Abstract
Retail poultry products are widely purported as the major infection vehicle for human campylobacteriosis. Numerous intervention strategies have sought to reduce Campylobacter contamination on broiler carcasses in the abattoir. This study reports the efficacy of bacteriophage in reducing the number of recoverable Campylobacter jejuni cells on artificially contaminated chicken skin.
Since their discovery in the late 1970s, campylobacters have been recognized as a major source of human gastrointestinal disease (11). Poultry, and especially chicken due to its preeminence in the market place, are considered major reservoirs of Campylobacter infection (14, 21). This is supported by reports of high numbers of campylobacters colonizing the avian gut (2, 17) and the high incidence of Campylobacter-positive poultry and poultry-derived products (15, 16). The rapid spread of Campylobacter in broiler house flocks has been previously reported, with one study showing total flock colonization in two days from the initial exposure (12). Although campylobacters are sensitive to desiccation, they are readily recovered from broiler fecal droppings (1). Moreover, this bacterial genus appears to be so well adapted to the avian gut that as few as 100 organisms can colonize (25). These two factors not only facilitate rapid flock colonization but also serve to disseminate the organism inside the broiler house and over the external surface of the birds.
Campylobacters can frequently be isolated from the feathers and skin of broiler chickens (3). One method of reducing broiler carcass contamination involves the use of hyperchlorite in scald water, for carcass washes, and chilled water tanks. Previous studies have shown this treatment to be ineffective in substantially reducing the numbers of human pathogens, including Campylobacter, associated with the chicken skin when used under standard commercial operating conditions (24). Increasing the concentration of hyperchlorite does marginally increase its efficacy but reduces the quality of the end product, which is unacceptable to consumers.
Host-specific bacteriophage have previously been used in the treatment of E. coli infections in piglets (22) and more recently in Enterococcus (5) and Vibrio (7) infections. Their efficacy in experimental enteric and systemic infections has already been demonstrated. Other studies have shown that the spoilage of beef by Pseudomonas spp. can be reduced by the application of phage (10). However, the ability of host-specific phage to reduce the presence of a food-borne pathogen which is not actively replicating on the surface of food has yet to be investigated.
The survival of the bacteriophage and its host independent of each other was determined on the surface of skin obtained from freshly sacrificed broiler chickens (n = 6) reared to be free of Campylobacter. The cecal contents and skin of these birds were established as Campylobacter negative by the direct plating of samples onto modified charcoal cefoperazone deoxycholate agar (mCCDA; code LAB 112; supplement code X112; LabM, Lancashire, United Kingdom), a selective medium used in the isolation of thermophilic campylobacters. Each chicken was sacrificed at 30 days of age and then was manually plucked prior to skin removal. Sections of chicken skin (2 cm2) were then cut by using clean, flame-sterilized scissors and were individually transferred to sterile petri dishes.
The Campylobacter strain used, C. jejuni NCTC 12662 phage type 14 (C. jejuni PT14), was prepared by uniformly inoculating plates of Columbia blood agar (CBA; Columbia agar base, code CM331, supplemented with 7% [vol/vol] laked horse blood, code SR48; Oxoid Ltd., Basingstoke, United Kingdom) and incubating them in a microaerobic atmosphere (5% O2, 5% H2, 10% CO2, 80% N2) at 42°C for 24 h. The resulting growth was harvested into 20-ml volumes of sterile 10 mM MgSO4 solution, and the cell density was adjusted to McFarland standard no. 3 (approximately 109 CFU per ml). All subsequent serial dilutions for the experimental inoculations were prepared in maximum recovery diluent (MRD; code CM733; Oxoid Ltd.). Bacteriophage φ2 was propagated on its host (C. jejuni NCTC 12662 PT14) by using the methods of Frost et al. (9) prior to adjusting the titer to 108 PFU per ml by dilution in SM buffer (50 mM Tris-Cl, pH 7.5, supplemented with 0.1 M NaCl, 8 mM MgSO40 · 7H2O, and 0.01% gelatin [Sigma G-1393]). Each section of chicken skin was inoculated in triplicate with either 106 CFU of C. jejuni NCTC 12662 PT14 (estimated using McFarland turbidity standards and subsequently titered by dilution) or 107 PFU of bacteriophage φ2 (NCTC 12674, ACTC 35922-B2) suspension (titer determined on the C. jejuni NCTC 12662 host strain) for each sampling time point. The skin sections were then stored individually in sterile containers at either 4°C (fresh) or −20°C (frozen).
Sections of skin from each storage condition were removed for the recovery of both Campylobacter and bacteriophage, initially 1 h after inoculation (day 1) and then at 24-h intervals thereafter for a period of 10 days. Campylobacters were recovered by stomaching each chicken skin section in 20 ml of MRD for 5 min in sterile filter stomacher bags using a Seward Lab Blender 400. The stomachate was then serially diluted in MRD before spread plating 100-μl volumes of each dilution onto the surface of mCCDA plates in triplicate. These plates were subsequently incubated in microaerobic conditions for 24 to 48 h before the enumeration of typical Campylobacter colonies. Representative colonies from each sample were subcultured onto CBA plates for 18 h before Gram staining and confirmation of typical Campylobacter cell morphology. Bacteriophage were recovered by stomaching the skin sections for 1 min in 20 ml of SM buffer in a sterile filter stomacher bag using a Seward Lab Blender 400. An aliquot of the stomachate (1 ml) was centrifuged at 13,000 × g for 3 min in a Centaur microcentrifuge to remove bacterial cells. Serial dilutions of the supernatant were used to inoculate a lawn of susceptible Campylobacter host cells (C. jejuni NCTC 12662) using a modification of the surface droplet technique (20) with enumeration performed according to the method of Miles and Misra (18). Bacterial lawns were prepared by adding 0.5 ml of a 109-CFU/ml suspension of C. jejuni PT14 to 5 ml of molten NZCYM overlay agar (NZCYM broth [code 241510; Difco, Oxford, United Kingdom] with 0.6% bacteriological agar [Oxoid Agar No. 1, code L13]) and then pouring it over NZCYM base agar (NZCYM broth with 1% bacteriological agar) to ensure an even distribution. Plates were left to set for 20 min prior to the spotting of phage suspensions onto the surface. After inoculation of the phage the plates were left to dry for 20 min before incubation in microaerobic conditions at 42°C for 24 h and then were examined for the presence of plaques.
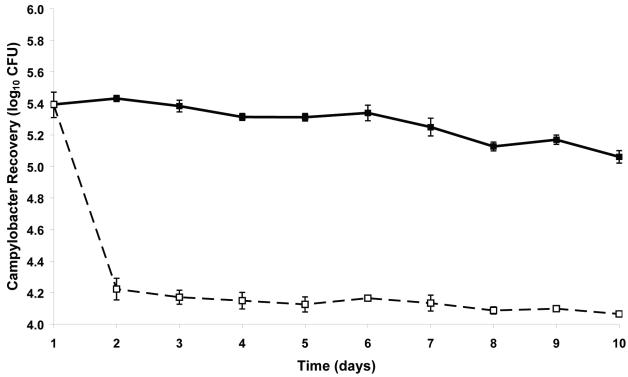
The recovery of Campylobacter NCTC 12662 from the surface of chicken skin is presented in Fig. 1. Campylobacter recovery at the initial time point was identical (log10 5.4 CFU) for skin samples stored at 4 and −20°C. However, recovery from the fresh samples continued to gradually decline to reach a base of log10 5.1 ± 0.04 CFU at the end of the experiment. In contrast, the freeze-thaw treatment of the skin resulted in a fall in Campylobacter recovery to log10 4.2 ± 0.08 CFU on day 2 versus log10 5.4 ± 0.02 CFU for the fresh chicken skin at the same time point. On frozen skin, Campylobacter recovery was sustained at approximately the same level for the rest of the experiment with only a slight decline to log10 4.1 ± 0.01 CFU on day 10. Numerous studies have shown the prevalence and scale of Campylobacter contamination of retail poultry products (4, 14-16). Investigations into the survival of clinical Campylobacter isolates stored at different temperatures noted a log10 3.0 CFU drop in viable cells upon freezing (8). Our data suggest this may not be the case for campylobacters on the surface of chicken skin, where the reduction was closer to log10 1.0 or log10 2.0 CFU for skin stored at 4 and −20°C, respectively. Considering these data, it is possible that chicken skin has a protective effect on the Campylobacter cells. Similar findings have been reported for other physical and chemical methods of removing Campylobacter from chicken carcasses (13). Studies with Salmonella also suggest that the chicken skin provides an environment that confers nonspecific protection to bacteria from potentially harmful treatments (13, 24). This is most likely due to the presence of feather follicles and folds on the skin surface that, along with oils and fats present, offer some protection from ice crystal formation, which is known to be a major cause of damage to cells undergoing the freezing process. Chicken carcasses have been reported to be contaminated with up to 109 CFU of Campylobacter (15). Since the infective dose of Campylobacter for humans is thought to be ≤500 cells (6), the information on the enhanced survival of Campylobacter on chicken skin under these conditions is of critical importance in food safety.
FIG. 1.
Graph showing the recovery of C. jejuni NCTC 12662 from the surface of chicken skin inoculated with 106 CFU and stored at 4°C (▪) and −20°C (□) over a 10-day period. Recovery is presented as the mean log10 CFU ± standard deviation.
Bacteriophage φ2 recovery from chicken skin stored at 4°C remained relatively constant at log10 6.7 ± 0.14 PFU of the initial inoculated titer (log10 7.0 PFU) for the duration of the experiment, falling slightly to log10 6.4 ± 0.1 PFU at day 10. The recovery of phage inoculated on chicken skin stored at −20°C was initially similar to recovery from skin stored at 4°C (log10 6.8 ± 0.11 PFU) in parallel with the pattern observed for its host, but following freeze-thaw the recovery fell to log10 6.1 ± 0.2 PFU, where it remained for the rest of the experiment. The fact that phage are able to survive on the surface of chicken skin for a period of at least 10 days when stored under either fresh or frozen conditions is important when considering the potential efficacy and environmental impact of phage therapy on animals that carry Campylobacter. Phage of Campylobacter along with their hosts can potentially survive on the retail product until well after its prescribed shelf life. A concurrent study has shown that Campylobacter phage are indeed able to survive and be isolated from retail poultry products.
To establish whether bacteriophage φ2 bound nonspecifically to other Campylobacter species at the refrigerated temperature required for retail chicken storage (4°C), the phage was mixed with Campylobacter coli NCTC 12667, which is not susceptible to infection by this phage. A 107-PFU suspension of φ2 was mixed with a 106-CFU/ml suspension of NCTC 12667 in MRD to give a multiplicity of infection (MOI) of 10. Triplicates of this suspension were stored statically at 4°C. Campylobacter and bacteriophage titers were recorded at 24-h intervals for 10 days. Campylobacters were enumerated by using serial-dilution mCCDA spread plates as described previously. For bacteriophage enumeration, 1 ml of the suspension was centrifuged at 13,000 × g for 3 min in a Centaur microcentrifuge to remove bacterial cells. Serial dilutions of the supernatant were used to inoculate a lawn of susceptible Campylobacter host cells (C. jejuni PT14) for enumeration using the surface droplet technique described previously. This experiment was then repeated for φ2 and susceptible host C. jejuni PT14 for comparison. There was no recorded fall in either the Campylobacter or bacteriophage titers when φ2 was mixed with C. coli NCTC 12667 compared to titers of the controls. In contrast, when φ2 was mixed with the susceptible host (C. jejuni PT14), falls of log10 0.8 ± 0.1 PFU/ml and log10 0.5 ± 0.2 CFU/ml were observed for phage and Campylobacter populations, respectively. There was no increase in phage titer over the 10-day course of the experiment with either Campylobacter species used. After the initial decrease in Campylobacter and phage titers were recorded, no further significant changes in their numbers were observed for the remainder of the experiment at this temperature.
Sections of chicken skin (2 cm2), prepared as described above, were inoculated with combinations of different bacteriophage and host titers in order to determine the effect of phage application on the recovery of Campylobacter from artificially contaminated chicken skin stored at 4 and −20°C. Each skin section was inoculated with a combination of phage and Campylobacter titers in the form of a simple 3 by 3 matrix to obtain MOIs ranging from 0.001 to 100,000 (Table 1). Each specific MOI combination was inoculated in triplicate for each sampling time point for each storage condition. Both phage and host were prepared for inoculation as described above. The skin was first inoculated with C. jejuni NCTC 12662, and following a 30-min drying period the phage (φ2) was inoculated. A further 30-min period was allowed for the phage inoculum to dry before storage at 4°C (fresh) or −20°C (frozen). The initial (day 1) samples were removed for Campylobacter and phage enumeration immediately following the final 30-min drying period and thereafter on days 3 and 5 for fresh samples and day 5 only for frozen samples. Campylobacter and bacteriophage populations were enumerated by using the methods described above. The significance of the effect of phage treatment on Campylobacter numbers was assessed with a one-tailed paired t test with a confidence interval of 95% using the Data Analysis Toolkit for Microsoft Excel XP. The recovery of C. jejuni from the surface of chicken skin following treatment with different titers of bacteriophage is presented in Table 2. Campylobacter recovery from controls inoculated with 106 and 104 CFU remain consistent for the course of the experiment, with recovery ranging from log10 5.2 to log10 5.4 CFU (106-CFU inoculation) and log10 3.2 to log10 3.4 CFU (104-CFU inoculation). With the lowest phage titer applied (103 PFU) there was no significant fall in the numbers of Campylobacter recovered from fresh or frozen chicken skin compared to those of the control. However, in all cases of the highest-titer phage treatment (107 PFU) there was a significant reduction (P < 0.0001) in Campylobacter recovery at all sampling points, falling by log10 1.1 to log10 1.2 CFU (106 CFU inoculation) and log10 1.1 to log10 1.3 (104 CFU inoculation) versus those of their respective controls. The difference in recovery was even greater in the frozen chicken skin samples. When 107 PFU was applied, a reduction of log10 2.3 and log10 2.5 CFU compared to those of the controls (log10 4.1 ± 0.6 and log10 2.5 ± 0.6 CFU) was recorded for Campylobacter inoculations of 106 and 104 CFU, respectively. These reductions were also significant (P < 0.0001) using a one-tailed paired t test.
TABLE 1.
Matrix of Campylobacter and bacteriophage titers used for chicken skin inoculation
| Amt. of Campylobacter inoculum (CFU) | Amt. of bacteriophage inoculum (PFU) and MOIa
|
||
|---|---|---|---|
| 107 | 105 | 104 | |
| 106 | 10 | 0.1 | 0.01 |
| 104 | 1,000 | 10 | 1 |
| 102 | 100,000 | 1000 | 10 |
The MOI for each phage-host combination is displayed.
TABLE 2.
Recovery of C. jejuni NCTC 12662 from the surface of chicken skin treated with bacteriophage φ2 and stored at 4°Ca
| Amt. of Campylobacter inoculum (CFU) | Amt. of bacteriophage inoculum (PFU) and day recorded
|
Control recovery at day:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 107
|
105
|
103
|
||||||||||
| 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | |
| 106 | 4.2 ± 0.2 | 4.2 ± 0.1 | 4.2 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.1 | 5.0 ± 0.1 | 5.3 ± 0.1 | 5.4 ± 0.1 | 5.2 ± 0.1 |
| 104 | 1.9 ± 0.1 | 2.1 ± 0.0 | 2.3 ± 0.0 | 3.4 ± 0.2 | 3.5 ± 0.0 | 3.1 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.2 | 3.4 ± 0.1 | 3.1 ± 0.4 | 3.4 ± 0.2 | 3.4 ± 0.2 |
| 102 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.8 ± 1.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 1.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Results are shown as log10 CFU recovered ± standard deviations for different combinations of phage and host innocula recorded at days 1, 3, and 5.
The application of Campylobacter-specific bacteriophage in high enough titers to the surface of chicken skin inoculated with Campylobacter clearly reduced the number of recoverable cells. This reduction was consistent over the course of the experiment, with a fall in recovery of log10 1.0 CFU for inoculated skin stored at 4°C. A larger reduction would be desirable if this practice was to yield commercial benefit in the future as a sole control measure. However, in its present state it might be useful alongside other contamination control practices. There was a significantly greater reduction in Campylobacter recovery associated with the combination of freezing and phage treatment. Countries such as Iceland routinely freeze Campylobacter-positive carcasses in an attempt to reduce contamination, and recent studies suggest this approach is effective (23). Combining this freezing with phage treatment could result in further falls in Campylobacter prevalence on broiler carcasses. It is generally accepted that campylobacters do not replicate when incubated at 4°C. Our data support this, since the number of campylobacters which could be recovered from the surface of the skin fell over time. There was also no increase in bacteriophage numbers on any of the chicken skin samples inoculated with Campylobacter. Recovery of bacteriophage on all of the samples fell over time but did not differ significantly from that of the control. In the absence of active replication, the phage are unlikely to reduce the number of Campylobacter cells in situ. Consequently, the most likely explanation for the observed reduction in Campylobacter recovery is that upon inoculation a proportion of the phage successfully adsorb to the surface of the bacteria but do not replicate until the bacterium itself increases its metabolic activity. This is supported by the data showing there is no reduction in either bacteriophage or Campylobacter titer when the phage is mixed with a nonpermissive host, suggesting that nonspecific adsorption or lysis by the phage is not taking place. This has implications for using phage as decontaminating agents, as only populations of susceptible bacteria would be affected. Effective cocktails of broad-host-range phage would need to be used for this technique to be practically applicable.
In our experiments we have diluted the campylobacters recovered after phage treatment of the chicken skin before placing them under conditions that will allow growth. This procedure enabled us to enumerate the surviving bacteria. Under these circumstances any bacterial cells that are phage adsorbed will perish, but the phage from this burst will not easily disseminate to other host bacteria. However, in a situation where the phage-adsorbed bacteria are part of a larger, localized population of host bacteria, the phage burst will initiate new infection cycles. If the conditions permissive for growth were indeed a human or animal gut, then the initial infective dose would be reduced with the prospect of further control by the phage on the growth of the host bacterium.
To determine if the campylobacters recovered after phage treatment were resistant to further phage infection, these isolates were subcultured onto CBA plates under microaerobic conditions at 42°C for 24 h and were used to prepare bacterial lawns on NZCYM plates. The lawns were then inoculated with serial dilutions of a φ2 bacteriophage suspension, and plaques were enumerated using the surface droplet technique described above. The phage titers obtained from lawns of the recovered campylobacters were compared with titers determined with the initial strain. All of the campylobacters recovered from phage-treated chicken skin remained sensitive to φ2. The titers obtained on lawns of these bacteria did not differ significantly from those of the original strain. The recovered campylobacters were then examined for their genetic similarity to the inoculated strain by using pulsed-field gel electrophoresis (PFGE) to compare SmaI restriction fragments of genomic DNA using the method described by Ribot et al. (19). The PFGE macrorestriction profiles produced by SmaI digestion of the recovered Campylobacter strains were indistinguishable from that of the original inoculated strain. Adaptation of the host to become resistant to bacteriophage can readily be demonstrated in the laboratory, and this is postulated to be a major drawback in the use of phage as control agents in general. There was no evidence of resistance arising in the Campylobacter recovered after phage treatment in this study. The campylobacters recovered were confirmed as being identical to the inoculation strain by PFGE of SmaI restriction fragments of genomic DNA. However, in the absence of replication these studies do not rule out the generation of new mutational events selected posttreatment.
In conclusion, we have demonstrated the ability of a characterized NCTC Campylobacter and its bacteriophage predator to survive independently on the surface of chicken skin. Phage inoculated onto the surface of skin contaminated with Campylobacter exhibit a control effect even in the absence of host growth. Further development of this study could lead to the use of bacteriophage in connection with other measures to control chickens contaminated with Campylobacter.
Acknowledgments
This study was supported in part by the United Kingdom Department for the Environment, Food and Rural Affairs. R.J.A. acknowledges the financial support of the University of Nottingham.
We thank Jenny Frost and colleagues at the Central Public Health Laboratory, Colindale, London, for their donation of the phage and Campylobacter strains.
REFERENCES
- 1.Berndtson, E., M. L. Danielsson-Tham, and A. Engvall. 1996. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int J. Food Microbiol. 32:35-47. [DOI] [PubMed] [Google Scholar]
- 2.Berndtson, E., M. Tivemo, and A. Engvall. 1992. Distribution and numbers of Campylobacter in newly slaughtered broiler chickens and hens. Int J. Food Microbiol. 15:45-50. [DOI] [PubMed] [Google Scholar]
- 3.Berrang, M. E., R. J. Buhr, and J. A. Cason. 2000. Campylobacter recovery from external and internal organs of commercial broiler carcass prior to scalding. Poult. Sci. 79:286-290. [DOI] [PubMed] [Google Scholar]
- 4.Berrang, M. E., S. R. Ladely, and R. J. Buhr. 2001. Presence and level of Campylobacter, coliforms, Escherichia coli, and total aerobic bacteria recovered from broiler parts with and without skin. J. Food Prot. 64:184-188. [DOI] [PubMed] [Google Scholar]
- 5.Biswas, B., S. Adhya, P. Washart, B. Paul, A. N. Trostel, B. Powell, R. Carlton, and C. R. Merril. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 7.Cerveny, K. E., A. DePaola, D. H. Duckworth, and P. A. Gulig. 2002. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 70:6251-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, K. F., H. Le Tran, R. Y. Kanenaka, and S. Kathariou. 2001. Survival of clinical and poultry-derived isolates of Campylobacter jejuni at a low temperature (4°C). Appl. Environ. Microbiol. 67:4186-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost, J. A., J. M. Kramer, and S. A. Gillanders. 1999. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greer, G. 1986. Homologous bacteriophage control of Pseudomonas growth and beef spoilage. J. Food Prot. 49:104-109. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths, P. L., and R. W. A. Park. 1990. Campylobacters associated with diarrhoeal disease. J. Appl. Bacteriol. 69:281-301. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey, T. J., F. Jorgensen, and K. L. Mattick. 2000. Fit to eat? Food scares and safe food production. Microbiol. Today 27:10-12. [Google Scholar]
- 13.Humphrey, T. J., and D. G. Lanning. 1987. Salmonella and Campylobacter contamination of broiler chicken carcasses and scald tank water: the influence of water pH. J. Appl. Bacteriol. 63:21-25. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs-Reitsma, W. 2000. Campylobacter in the food supply, p. 467-481. In M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 15.Jorgensen, F., R. Bailey, S. Williams, P. Henderson, D. R. Wareing, F. J. Bolton, J. A. Frost, L. Ward, and T. J. Humphrey. 2002. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int. J. Food Microbiol. 76:151-164. [DOI] [PubMed] [Google Scholar]
- 16.Kramer, J. M., J. A. Frost, F. J. Bolton, and D. R. Wareing. 2000. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J. Food Prot. 63:1654-1659. [DOI] [PubMed] [Google Scholar]
- 17.Mead, G. C., W. R. Hudson, and M. H. Hinton. 1995. Effect of changes in processing to improve hygiene control on contamination of poultry carcasses with Campylobacter. Epidemiol. Infect. 115:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles, A. A., and S. S. Misra. 1938. The estimation of the bacterial power of the blood. J. Hyg. 38:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama, S. M., F. J. Bolton, and D. N. Hutchinson. 1990. Evaluation of methods for the enumeration of Campylobacter jejuni and Campylobacter coli bacteriophages. Lett. Appl. Microbiol. 10:193-195. [Google Scholar]
- 21.Shane, S. M. 2000. Campylobacter infection of commercial poultry. Rev. Sci. Technol. 19:376-395. [DOI] [PubMed] [Google Scholar]
- 22.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 23.Stern, N. J., K. L. Hiett, G. A. Alfredsson, K. G. Kristinsson, J. Reiersen, H. Hardardottir, H. Briem, E. Gunnarsson, F. Georgsson, R. Lowman, E. Berndtson, A. M. Lammerding, G. M. Paoli, and M. T. Musgrove. 2003. Campylobacter spp. in Icelandic poultry operations and human disease. Epidemiol. Infect. 130:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whyte, P., J. D. Collins, K. McGill, C. Monahan, and H. O'Mahony. 2001. Quantitative investigation of the effects of chemical decontamination procedures on the microbiological status of broiler carcasses during processing. J. Food Prot. 64:179-183. [DOI] [PubMed] [Google Scholar]
- 25.Young, C. R., R. L. Ziprin, M. E. Hume, and L. H. Stanker. 1999. Dose response and organ invasion of day-of-hatch Leghorn chicks by different isolates of Campylobacter jejuni. Avian Dis. 43:763-767. [PubMed] [Google Scholar]