Abstract
Teschoviruses specifically infect pigs and are shed in pig feces. Hence, their presence in water should indicate contamination with pig fecal residues. To assess this hypothesis, we have developed a real-time reverse transcriptase PCR (RT-PCR) method that allows the quantitative detection of pig teschovirus (PTV) RNA. The method is able to detect 92 fg of PTV RNA per ml of sample. Using this method, we have detected the presence of PTV RNA in water and fecal samples from all pig farms examined (n = 5). Feces from other animal species (cattle, sheep, and goats) were negative in this test. To compare the PTV RNA detection method with conventional chemical determinations currently in use for evaluation of water contamination, we analyzed water samples collected downstream from a pig slurry spillage site. We have found a positive correlation within both types of determinations. The sensitivity of the PTV detection assay was similar to that achieved by unspecific organic matter determination and superior to all other conventional chemical analyses performed. Furthermore, the new method is highly specific, revealing the porcine origin of the contamination, a feature that is lacking in currently available methods for the assessment of water contamination.
The pig industry produces large amounts of residues worldwide, representing an important environmental concern (14). Accidental or deliberate spills, overuse as fertilizer, and emissions of incompletely depurated pig wastes, constitute major environmental risks (6). Although alternatives for sustainable use of these wastes are available (12), overproduction in certain areas creates economic constraints, preventing environmentally acceptable management. Organic loads, nutrients (17), and metals (5) are considered the main environmental hazards derived from pig waste contamination of water, and the presence of antimicrobial residuals is receiving increasing attention (1). The characteristic odor of pig manure disappears rapidly, and all major components are common to other wastes, including composted urban wastes, making it difficult to attribute the contamination of surface waters to pig waste disposal. Therefore, the availability of techniques allowing the identification of pig waste as the pollution source is highly desirable.
Recently, we have shown that the detection of bovine enteroviruses in surface waters can be useful to monitor water contamination by cattle residues (10). The same rationale may be applied to the problem of determination of animal waste contamination for any livestock species, given an adequate viral candidate to detect. Optimally, the candidate must fulfill the following characteristics. (i) It must be specific for the livestock species under consideration. (ii) It must be endemic. (iii) It must be excreted in feces in detectable amounts. (iv) It must be stable in the environment. Accordingly, provided a swine-specific virus meets all these characteristics, it should be possible to exploit its presence in water as a reliable marker to determine if a given contamination of water is caused by pig waste. In the present study, we have investigated whether the members of the teschovirus group can be used for this purpose. Although originally described as the causative agents of a neurological disorder known as Teschen-Talfan disease (4), teschoviruses are generally nonpathogenic, and infections with them remain unapparent (7). This characteristic is frequently found in endemic viruses. Teschoviruses were previously classified as enteroviruses due to the physicochemical properties of their virions, which resemble those of enteroviruses. They comprise 11 serotypes, and analysis of their nucleotide sequences has prompted reclassification of them into a new genus, Teschovirus, within the family Picornaviridae (3, 7-9, 18).
In order to assess if the presence of teschoviruses in surface waters can be used as a marker of contamination with pig fecal residues, we developed a real-time reverse transcriptase PCR (RT-PCR) method that allows the specific and quantitative detection of pig teschovirus (PTV) RNA in environmental waters and biological samples. The design of the oligonucleotide primers and the 5′-6-FAM-3′-TAMRA-labeled TaqMan probe was achieved by identifying conserved regions through the aligned RNA sequences of all prototype strains of teschoviruses (PTV-1 to -11, GenBank accession no. AF231769, AB038528, AF296087, AF296088, AF296089, AF296090, AF296091, AF296092, AF296093, AF296094, AF296119, AF296095, and AF296096). The highly conserved 5′ noncoding region (nucleotides [nt] 329 to 394, nucleotide numbering according to PTV-1 Talfan prototype strain sequence; GenBank accession no. AF231769) was chosen as the target for the forward and reverse primers and the fluorogenic TaqMan probe, whose sequences were, respectively, 5′-CACCAGCGTGGAGTTCCTGTA-3′, 5′-AGCCGCGACCCTGTCA-3′, and 5′-TGCAGGACTGGACTTG-3′.
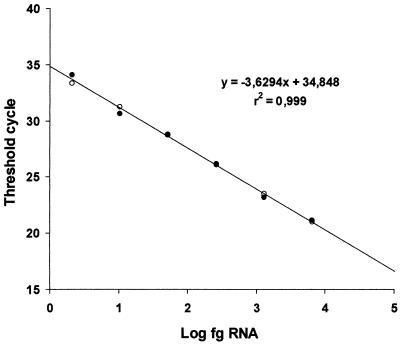
We developed a quantitative real-time RT-PCR assay for porcine teschoviruses by using a standard PTV-1 (isolate Vir 1626/89; kindly provided by R. Zell, Jena University, Jena, Germany) in an ABI Prism 7700 sequence detection system. The virus, propagated in PK-15 cell monolayers, was concentrated and purified by ultracentrifugation of a clarified infection supernatant for 2 h at 130,000 × g through a 2-ml 20% sucrose cushion. The viral RNA was isolated with the Qiamp viral RNA mini kit (Qiagen) according to the manufacturer's instructions (excluding RNA carrier to avoid interference with the RNA quantification). The RNA concentration was determined spectrophotometrically by measuring the A260 of the preparation (in which 1 optical density unit corresponds to 40 μg of RNA per ml). Dilutions of this viral RNA stock were used as a standard to quantify PTV RNA in water and feces samples. In order to optimize the method for maximum sensitivity and specificity, we tested several concentrations of primers and fluorogenic TaqMan probe, as well as different numbers of cycles and hybridization temperatures. The amplification was carried out with a commercial RT-PCR amplification kit (TaqMan One-Step RT-PCR master mix reagents; Applied Biosystems, Branchburg, N.J.), according to the manufacturer's instructions. The final TaqMan RT-PCR protocol consisted of the addition of 10 μl of isolated RNA to 40 μl of RT-PCR mix (25 μl of TaqMan 2× universal PCR master mix, 1.25 μl of 40× Multiscribe and RNase inhibitor mix from the TaqMan RT-PCR kit mentioned above, plus primers, to a final concentration of 0.5 μM; fluorogenic TaqMan probe, to a final concentration of 0.25 μM; and RNase-free water up to 40 μl), and then the tubes were subjected to a first RT step at 48°C for 30 min, followed by 10 min at 95°C (hot start) and 50 cycles of 15 s at 95°C and 1 min at 60°C. All PTV RNA determinations throughout this study were performed in duplicate. Figure 1 shows the linearity and efficiency of the real-time RT-PCR when serially diluted RNA from the standard PTV was used as a template. The assay was linear over a 105-dilution range with a correlation coefficient of 0.999, indicating that it was both reproducible and sensitive. Using this standard PTV-1 RNA, the assay was able to detect 2.8 fg of viral RNA, equivalent to 208 RNA molecules (approximately 7.4 × 10−3 50% tissue culture infective doses) per test tube, corresponding to 92 fg (6.8 × 103 molecules) of PTV RNA per ml of initial sample.
FIG. 1.
Linearity and efficiency of the real time RT-PCR assay for PTV. Serial fivefold dilutions of PTV RNA were made in duplicate assays, and the data represent the threshold cycle calculated for each reaction and plotted against the quantity (femtograms) of RNA on a log scale. Open and solid circles represent data from each duplicate.
To assess whether the assay described above was suitable for the detection of PTV RNA molecules in environmental samples (water and feces), we analyzed samples consisting of sewage water, water from pigsty ponds, and pig slurry, collected from five different pig farms from the western, eastern, and central parts of Spain (provinces of Badajoz, Barcelona, and Madrid, respectively) that were at least 50 km apart from each other (Table 1). Water samples (1.5 liters each) were tested without any concentration procedure or after concentration by filtration-elution through electropositive filters, as described elsewhere (11). RNA was isolated from 140 μl of water samples, water concentrates, or pig slurry with the Qiamp viral RNA mini kit (Qiagen) according to the manufacturer's instructions. The RT-PCR assay was performed as described above. The specificity of the assay was assessed in parallel by using aqueous extracts of feces from cattle, sheep, and goats that were positive for bovine enterovirus (data not shown). Results for samples from all pig farms examined were positive for PTV RNA, although it was necessary in some cases (such as samples APOBA1 and APOBA2) to concentrate the samples (water effluents) by filtration-elution to observe a positive result (Table 1). Conversely, none of the nonporcine fecal samples was positive for PTV RNA in this analysis,
TABLE 1.
Analysis of PTV in water and fecal samples potentially contaminated by pig fecal waste
| Farm no. | Sample | Location | Type of sample | Source | PTV RNA
|
|
|---|---|---|---|---|---|---|
| Result | Concna | |||||
| 1 | APOBA1 | Badajoz | Water | Sewage | + | Required |
| 2 | APOBA2 | Badajoz | Water | Pigsty pond | + | Required |
| 3 | APOBA3 | Badajoz | Water | Pigsty pond | + | None |
| 4 | PUOS1 | Barcelona | Pig slurry | Farm soil | + | None |
| PUOS2 | Pig slurry | Farm soil | + | None | ||
| 5 | APOJA1 | Madrid | Spillage | River | + | None |
| Control samples | Feces | Bovine | − | None | ||
| Feces | Goat | − | None | |||
| Feces | Ovine | − | None | |||
Concentration of water by filtration-elution.
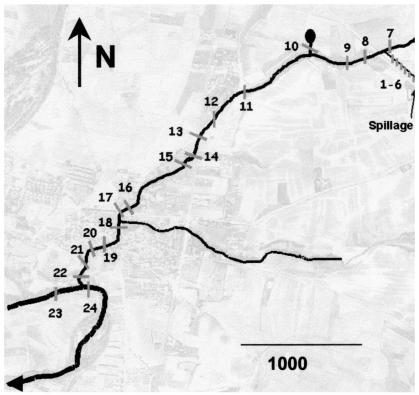
To validate this method, we analyzed 100-ml water samples collected downstream from a pig slurry spillage at 24 different points (Fig. 2). Samples were collected in the open duct and at different sites in the receiving streams. All samples were physicochemically analyzed, in parallel with the PTV RNA quantitative analysis. Sample collection and physicochemical analyses were performed by standard methods (routinely employed in the Laboratory of Ecotoxicology, Instituto Nacional de Investigación y Tecnologia Agraria y Alimentaria) (15), including the analysis of metals by atomic absorption spectrophotometry (2). PTV RNA was quantified by the real-time RT-PCR TaqMan protocol described above, using PTV-1 RNA (quantified spectrophotometrically) as the standard. Negative samples were reanalyzed after concentration by ultracentrifugation at 130,000 × g for 2 h at 10°C, a concentration method more suited for small volumes (100 ml) than the filtration-elution method used above. The concentration factor (16×) achieved with this method was estimated by measuring in parallel PTV RNA from two positive samples (samples 1 and 2) before and after concentration.
FIG. 2.
Map of the survey area. Water samples were collected and numbered correlatively after a pig slurry spillage. Samples 1 to 6 were collected from the open duct of the farm, sample 7 was collected upstream of the point where the open duct empties into the receiving stream, and sample 10 was collected from a spring source 100 m away from the stream. Samples 23 and 24 were taken from the river, upstream and downstream of the point where the stream flows into the river, respectively. (Background photo courtesy of the Departamento de Biotecnologia, Instituto Nacional de Investigaciones Agrarias, Madrid, Spain.).
Table 2 shows the comparison between PTV-RNA quantification and chemical determinations. The results reveal high concentrations of PTV (between 25,000 and 125,000 particles per ml) in samples 1 to 6, collected from an open duct of the farm, a result that is consistent with a considerable amount of pig waste. In the remaining samples collected from the receiving stream, PTV RNA concentrations were 15 to 1,000 times lower, due to the dilution effect. The PTV RNA became undetectable approximately 3,100 m downstream from the spillage, exactly at the same point organic matter dropped to background levels. Sample 7, collected upstream of the point at which the stream meets the duct draining the farm, was negative in the assay, as was sample 10, collected from a spring source 100 m away from the stream.
TABLE 2.
Analysis of PTV in water from a river contaminated by a pig slurry spillage: comparison with standard parameters of contamination
| Sample no. | Site | Concn of PTV RNA (molecules/ml) | Chemical parameters
|
||||
|---|---|---|---|---|---|---|---|
| Organic matter (mg/liter) | Nitrates (mg/liter) | Nitrites (mg/liter) | Potassium (mg/liter) | Copper (mg/liter) | |||
| Open duct | |||||||
| 1 | 5 m from slurry tank | 42,945 | 1,100 | 7.12 | 2.89 | 1,268 | 2.53 |
| 2 | 20 m from slurry tank | 76,380 | 1,120 | 0.93 | 3.02 | 1,170 | 2.79 |
| 3 | 40 m from slurry tank | 68,430 | 1,110 | 9.63 | 2.61 | 1,319 | 4.43 |
| 4 | 100 m from slurry tank | 26,005 | 1,120 | 1.48 | 2.47 | 1,404 | 2.51 |
| 5 | 150 m from slurry tank | 49,335 | 1,260 | 2.73 | 2.71 | 1,302 | 2.93 |
| 6 | 200 m from slurry tank | 125,270 | 2,120 | 18.16 | 6.3 | 1,398 | 3.45 |
| Stream | |||||||
| 7 | Upstream | NDa | 2.61 | 26.87 | 0.05 | 6.91 | 0.007 |
| 8 | 100 m | 490 | 4.04 | 137.69 | 0.05 | 6.10 | 0.006 |
| 9 | 500 m | 1,649 | 5.15 | 65.89 | 0.14 | 6.65 | 0.001 |
| 11 | 2,000 m | 126 | 4.28 | 30.97 | 0.02 | 5.07 | 0.007 |
| 12 | 2,500 m | 245 | 4.2 | 27.61 | 0.03 | 5.76 | 0.001 |
| 13 | 2,800 m | 334 | 4.43 | 45.52 | 0.05 | 5.72 | 0.002 |
| 14 | 3,000 m | 141 | 4.83 | 33.59 | 0.02 | 5.75 | 0.001 |
| 15 | 3,100 m | ND | 2.3 | 55.09 | 0.04 | 5.75 | 0.004 |
| 16 | 4,000 m | ND | 2.06 | 61.06 | 0.05 | 2.71 | 0.005 |
| 17 | 4,100 m | ND | 3.09 | 64.91 | 0.02 | 2.76 | 0.004 |
| 18 | 4,300 m | ND | 2.69 | 33.58 | 0.03 | 3.14 | 0.003 |
| 19 | 4,600 m | ND | 1.82 | 43.41 | 0.01 | 3.04 | 0.002 |
| 20 | 4,800 m | ND | 2.45 | 68.72 | 0.03 | 2.12 | 0.000 |
| 21 | 5,000 m | ND | 3.25 | 38.83 | 0.04 | 3.18 | 0.000 |
| 22 | 5,200 m | ND | 2.3 | 38.36 | 0.04 | 4.27 | 0.000 |
| Jarama River | |||||||
| 23 | Upstream | ND | 0.63 | NAb | 0.03 | NA | 0.005 |
| 24 | Mixing zone | ND | 1.42 | 0.12 | 0.03 | 0.79 | 0.003 |
| Spring water | |||||||
| 10 | 100 m to stream | ND | 1.9 | 54.41 | 0 | 0.99 | 0.002 |
ND, not detectable.
NA, not analyzed.
Statistical comparison of the data corresponding to PTV-positive samples (1 to 6 and 8 to 14; n = 12) reveals a significant correlation between the quantity of PTV RNA and levels of organic matter, nitrites, potassium, and copper (Pearson correlation coefficients, r = 0.94, 0.96, 0.82, and 0.86, respectively; n = 12). The lack of correlation with nitrates (r = −0.50) could be due to the rapid oxidation of nitrates to nitrites, which is expected, considering that dissolved oxygen was over the saturation value in the receiving water. In the same way, the large differences in the concentration of nitrates could be explained by a combination of oxidation at the surface and reduction in the waste bulk, in accordance with the significant concentration of nitrites and the absence of dissolved oxygen in the bulk effluent. The waste discharge increases the concentration of organic matter and nitrates in the stream. Additional peaks of nitrites and nitrates were observed several meters downstream from the discharge, likely originated by the mineralization of organic nitrogen (16). Nitrates, potassium, and copper were still found at high levels upstream from the discharge, suggesting additional pollution sources probably caused by the use of pig manure as fertilizer. In the mixing zone, an increase in organic matter was observed, which cannot be explained by a direct organic matter load except for the indirect effect on algal growth associated with the presence of nitrates and other nutrients in the stream, a fact that highlights the lack of specificity of the organic matter determinations.
Taken together, the results presented here indicate that PTVs can be highly sensitive and specific markers of water contamination due to pig waste. PTV RNA was detected up to 3 km downstream of the discharge, where the impacts on nitrates and nitrites were no longer observed. The analytical protocol described in this work can detect specific PTV RNA in amounts as low as 208 viral RNA molecules per test tube and is thus more sensitive than other protocols described recently in the literature for the detection of teschoviruses based on conventional RT-PCR (13). Although the prevalence of PTV in pig populations remains to be clearly established, our results point out to a wide distribution, at least in Spain, since PTV was found in all samples analyzed, collected from five points up to 1,000 km distant from each other. A survey to assess the endemicity of these viruses is currently being conducted in our laboratory to assess the adequacy of teschoviruses as universal markers of pig fecal contamination in environmental water.
Acknowledgments
We thank Ronald Zell for providing the PTV Vir 1626/89 isolate, nucleotide sequences, and advice on the design of the study.
Funding was provided by CICYT (Spain) grant no. RTA 02-035 and Comunidad de Madrid (Spain) grant no. 07 M/0003/2001.
REFERENCES
- 1.Campagnolo, E. R., K. R. Johnson, A. Karpati, C. S. Rubin, D. W. Kolpin, M. T. Meyer, J. E. Esteban, R. W. Currier, K. Smith, K. M. Thug, and M. McGeehin. 2002. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 299:89-95. [DOI] [PubMed] [Google Scholar]
- 2.Carbonell, G., J. A. Martinez-Pereda, J. V. Tarazona, and M. J. Muñoz. 1998. Mobilization of essential metals during and after short-term lethal cadmium exposure in rainbow trout, Oncorhynchus mykiss. Ecotoxicol. Environ. Restor. 1:85-91. [Google Scholar]
- 3.Doherty, M., D. Todd, N. McFerran, and E. M. Hoey. 1999. Sequence analysis of a porcine enterovirus serotype 1 isolate: relationships with other picornaviruses. J. Gen. Virol. 80:1929-1941. [DOI] [PubMed] [Google Scholar]
- 4.Harding, J. D. J., J. T. Done, and G. F. Kershaw. 1957. A transmissible polio-encephalomyelitis of pigs (Talfan disease). Vet. Rec. 69:824-832. [Google Scholar]
- 5.Hsu, J. H., and S. L. Lo. 2001. Effect of composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environ. Pollut. 114:119-127. [DOI] [PubMed] [Google Scholar]
- 6.Jongbloed, A. W., and N. P. Lenis. 1998. Environmental concerns about animal manure. J. Anim. Sci. 76:2641-2648. [DOI] [PubMed] [Google Scholar]
- 7.Kaku, Y., A. Sarai, and Y. Murakami. 2001. Genetic reclassification of porcine enteroviruses. J. Gen. Virol. 82:417-424. [DOI] [PubMed] [Google Scholar]
- 8.Kaku, Y., S. Yamada, and Y. Murakami. 1999. Sequence determination and phylogenetic analysis of RNA-dependent RNA polymerase (RdRp) of the porcine enterovirus 1 (PEV-1) Talfan strain. Arch. Virol. 144:1845-1852. [DOI] [PubMed] [Google Scholar]
- 9.Krumbholz, A., M. Dauber, A. Henke, E. Birch-Hirschfeld, N. J. Knowles, A. Stelzner, and R. Zell. 2002. Sequencing of porcine enterovirus groups II and III reveals unique features of both virus groups. J. Virol. 76:5813-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley, V., J. Higgins, and R. Fayer. 2002. Bovine enteroviruses as indicators of fecal contamination. Appl. Environ. Microbiol. 68:3455-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehnert, D. U., K. E. Stewien, C. M. Harsi, A. P. Queiroz, J. M. Candeias, and J. A. Candeias. 1997. Detection of rotavirus in sewage and creek water: efficiency of the concentration method. Mem. Inst. Oswaldo Cruz 92:97-100. [DOI] [PubMed] [Google Scholar]
- 12.Miner, J. R. 1999. Alternatives to minimize the environmental impact of large swine production units. J. Anim. Sci. 77:440-444. [DOI] [PubMed] [Google Scholar]
- 13.Palmquist, J. M., S. Munir, A. Taku, V. Kapur, and S. M. Goyal. 2002. Detection of porcine teschovirus and enterovirus type II by reverse transcription-polymerase chain reaction. J. Vet. Diagn. Investig. 14:476-480. [DOI] [PubMed] [Google Scholar]
- 14.Pellini, T., and J. Morris. 2001. A framework for assessing the impact of the IPPC directive on the performance of the pig industry. J. Environ. Manag. 63:325-333. [DOI] [PubMed] [Google Scholar]
- 15.Tarazona, J. V., M. J. Muñoz, G. Carbonell, M. Carballo, J. A. Ortiz, and A. Castaño. 1991. A toxicological assessment of water pollution and relationship with aquaculture development in Algeciras Bay (Cadiz, Spain). Arch. Environ. Contam. Toxicol. 20:480-487. [Google Scholar]
- 16.Tarazona, J. V., and M. J. Muñoz. 1989. A simple and practical model for toxicological assesment of nitrification byproducts in rivers. Toxicol. Environ. Chem. 24:9-15. [Google Scholar]
- 17.Vagstag, N., J. V., E. Loigu, and J. Deelstra. 2000. Nutrient losses from agricultural areas in the Gulf of Riga drainage basin. Ecol. Eng. 14:435-441. [Google Scholar]
- 18.Zell, R., M. Dauber, A. Krumbholz, A. Henke, E. Birch-Hirschfeld, A. Stelzner, D. Prager, and R. Wurm. 2001. Porcine teschoviruses comprise at least eleven distinct serotypes: molecular and evolutionary aspects. J. Virol. 75:1620-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]