar ratio of monosaccharides measured after harsh and mild hydrolysis, about 75% of the EPS collected during growth on sucrose was in the form of gluconacetan and 25% was in the form of levan. This ratio remained constant throughout the course of the culture (Fig. 3d). Correction of the levan yield for the soluble EPS yield showed that the gluconacetan yield was identical during growth on fructose and sucrose (0.35 C-mol/C-mol).
Activities of enzymes involved in EPS biosynthesis.
To determine whether the EPS yield can be related to precursor availability, the activity of six enzymes involved in EPS synthesis was assayed (Fig. 1 and Table 2). For all experiments, broth samples were collected during the second phase of growth, during which the biomass concentration remained essentially constant (Fig. 2).
TABLE 2.
Activity of some key enzymes involved in the synthesis of sugar nucleotide precursors of gluconacetan polymerizationa
| Enzyme (location) | Activityb of enzyme during growth on:
|
||
|---|---|---|---|
| Glucose | Sucrose | Fructose | |
| Levansucrase | |||
| Levan synthetase (medium) | 1,206 ± 56 | 970 ± 14 | 1,150 ± 21 |
| Levan synthetase (membrane) | 4,627 ± 275 | 24,432 ± 2,310 | 18,782 ± 3,960 |
| Levan synthetase (cytosol) | 20 ± 2 | 395 ± 45 | 138 ± 9 |
| Invertase (medium) | 15 ± 10 | 4 ± 3 | 0 ± 2 |
| Invertase (membrane) | 2,149 ± 407 | 6,627 ± 785 | 4,110 ± 660 |
| Invertase (cytosol) | 7 ± 5 | 290 ± 45 | 64 ± 5 |
| Glucose dehydrogenase (cytosol) | 0.80 ± 0.2 | 0.87 ± 0.2 | 0.42 ± 0.2 |
| Glucose-6-P isomerase (cytosol) | 941 ± 200 | 1,257 ± 200 | 1,435 ± 200 |
| Glucose-1-P thymidylyltransferase (cytosol) | 31 ± 5 | 37 ± 5 | 123 ± 5 |
| UDP-glucose pyrophosphorylase (cytosol) | 162 ± 100 | 210 ± 100 | 558 ± 100 |
| Mannose-6-P isomerase (cytosol) | 3 ± 1 | 3 ± 1 | 2 ± 1 |
Results are from analysis of samples that were collected from culture during broth growth on glucose, sucrose, or fructose.
Activity values are given in units per gram of total protein.
Levan synthase and invertase activities were measured in culture medium samples and in cell membrane and cytosol fractions (Table 2). The activities of levan synthase and invertase were, in decreasing order, cell membrane, culture medium, and cytosolic fraction. The highest levan synthase and invertase activities were measured during growth on sucrose, although some activity was also found during growth on glucose and fructose, even though neither of them are substrates of these enzymes.
Cell extracts collected during growth on glucose showed a glucose dehydrogenase activity of 0.8 U/g of total protein (Table 2). Similar activity was assayed in cell extracts collected during growth on sucrose. This activity can be correlated with the presence of residual glucose (less than 1 g/liter) detected during cultivations on sucrose. When fructose was used as the carbon source, glucose dehydrogenase still showed a 0.4 U/g of total protein activity, even though fructose is not a substrate for this enzyme. The maximum glucose-6-phosphate isomerase activity (1,435 U/g of total protein) was measured in cell extracts collected during fermentations on fructose. This activity is comparable to that found during growth on sucrose but higher than that found during growth on glucose (941 U/g of total protein). Glucose-1-phosphate thymidylyltransferase had the same activity during growth on either glucose or sucrose (33 U/g of total protein), although the activity on fructose was threefold higher. Similarly, UDP glucose pyrophosphorylase analysis showed the highest activity (558 U/g of total protein) for growth on fructose and an activity that was threefold lower with sucrose or glucose. In contrast to other enzyme activities, mannose-6-phosphate isomerase activity was low and relatively constant, regardless of the substrate.
DISCUSSION
Two phases during batch cultivation on mixed substrates.
Regardless of the carbohydrate source, two main phases could be identified during batch growth of G. xylinus I-2281 on mixed substrates (Fig. 2). The first phase corresponds to acetate oxidation and production of biomass. The second phase is related to the assimilation of the sugar alone. The first phase ends after the exhaustion of acetate. A severe reduction in bacterial growth in the absence of acetate is in accordance with previous results on G. xylinus, which showed that medium containing glucose must be supplemented with ethanol or acetate in order to allow growth of G. xylinus I-2281 (27; H. Kornmann, P. Duboc, P. Niederberger, I. Marison, and U. von Stockar, submitted for publication). The second phase ends after the exhaustion of the sugar. In contrast to some other Acetobacter species (41), neither biomass nor EPS were synthesized from accumulated gluconic acid. Instances of non-growth-associated EPS and glucose oxide production mostly occur during the second phase.
According to a 14C study regarding Acetobacter xylinum cellulose synthesis (38), EPS and glucose oxides are synthesized from sugars and not from acetate. As discussed in another study (Kornmann et al., submitted), the overall catabolism may be divided into two independent parts: (i) the upper catabolism, related to sugar assimilation and production of EPS and glucose oxides, and (ii) the lower catabolism, related to acetate oxidation. In Fig. 2, these two independent parts run concomitantly during the first phase. The lower catabolism stops after the acetate depletion, whereas the upper one continues through the second phase until the carbohydrate source is also exhausted.
The values of EPS yields measured during growth of G. xylinus I2281 on sucrose or fructose (Table 1) are of the same order of magnitude as the yield of xanthan gum, an EPS produced by the plant pathogen Xanthomonas campestris, which is widely used in the food industry (25).
Glucose flux: glucose oxides versus gluconacetan.
Gluconic acid, 2-ketogluconic acid, and 5-ketogluconic acid, in addition to EPS, are major metabolites produced during growth of G. xylinus. These metabolites result from the oxidation of glucose by glucose and gluconate dehydrogenases (Fig. 1). Production of glucose oxides has been measured during growth not only on glucose but also on sucrose (Table 1). This last result can be explained by the release of glucose by invertase activity during growth on sucrose (Fig. 1).
Acetobacter species are capable of producing large amounts of gluconic acid. For example, Acetobacter xylinum BRC5 (15, 21) and IFO 13693 (41) oxidize up to 80 and 72% of the glucose supplied, respectively. This effect has been also observed in G. xylinus (Table 1). In Acetobacter species, glucose and gluconate dehydrogenases are mainly located in the cell membrane (1, 22, 29). This is in agreement with the low cytosolic glucose dehydrogenase activity (0.8 μmol/min/g of total protein; Table 2) compared to the specific production rate of gluconic acid observed on glucose (3,850 μmol/min/g of total protein).
Since (keto)-gluconates are not further metabolized, glucose is not an appropriate substrate for EPS production. This is clearly illustrated by a comparison of the glucose oxides and EPS yields obtained during growth on fructose and glucose (Table 1). However, by controlling a low glucose concentration in the bioreactor by using an appropriate feeding strategy of glucose solution, the production of (keto)-gluconate should be reduced (23, 24) and the yield of gluconacetan should increase. The same result can obviously be achieved by using either sucrose or fructose as the carbohydrate source.
Levan production from sucrose.
Mild hydrolysis of EPS pellets (Fig. 3d) and enzymatic assays (Table 2) showed that levan was detected only during growth on sucrose. No levan was measured after mild hydrolysis of EPS pellets produced on glucose or fructose (data not shown). Considering a 25% yield of levan in the total EPS collected during growth on sucrose (Fig. 3d), the yield of gluconacetan (0.35 C-mol/C-mol) is then identical during growth on fructose or sucrose.
Levan is a nonthickening molecule which is reported to have hypocholesterolemic (40), antitumoral (4), calorie-free, noncarious, and probiotic (42) properties. Production of levan has been reported for other Acetobacter strains (14, 33). However, until now, there have been no reports describing the production of levan in G. xylinus.
This study shows that levan synthetase is mainly cell surface associated (Table 2), while gluconacetan results from cytosolic activity. The constant ratio between gluconacetan and levan (Fig. 3d) suggests that the specific activity of levan synthase, and that of the enzymes involved in the biosynthetic pathway of gluconacetan precursors, remained constant during batch cultures on sucrose. In G. xylinus I-2281, invertase and levan synthase activities may be the result of the same enzyme, levan sucrase. According to a previous study (37), enzyme expression was repressed by glucose but not by fructose (Table 2).
Cytosolic enzyme activity.
In parallel to EPS yields and composition analysis, the biosynthetic pathway of gluconacetan was explored through analysis of the activity of enzymes specific to the different pathways involved in the activation of gluconacetan precursors.
Although the gluconacetan composition remained constant for the different carbohydrate substrates (Fig. 3), large differences were measured in the key enzyme activities (Table 2). Activities of glucose-1-phosphate thymidylyltransferase and UDP-glucose pyrophosphorylase were higher during growth on fructose than they were on other substrates. A ratio of 1/5 between the activities of these two enzymes was maintained, regardless of the carbon source, which would agree with the proportion of rhamnose present in gluconacetan.
Regardless of the source of carbohydrate, glucose-6-phosphate isomerase activity is higher than that of the other cytosolic enzymes (Table 2). As a matter of fact, this enzyme plays a central role in the pentose phosphate pathway, which runs concomitantly with gluconacetan production (19, 38). However, glucose-6-phosphate isomerase activity is higher on fructose than on the other substrates. As a matter of fact, the metabolism of fructose requires the isomerization of large amounts of fructose-6-phosphate to activate glucose-6-phosphate, which is in turn required to produce UDP-glucose, UDP-glucuronate, and TDP-rhamnose—conditions which are not met during growth on glucose.
In conclusion, this study shows the potential of Gluconacetobacter strains to produce large quantities of complex EPS. It has been shown that nutritional factors have a strong influence on the yield and nature of the EPS produced. The quantitative analysis of EPS production in a bioreactor carried out during the course of this study shows that glucose is not an appropriate substrate for EPS production by G. xylinus, since most of it is oxidized into (keto)-gluconates. By growing G. xylinus on acetate and either sucrose and fructose, however, gluconacetan with a yield of 0.35 C-mol/C-mol was obtained. On sucrose, G. xylinus also produced 0.07 C-mol/C-mol of levan, which has previously been found only in a few Acetobacter strains.
G. xylinus I-2281 shows many advantages for process integration, since biomass and EPS are dissociated. Furthermore, by-product formation may be reduced by controlling the residual glucose concentration at a low level. Compared to other EPS-producing strains such as lactic acid bacteria, the yield of EPS is extremely high. However, it should be possible to increase the EPS yield even further through metabolic engineering of the sugar nucleotide pathway. Indeed, EPS clusters have already been analyzed by Griffin et al. (12) for some Acetobacter strains. In G. xylinus I-2281, the overexpression of genes coding for mannose-6-phosphate isomerase should also lead to a higher level of EPS. Given the fact that only very low mannose-P isomerase activities were measured in G. xylinus I-2281 (Table 2), the overexpression of this gene could also lead to higher levels of EPS synthesis, as is true for microbial alginate production (34).
Acknowledgments
The Swiss Commission for Technical Innovation (CTI) and the Swiss National Foundation for Scientific Research (FNS) are gratefully acknowledged for their support.
REFERENCES
- 1.Alvarez, B., and G. Martinez-Drets. 1995. Metabolic characterization of Acetobacter diozotrophicus. Can. J. Microbiol. 41:918-924. [Google Scholar]
- 2.Bergmeyer, H. U., K. Gawehn, and M. Grassl. 1974. Uridine-5′-diphosphoglucose pyrophosphorylase, p. 519-520. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, vol. 1. Academic Press, Inc., New York, N.Y.
- 3.Blanc, P. J. 1996. Characterization of the tea fungus metabolites. Biotechnol. Lett. 18:139-142. [Google Scholar]
- 4.Calazans, G. M. T., R. C. Lima, F. P. de Franca, and C. E. Lopes. 2000. Molecular weight and antitumour activity of Zymomonas mobilis levans. Int. J. Biol. Macromol. 27:245-247. [DOI] [PubMed] [Google Scholar]
- 5.Chung, Y. C., and Y. Shyu. 1999. The effects of pH, salt, heating and freezing on the physical properties of bacterial cellulose—nata. Int. J. Food Sci. Technol. 34:23-26. [Google Scholar]
- 6.Degeest, B., and L. De Vuyst. 2000. Correlation of activities of the enzymes α-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl. Environ. Microbiol. 66:3519-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Iannino, I. N., R.-O. Couso, and M.-A. Dankert. 1988. Lipid-linked intermediates and the synthesis of acetan in Acetobacter xylinum. J. Gen. Microbiol. 134:1731-1736. [Google Scholar]
- 8.De Ley, J., M. Gillis, and J. Swings 1984. Family VI: Acetobacteraceae, p. 267-278. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md.
- 9.Embuscado, M. E., J. S. Marks, and J. N. Bemiller. 1994. Bacterial cellulose. 1. Factors affecting the production of cellulose by Acetobacter xylinum. Food Hydrocol. 8:407-418. [Google Scholar]
- 10.Fett, W. F., S. F. Osman, M. L. Fishman, and K. Ayyad. 1996. Biopolymers from fermentation, p. 76-87. In G. Fuller, T. A. McKeon, and D. D. Bills (ed.), Agricultural materials as renewable resources, vol. 647. ACS Symposium Series, Washington, D.C.
- 11.Gracy, R. W., and E. A. Noltman. 1968. Studies on phosphomannose isomerase I. Isolation, homogoneity measurements, and determination of some physical properties. J. Biol. Chem. 243:3161-3168. [PubMed] [Google Scholar]
- 12.Griffin, A. M., K. J. Edwards, V. J. Morris, and M. J. Gasson. 1997. Genetic analysis of acetan biosynthesis in Acetobacter xylinum—DNA-sequence analysis of the acem gene encoding a UDP-glucose dehydrogenase. Biotechnol. Lett. 19:469-474. [Google Scholar]
- 13.Grobben, G. J., M. R. Smith, J. Sikkema, and J. A. M. deBont. 1996. Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. Appl. Microbiol. Biotechnol. 46:279-284. [Google Scholar]
- 14.Hernandez, L., J. Arrieta, C. Menedez, R. Vasquez, R. Coego, V. Suarez, G. Selman, M. F. Petit-Glatron, and R. Chambert. 1995. Isolation end enzymatic properties of levansucrase secreted by Acetobacter diazotrophicus SRT4, a bacterium associated with sugar cane. Biochem. J. 309:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang, J. W., Y. K. Yang, J. K. Hwang, Y. R. Pyun, and Y. S. Kim. 1999. Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J. Biosci. Bioeng. 88:183-188. [DOI] [PubMed] [Google Scholar]
- 16.Jansson, P. E., J. Lindberg, K. M. S. Wimalasiri, and M. A. Dankert. 1993. Structural studies of acetan, an exopolysaccharide elaborated by Acetobacter xylinum. Carbohydr. Res. 245:303-310. [DOI] [PubMed] [Google Scholar]
- 17.Kent, R. A., R. S. Stephens, and J. A. Westland. 1991. Bacterial cellulose fiber provides an alternative for thickening and coating. Food Technol. 45:108. [Google Scholar]
- 18.Kojima, Y., A. Seto, N. Tonouchi, T. Tsuchida, and F. Yoshinaga. 1997. High-rate production in static culture of bacterial cellulose from sucrose by a newly isolated Acetobacter strain. Biosci. Biotechnol. Biochem. 61:1585-1586.
- 19.Macauley, S., B. McNeil, and L. M. Harvey. 2001. The genus Gluconobacter and its applications in biotechnology. Crit. Rev. Microbiol. 21:1-25. [DOI] [PubMed] [Google Scholar]
- 20.MacCormick, C. A., J. E. Harris, A. P. Gunning, and V. J. Morris. 1993. Characterization of a variant of the polysaccharide acetan produced by a mutant of Acetobacter xylinum strain CR1/4. J. Appl. Bacteriol. 74:196-199. [DOI] [PubMed] [Google Scholar]
- 21.Masaoka, S., T. Ohe, and N. Sakota. 1993. Production of cellulose from glucose by Acetobacter xylinum. J. Ferm. Bioeng. 75:18-22. [Google Scholar]
- 22.Matsushita, K., H. Toyama, and O. Adachi. 1994. Respiratory chains and bioenergetics of acetic acid bacteria. Adv. Microb. Physiol. 36:247-301. [DOI] [PubMed] [Google Scholar]
- 23.Olsthoorn, A. J. J., T. Otsuki, and J. A. Duine. 1998. Negative cooperativity in the steady-state kinetics of sugar oxidation by soluble quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus. Eur. J. Biochem. 255:255-261. [DOI] [PubMed] [Google Scholar]
- 24.Pire, C., M. L. Camacho, J. Ferrer, D. W. Hough, and M. J. Bonete. 2000. NAD(P)+-glucose dehydrogenase from Haloferax mediterranei: kinetic mechanism and metal content. J. Mol. Catal. B Enzym. 10:409-417. [Google Scholar]
- 25.Quinn, F. X. 1996. Xanthan. In J. C. Salamone (ed.), The polymeric materials encyclopedia. CRC Press, Boca Raton, Fla.
- 26.Roller, S., and I. C. M. Dea. 1992. Biotechnology in the production and modification of biopolymers for foods. Crit. Rev. Biotechnol. 12:261-277. [Google Scholar]
- 27.Schüller, G., C. Hertel, and W. P. Hammes. 2000. Gluconacetobacter entanii sp. nov., isolated from submerged high-acid industrial vinegar fermentations. Int. J. Syst. Evol. Microbiol. 50:2013-2020. [DOI] [PubMed] [Google Scholar]
- 28.Schulze, U. 1995. Ph.D. thesis. Technical University of Denmark, Lyngby, Denmark.
- 29.Shinagawa, E., K. Matsushita, H. Toyama, and O. Adachi. 1999. Production of 5-ketogluconate by acetic acid bacteria is catalyzed by pyrroloquinoline quinone (pqq)-dependent membrane-bound d-gluconate dehydrogenase. J. Mol. Catal. B. Enzym. 6:341-350. [Google Scholar]
- 30.Smith, L. D., N. Budgen, S. J. Bungard, M. J. Danson, and D. W. Hough. 1989. Purification and characterization of glucose dehydrogenase from the thermoacidophilic archaebacterium Thermoplasma acidophilum. Biochem. J. 261:973-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephanopoulos, G. N., A. A. Aristidou, and J. Nielsen. 1998. Metabolic engineering, Academic Press ed., vol. 1. Academic Press, San Diego, Calif.
- 32.Sutherland, I. W. 1990. Bio/Technology of microbial exopolysaccharides, vol. 9. Cambridge University Press, Cambridge, United Kingdom.
- 33.Tajima, K., N. Uenishi, M. Fujiwara, T. Erata, M. Munekata, and M. Takai. 1997. The production of a new water-soluble polysaccharide by Acetobacter xylinum NCI 1005 and its structural analysis by NMR spectroscopy. Carbohydr. Res. 305:117-122. [DOI] [PubMed] [Google Scholar]
- 34.Tatnell, P. J., N. J. Russell, and P. Gacesa. 1994. GDP-mannose dehydrogenase is the key regulatory enzyme in alginate biosynthesis in Pseudomonas aeruginosa—evidence from metabolite studies. Microbiology 140:1745-1754. [DOI] [PubMed] [Google Scholar]
- 35.Tonouchi, N., T. Tsuchida, F. Yoshinaga, T. Beppu, and S. Horinouchi. 1996. Characterization of the biosynthetic pathway of cellulose from glucose and fructose in Acetobacter xylinum. Biosci. Biotechnol. Biochem. 60:1377-1379. [Google Scholar]
- 36.Valla, S., D. H. Coucheron, E. Fjaervik, J. Kjosbakken, H. Weinhouse, P. Ross, D. Amikam, and M. Benziman. 1989. Cloning of a gene involved in cellulose biosynthesis in Acetobacter xylinus: complementation of cellulose-negative mutant by UDPG pyrophosphorylase structure gene. Mol. Gen. Genet. 217:26-30. [DOI] [PubMed] [Google Scholar]
- 37.Viikari, L. 1988. Carbohydrate metabolism in Zymonas. Crit. Rev. Biotechnol. 7:237-261. [Google Scholar]
- 38.Weinhouse, H., and M. Benziman. 1974. Regulation of hexose phosphate metabolism in Acetobacter xylinum. Biochem. J. 138:537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada, Y., K. Hoshino, and T. Ishikawa. 1997. The phylogeny of acetic acid bacteria based on the partial sequences of 16s ribosomal RNA: the elevation of the subgenus Gluconoacetobacter to the generic level. Biosci. Biotechnol. Biochem. 31:1244-1251. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto, Y., Y. Takahashi, M. Kawano, M. Iizuka, T. Matsumoto, S. Saeki, and H. Yamaguchi. 1999. In vitro digestibility and fermentability of levan and its hypocholesterolemic effects in rats. J. Nutr. Biochem. 10:13-18. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Y. K., S. H. Park, J. W. Hwang, W. R. Pyun, and Y. S. Kim. 1998. Cellulose production by Acetobacter xylinum BRC5 under agitated condition. J. Ferment. Bioeng. 85:312-317. [Google Scholar]
- 42.Yun, J. W. 1996. Fructooligosaccharides—occurrence, preparation, and application. Enzyme Microb. Technol. 19:107-117. [Google Scholar]