Abstract
Pluripotent hemopoietic stem cells (P-HSCs) were thought to be c-kit+, but recent reports indicate that they are c-kitlow. In the present report, we provide evidence using Ly5 congenic mice that P-HSCs are c-kit<low. Lineage-negative (Lin−)/CD71− cells among bone marrow cells (BMCs) from C57BL/6 Ly5.1 mice were separated into major histocompatibility complex class Ihigh (class Ihigh)/c-kitlow and class Ihigh/c-kit<low populations. Each population (500 cells) was transplanted into lethally (9.0 Gy) irradiated C57BL/6 Ly5.2 congenic mice along with Ly5.2 (2 × 105) compromised cells. Donor-derived Ly5.1+ cells were detected 6 months after transplantation in primary recipients reconstituted with either class Ihigh/c-kitlow or class Ihigh/c-kit<low cells. BMCs (1 × 106) from the primary recipients were further transplanted into secondary recipients (Ly5.2 mice) to assess their long term repopulating activity. Six months after bone marrow transplantation, Ly5.1+ cells in all lineages were detected only in secondary recipients that had been given BMCs from the primary recipients reconstituted with class Ihigh/c-kit<low cells but not in cells that were class Ihigh/c-kitlow. When the BMCs (1 × 106) of these secondary recipients were further transplanted into tertiary recipients, all tertiary recipients that had been given BMCs from the secondary recipients originally reconstituted with Lin−/CD71−/class Ihigh/c-kitlow cells died within 10 days whereas all six tertiary recipients originally reconstituted with Lin−/CD71−/class Ihigh/c-kit<low cells showed donor (Ly5.1+)-derived cells in their peripheral blood. In the single tertiary recipient that was killed, donor-derived T cells, B cells, macrophages, and granulocytes also were detected in several major hematolymphoid organs. The remaining five mice continue to survive more than 6 months after the tertiary bone marrow transplantation.
Pluripotent hemopoietic stem cells (P-HSCs) are defined as cells with the capacity to eternally self-renew and to differentiate into cells of every hemopoietic lineage. Various efforts have been made to purify P-HSCs (reviewed in ref. 1). Visser et al. (2) have reported that murine HSCs express high levels of major histocompatibility class I and that they have the capacity to bind wheat germ agglutinin. We also have reported that wheat germ agglutinin-binding cells in the Go phase [obtained after 5-fluorouracil (5-FU) treatment both in vivo and in vitro] show 150 times the number of spleen colony-forming units on day 14 as the original bone marrow cells (BMCs) (3). Spangrude et al. (4) purified murine multipotent progenitors with radio-protective ability by defining the Thy-1low/Sca-1+/lineage-negative (Lin−) population of BMCs. Uchida et al. (5, 6) also have reported that Thy-1low/Sca-1+/Lin− cells are multipotent progenitors. Morrison and Weissman (7) have further purified P-HSCs from Thy-1low/Sca-1+/Lin− cells based on the expression of CD4 and Mac-1 molecules; they have shown by clonal analyses that only Lin−/Mac-1−/CD4− cells possess long term repopulating activity (LTRA). P-HSCs in the mouse bone marrow (8–10) and in the adult liver (11) have been considered to be c-kit+ but recently have been shown to be c-kitlow (12, 13). Very recently, we established a new method for the purification of P-HSCs from mouse bone marrow; Lin−/CD71−/class Ihigh cells in a low density fraction were obtained from the BMCs of mice 4 days after 5-FU injection (14). As few as four of these cells purified from male mice reconstituted cells of all lineages over long periods in irradiated female recipients. Donor-derived cells from the peripheral blood were detected by a PCR technique using male-specific primers in cells of both myeloid and lymphoid lineages as long as 180 days after reconstitution. Moreover, original donor-derived cells were detected in the peripheral blood 60 days after transplantation in secondary recipients that had been given BMCs from primary recipients, indicating that Lin−/CD71−/class Ihigh cells have LTRA. In addition, the cells were found to be c-kit− or c-kitlow (14). In the present study, we provide evidence using Ly5 congenic mice that P-HSCs are c-kit<low.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6 Ly 5.2) mice were purchased from CLEA Japan (Osaka) and maintained in our animal facility until used. Congenic C57BL/6-Ly5.1-Pep3b (B6 Ly5.1) mice were obtained from The Jackson Laboratory. These mice were bred and maintained in our animal facility. All mice were used at 8–12 weeks of age.
Antibodies.
Purified rat mAbs against CD4 (GK1.5), CD8 (53–6.72), CD45R (B220, RA3–6B2), granulocytes (Gr-1, RB6), macrophages (Mac-1, M1/70), and transferrin receptor (CD71) were purchased from PharMingen, and mAb against erythroid lineage cells (TER119) was kindly donated by T. Kina (Chest Disease Institute, Kyoto University, Kyoto). These mAbs were used to deplete myeloid/lymphoid lineage cells and CD71+ cells using specific magnetic beads conjugated with sheep anti-rat IgG antibody (Dynabeads M-450, Dynal, Oslo). Fluorescein isothiocyanate (FITC)-coupled anti-H-2Kb mAb from PharMingen and biotinylated anti-c-kit mAb (ACK4; kindly donated by S.-I. Nishikawa, Kyoto University) were used to further purify the P-HSCs. FITC-coupled rabbit anti-mouse μ-chain antibody and biotinylated anti-Ly5.1 mAb were purchased from Coulter, and phycoerythrin–streptavidin was purchased from Tago. FITC-coupled mAbs against B220, CD4, CD8, granulocytes (Gr-1), and macrophages (Mac-1) also were purchased from PharMingen for analyzing cell surface phenotypes.
Purification of P-HSCs.
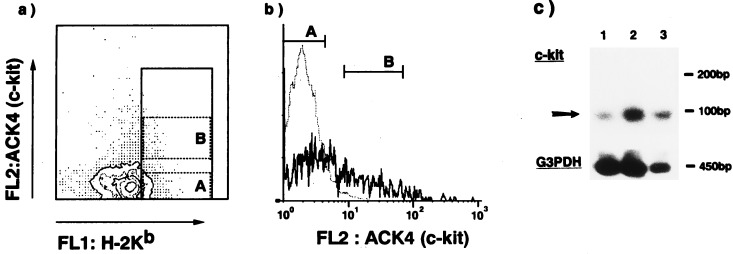
B6 Ly5.1 mice were treated with 5-FU (150 mg/kg) and, 3 days later, the BMCs were collected and applied to Percoll (Pharmacia) discontinuous density gradient. After centrifugation, the cells with a density of 1.063 < ρ < 1.071 were collected as reported (3, 14). The low density cells were treated with a mixture of mAbs against CD4, CD8, Mac-1, Gr-1, TER119, and CD71, followed by anti-rat IgG-conjugated magnetic beads (Dynabeads) to deplete the cells bearing myeloid/lymphoid lineage markers and CD71 molecules. The cells thus prepared (Lin−/CD71− cells) were stained with biotinylated anti-c-kit mAb (ACK4) followed by phycoerythrin–streptavidin and FITC anti-H-2Kb mAb to identify cells with class Ihigh/c-kitlow and class Ihigh/c-kit<low. Both populations showed low side light scattering and moderate forward light scattering and were sorted using a FACStar (Becton Dickinson) as shown in Fig. 1.
Figure 1.
Staining profiles (a, b) and RT-PCR analyses (c) of Lin−/CD71−/class Ihigh/c-kit<low cells. Lin−/CD71− cells were stained with anti-H-2Kb and anti-c-kit mAbs, and cells with a high expression of major histocompatibility class I were collected (represented in a). These cells were further divided into two populations based on the expression of c-kit [a and b (histogram)] and c-kit<low (A), and c-kitlow (B) cells were sorted by a FACStar. The dotted line in b represents a negative control stained by isotype-matched rat mAb. (c) RT-PCR-amplified products from a c-kit gene (upper) and from a glyceraldehyde 3-phosphate dehydrogenase (G3PDH) gene as an internal standard (lower) were electrophoresed, transferred to a nylon membrane, and probed with internal oligonucleotides. Lanes: 1, Lin−/CD71−/class Ihigh/c-kit<low cells; 2, Lin−/CD71−/class Ihigh/c-kitlow cells; and 3, Lin−/CD71− unsorted BMCs.
Reverse Transcriptase PCR (RT-PCR).
BMCs (1.2 × 104) (unsorted Lin−/CD71− cells or sorted Lin−CD71−/class Ihigh/c-kitlow or Lin−/CD71−/class Ihigh/c-kit<low cells) were used for RT-PCR analyses. Total RNA was isolated using guanidine thiocyanate. First-strand cDNA was prepared with oligo(dT)20 as a primer using RT-PCR high (Toyobo, Osaka). The cDNA was amplified by PCR for 27 cycles. For the analyses of c-kit gene expression, the following specific primers were used: c-kit (5′-GGGCAAGAGTTCCGCCTTCTT-3′ and 3′-CCGAAACACCAGCGTCG-5′) generating a 100-bp fragment (15). For the analyses of glyceraldehyde 3-phosphate dehydrogenase as an internal standard gene expression, primers (generating a 450-bp product) supplied by RT-PCR high (Toyobo) were used. Each PCR product was electrophoresed through a 2% agarose gel, transferred to a nylon membrane, and hybridized with a 32P-labeled oligonucleotide for c-kit cDNA corresponding to nucleotides 1561–1577 (5′-TAAAGAGCAAATCCAGG-3′).
Transplantation of P-HSCs.
Recipient (B6 Ly5.2) mice were treated with 9.0 Gy (137Cs) of total body irradiation and were injected i.v. 24 h later with 500 cells from the sorted class Ihigh/c-kitlow or class Ihigh/c-kit<low fraction plus 2 × 105 compromised cells from B6 Ly5.2 mice (BMCs from B6 Ly5.2 mice that had been compromised with respect to LTRA by having been subjected to two cycles of marrow regeneration) (14). Six months after the transplantation, cells from the bone marrow, thymus, spleen, lymph nodes, peritoneal cavity, and peripheral blood of the recipient mice were stained with a panel of FITC-conjugated mAbs (anti-sIgM, anti-B220, anti-CD4, anti-CD8, anti-granulocyte, and anti-macrophage) in combination with biotinylated anti-Ly5.1 mAb and phycoerythrin–streptavidin to check for donor-derived myeloid/lymphoid cells.
Secondary and Tertiary Transplantation.
Secondary transplantation was carried out to examine the LTRA of the donor P-HSCs. The secondary recipients (C57BL/6 Ly5.2 mice) were transplanted with 106 BMCs from primary recipients after 9.0 Gy of irradiation 6 months after the primary transplantation. A further 6 months after the transplantation, cells expressing the Ly5.1 phenotype, originating from the primary donor-derived P-HSCs, were detected in the bone marrow, thymus, spleen, lymph nodes, peritoneal cavity, and peripheral blood using mAbs against myeloid or lymphoid lineage cells along with anti-Ly5.1 mAb. Tertiary transplantation was carried out in the same way; 1 × 106 BMCs from the secondary recipients were transplanted into the tertiary recipients (B6 Ly5.2 mice).
RESULTS
Purification and Characterization of c-kitlow and c-kit<low HSCs.
The HSCs (Lin−/CD71−/class Ihigh cells) were further fractionated on the basis of the expression of c-kit. Fig. 1a shows the flow cytometric profile of Lin−/CD71− cells stained with anti-H-2Kb and anti-c-kit mAbs. H-2Kb high cells were gated, and two cell populations—c-kitlow and c-kit− (which are comparable to the negative control level)—were fractionated by sorting on the basis of c-kit expression. Sorting gates and histograms are shown in Fig. 1 a and b; the sorting gate of c-kit− cells was set not to overlap with that of c-kitlow cells. The FACS profile was very similar to that reported by Katayama et al. (13); the c-kitlow and c-kit− populations were identified as shown in Fig. 1 a and b. Purified Lin−/CD71−/class Ihigh/c-kitlow cells or Lin−/CD71−/class Ihigh/c-kit− cells were ≈0.006% or 0.008% of the 5-FU-treated whole BMCs, respectively.
The next step was to examine the expression of c-kit transcripts in c-kit− cells. The c-kit transcripts in Lin−/CD71− cells, Lin−/CD71−/class Ihigh/c-kitlow cells, and Lin−/CD71−/class Ihigh/c-kit− cells were therefore compared by RT-PCR analyses. As shown in Fig. 1c, substantial expression of c-kit mRNA was detected even in the class Ihigh/c-kit− cells although the level of c-kit mRNA in these cells was lower than that in class Ihigh/c-kitlow cells. Based on these findings, we defined the cells as “c-kit<low” instead of c-kit− although the cells appeared to be c-kit− in FACS analyses, as shown in Fig. 1 a and b.
Transplantation of c-kitlow or c-kit<low HSCs into Ly5 Congenic Recipients.
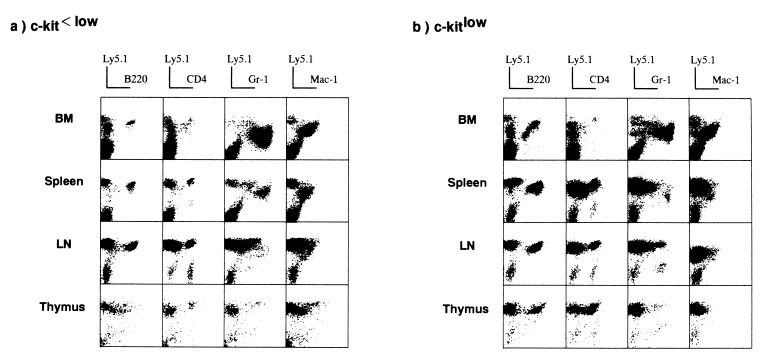
Five hundred cells of each fraction from 5-FU-treated B6 Ly5.1 mice were injected i.v. into irradiated B6 Ly5.2 recipients along with B6 Ly5.2 compromised cells. Six months after transplantation, cells from several organs were double-stained with a panel of mAbs against mature myeloid/lymphoid cells and anti-Ly5.1 mAb to check for donor-derived cells. Fig. 2 shows representative results for seven recipients. Ly5.1+ donor-derived mature cells [B220+ or surface Ig+ (data not shown) B cells, CD4+ or CD8+ (data not shown) T cells, Gr-1+ granulocytes, and Mac-1+ macrophages] were detected in recipients that had been given either Lin−/CD71−/class Ihigh/c-kitlow cells or Lin−/CD71−/class Ihigh/c-kit<low cells in all areas tested, including the bone marrow, thymus, spleen, lymph nodes, peritoneal cavity, and peripheral blood (data not shown). Ly5.1− cells might be derived from progenitors among the compromised B6 Ly5.2 cells. Thus, both populations contained progenitor cells with the potential to generate multilineage cells.
Figure 2.
Detection of donor-derived cells in the primary recipients. Five hundred Lin−/CD71−/class Ihigh/c-kitlow cells or Lin−/CD71−/class Ihigh/c-kit<low cells from 5-FU-treated B6 Ly5.1 mice were transplanted into irradiated B6 Ly5.2 recipients along with 2 × 105 compromised B6 Ly5.2 cells. Six months later, cells from various organs were stained with a panel of mAbs and donor-specific anti-Ly5.1 mAb.
Secondary Transplantation.
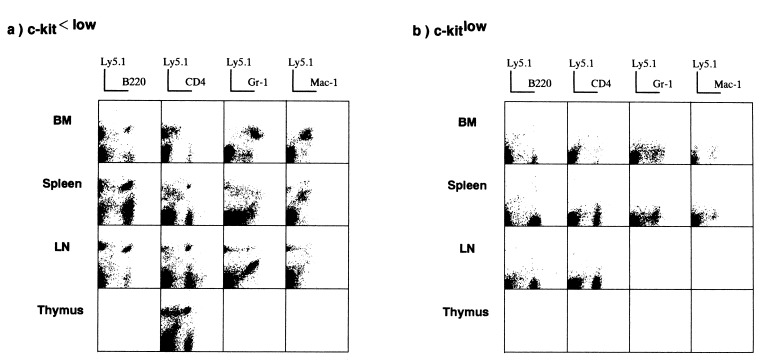
To examine the LTRA of these two cell populations, 1 × 106 BMCs from recipients that had either been transplanted with Lin−/CD71−/class Ihigh/c-kitlow cells or Lin−/CD71−/class Ihigh/c kit<low cells were retransplanted into irradiated B6 Ly5.2 mice. Six months later, several organs were examined again to check for original donor-derived Ly5.1+ cells. In contrast to the results observed in the primary recipients, original donor-derived Ly5.1+ cells could be detected only in secondary recipients that had received BMCs from the primary recipients that had been transplanted with Lin−/CD71−/class Ihigh/c-kit<low cells. As shown in Fig. 3a, Ly5.1+ cells were observed again among the myeloid and lymphoid cells in all organs tested. However, in the secondary recipients of BMCs from the mice originally transplanted with Lin−/CD71−/class Ihigh/c-kitlow cells, no Ly5.1+ donor-derived cells were detected at all (Fig. 3b). These findings clearly show that P-HSCs with LTRA are Lin−/CD71−/class Ihigh/c-kit<low cells but not Lin−/CD71−/class Ihigh/c-kitlow cells.
Figure 3.
Detection of donor-derived cells in the secondary recipients. Six months after the primary transplantation, BMCs from the primary recipients were transferred to the irradiated secondary B6 Ly5.2 recipients. Six months later, cells from various organs were stained with a panel of mAbs and donor-specific anti-Ly5.1 mAb to determine whether the original donor-derived cells could be detected among the multilineage cells.
Tertiary Transplantation.
Tertiary transplantation was then carried out to confirm LTRA of the original Lin−/CD71−/class Ihigh/c-kit<low cells or Lin−/CD71−/class Ihigh/c-kitlow cells. Six months after the secondary transplantation, BMCs (1 × 106) from the secondary recipients were transplanted again into irradiated B6 Ly5.2 tertiary recipients. All of the tertiary recipients that received BMCs from secondary recipients originally reconstituted with Lin−/CD71−/class Ihigh/c-kitlow cells died within 10 days. However, all of the tertiary recipients that were given BMCs from secondary recipients originally reconstituted with Lin−/CD71−/class Ihigh/c-kit<low cells showed donor (Ly5.1+)-derived cells among their peripheral blood cells. Furthermore, cells expressing the Ly5.1 phenotype were detected in the bone marrow, spleen, lymph nodes, thymus, and peritoneal cavity of a single mouse that was killed 1 month after the tertiary transplantation (Fig. 4). The remaining five mice of this tertiary transplantation group from the original mice transplanted with Lin−/CD71−/class Ihigh/c-kit<low cells continue to survive more than 6 months after the tertiary transplantation.
Figure 4.
Detection of donor-derived cells in a tertiary recipient from a secondary recipient originally reconstituted with Lin−/CD71−/class Ihigh/c-kit<low cells. Six months after the secondary transplantation, BMCs (1 × 106) from the secondary recipients originally reconstituted with either Lin−/CD71−/class Ihigh/c-kitlow or Lin-/CD71−/class Ihigh/c-kit<low cells were further retransplanted into the irradiated B6 Ly5.2 tertiary recipients. All tertiary recipients originally reconstituted with the former (c-kitlow) cells died within 10 days whereas all tertiary recipients (five mice) reconstituted with the latter cells survived more than 6 months; one mouse was killed 1 month after the transplantation, and the various hematolymphoid organs were examined for the phenotypes of hematolymphoid cells.
DISCUSSION
P-HSCs have been isolated on the basis of the expression of cell surface molecules: Sca-1 (16), Thy-1 (6), CD4 (7), Mac-1 (7), CD34 (17, 18), or retention of rhodamine 123 (19, 20). However, the function of these molecules in hemopoiesis remains unclear even though an adhesive interaction between CD34 and stromal cells has recently been reported (21). In this sense, the receptor for stem cell factor, c-kit, is thought to be an important protein that contributes to the differentiation of HSCs or multilineage progenitors (22–26). Therefore, it is critical to clarify the expression of c-kit on P-HSCs to ascertain the requirement for stem cell factor in the earlier stage of hemopoiesis.
In earlier studies of the expression of c-kit on hemopoietic progenitors, P-HSCs were reported to be c-kit+ cells (8–11, 27). However, the assays used in these studies to detect the activity of P-HSCs were inadequate; although they included the activity of spleen colony formation and long term multilineage reconstitution, they did not include retransplantation of bone marrow cells from the primary recipients to secondary recipients and tertiary recipients nor in vitro proliferation in the presence of hemopoietic cytokines. Recently, it has been reported that both murine dormant primitive hemopoietic progenitors (after treatment with 5-FU) and human CD34+ primitive hemopoietic progenitors are enriched in a c-kitlow cell population when assayed by colony formation in vitro in the presence of stem cell factor, interleukin 3, and interleukin 11 in mice (13) or by assessing long term culture initiating cells under the influence of interleukin 3, interleukin 6, stem cell factor, granulocyte–macrophage colony-stimulating factor, and erythropoietin in humans (12). Furthermore, it has been shown that blast cells derived from more primitive c-kitlow cells obtained from 5-FU-treated mice become c-kithigh during a 5-day period in culture (13). This finding suggests that the c-kit expression may be altered depending on the phase of the cells in the cell cycle; primitive HSCs in the Go phase are c-kitlow whereas progenitors in the cycling phase show a higher expression of c-kit.
In B cell lymphopoiesis, it has been shown that cells in the most primitive stage are B220dull/c-kit− and are probably generated directly from P-HSCs (28, 29), suggesting that P-HSCs are c-kit−. In our previous experiments, only four Lin−/CD71−/class Ihigh cells from male mice (after 5-FU injection) were able to reconstitute multilineage cells in irradiated female recipients for more than 180 days, and donor male cells were detected in the peripheral blood 60 days after retransplantation in the secondary recipients. These findings suggest that the Lin−/CD71−/class Ihigh population contains highly purified P-HSCs. We analyzed previously whether this population is c-kit−, c-kitlow, or c-kit+, and we found it to be c-kit− or c-kitlow but not c-kit+ (14). By analyzing marker changes in this population after 5-FU treatment, we very recently have obtained evidence suggesting that P-HSCs are c-kit− based on the cell surface expression of c-kit using an anti-c-kit mAb, the ACK4 (30).
In the present study using RT-PCR analyses, we have clarified further by FACS analyses the presence or absence of c-kit transcripts in the cells that did not appear to express c-kit on the cell surface. As shown in Fig. 1c, substantial expression of c-kit mRNA was observed even in cells that did not appear to express c-kit on their surface although the levels of c-kit mRNA in these cells were much lower than those in c-kitlow cells. Based on these findings, we have defined the cells as c-kit<low.
We attempted next to further clarify whether P-HSCs are c-kit<low or c-kitlow by LTRA. As shown in Fig. 3, the original donor-derived Ly5.1+ cells were detected in the secondary recipients 6 months after the retransplantation of a small number (1 × 106) of BMCs from the primary recipients that had been reconstituted with Lin−/CD71−/H-2high/c-kit<low cells but not with Lin−/CD71−/class Ihigh/c-kitlow cells. Moreover, original donor-derived Ly5.1+ cells were detected even in the tertiary recipients originally reconstituted with Lin−/CD71−/class Ihigh/c-kit<low cells although the tertiary recipients originally reconstituted with Lin−/CD71−/class Ihigh/c-kitlow cells died within 10 days of the tertiary transplantation. Using this serial transplantation assay system, it has been possible to examine the self-renewal capacity of P-HSCs.
In conclusion, Lin−/CD71−/H-2high/c-kit<low cells fulfill completely the definition of P-HSCs, having both the ability to generate multilineage cells and the capacity for self-renewal.
Acknowledgments
We thank Mr. F. Ishida (Research Center of Kansai Medical University) for flow cytometry studies, Ms. K. Ando for preparing the manuscript, and Ms. Tazim Verjee for manuscript typing and editing. This work was supported in part by a grant from the Ministry of Health and Welfare of Japan, the Ministry of Education, Science and Culture, Japan, and the Japan Private School Promotion Foundation. This work also was supported by The National Institute of Aging Grant 05628-12 (R.A.G.).
ABBREVIATIONS
- P-HSCs
pluripotent hemopoietic stem cells
- 5-FU
5-fluorouracil
- Lin−
lineage-negative
- BMCs
bone marrow cells
- LTRA
long term repopulating activity
- FITC
fluorescein isothiocyanate
- RT-PCR
reverse transcriptase PCR
References
- 1.Morrison S J, Uchida N, Weissman I L. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 2.Visser J W, Bauman J G J, Mudler A H, Eliason J F, de Leeuw A M. J Exp Med. 1984;159:1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyama-Inaba M, Ogata H, Toki J, Kuma S, Sugiura K, Yasumizu R, Ikehara S. Biochem Biophys Res Commun. 1987;147:687–694. doi: 10.1016/0006-291x(87)90985-5. [DOI] [PubMed] [Google Scholar]
- 4.Spangrude G J, Heimfeld H, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 5.Uchida N, Fleming W H, Alpern E J, Weissman I L. Curr Opin Immunol. 1993;5:177–184. doi: 10.1016/0952-7915(93)90002-a. [DOI] [PubMed] [Google Scholar]
- 6.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 8.Ikuta K, Weissman I L. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlic D, Fischer R, Nishikawa S-I, Nienhuis A W, Bodine D M. Blood. 1993;82:762–770. [PubMed] [Google Scholar]
- 10.Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S-I, Kunisada T, Sudo T, Kina T, Sudo T, Nakauchi H, Nishikawa S-I. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 12.Gunji Y, Nakamura M, Osawa H, Nagayoshi K, Nakauchi H, Miura Y, Yanagisawa M, Suda T. Blood. 1993;82:3283–3289. [PubMed] [Google Scholar]
- 13.Katayama N, Shih J-P, Nishikawa S-I, Kina T, Clark S C, Ogawa M. Blood. 1993;82:2353–2360. [PubMed] [Google Scholar]
- 14.Ogata H, Bradley G W, Inaba M, Ogata N, Ikehara S, Good R A. Proc Natl Acad Sci USA. 1995;92:5945–5949. doi: 10.1073/pnas.92.13.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlic D, Anderson S, Biesecker L G, Sorrentino B P, Bodine D M. Proc Natl Acad Sci USA. 1995;92:4601–4605. doi: 10.1073/pnas.92.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spangrude G J, Brooks D M. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 17.Baum C M, Weissman I L, Tsukamoto A S, Buckle A-M, Peault B. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiGuiusto D, Chen S, Combs J, Webb S, Namikawa R, Tsukamoto A, Chen B P, Galy A H M. Blood. 1994;84:421–432. [PubMed] [Google Scholar]
- 19.Wolf N S, Kone A, Priestley G V, Bartelmez S H. Exp Hematol. 1993;21:614–622. [PubMed] [Google Scholar]
- 20.Spangrude G J, Brooks D M, Tumas D B. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 21.Healy L, May G, Gale K, Grosveld F, Greaves M, Enver T. Proc Natl Acad Sci USA. 1995;92:12240–12244. doi: 10.1073/pnas.92.26.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geissler E N, Ryan M A, Housman D E. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 23.Huang E, Nocka K, Beier D R, Chu T-Y, Buck J, Lahm H-W, Leder W P, Besmer P. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 24.McNiece I K, Langley K E, Zsebo K M. Exp Hematol. 1991;19:226–231. [PubMed] [Google Scholar]
- 25.Migliaccio G, Migliaccio A R, Valinsky J, Langley K, Zsebo K, Visser J W A, Adamson J W. Proc Natl Acad Sci USA. 1991;88:7420–7424. doi: 10.1073/pnas.88.16.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji K, Zsebo K M, Ogawa M. Blood. 1991;78:1223–1229. [PubMed] [Google Scholar]
- 27.Okada, S., Nakauchi, H., Nagayoshi, K., Nishikawa, S., Nishikawa, S.-I., Miura, Y. & Suda, T. Blood 78, 1706–1712. [PubMed]
- 28.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Era T, Nishikawa S, Sudo T, Fu-Ho W, Ogawa M, Kunisada T, Hayashi S-I, Nishikawa S-I. Immunol Rev. 1994;137:35–51. doi: 10.1111/j.1600-065x.1994.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 30.Nishio N, Hisha H, Ogata H, Inaba M, Yamamoto Y, Amoh Y, Yasumizu R, Hanada K, Hamada H, Ikehara S. Stem Cells. 1996;14:584–591. doi: 10.1002/stem.140584. [DOI] [PubMed] [Google Scholar]