Abstract
Among Bacillus subtilis IFO13722 spores sporulated at 30, 37, and 44°C, those sporulated at 30°C had the highest resistance to treatments with high hydrostatic pressure (100 to 300 MPa, 55°C, 30 min). Pressure resistance increased after demineralization of the spores and decreased after remineralization of the spores with Ca2+ or Mg2+, whereas the resistance did not change when spores were remineralized with Mn2+ or K+, suggesting that former two divalent ions were involved in the activation of cortex-lytic enzymes during germination.
High temperature is employed to sterilize foods contaminated with spores, because of their higher resistance to physical and chemical treatments. However, under high-temperature sterilizing conditions, foods may lose not only their organoleptic qualities, such as flavor, color, and texture, but also nutritional value (15). High-hydrostatic-pressure (HHP) sterilization is one of the novel techniques that are able to inactivate microorganisms without altering the flavor and nutrient content of food (8). However, bacterial spores are more resistant than vegetative bacteria to hydrostatic pressure sterilization (8, 32, 34).
Some researchers have been attempting to determine the optimal conditions for pressure sterilization with a combination of heat (11, 14, 18), bacteriocin (30), pH (29, 32, 35), and oscillatory decompression procedures (12). There are, however, few studies addressing the effect of the conditions under which bacteria sporulate on the effectiveness of hydrostatic pressure sterilization (28, 29), even though the spores contaminating foods might have sporulated under various environments.
In this study, we investigated the resistance of Bacillus subtilis spores sporulated at three different temperatures to hydrostatic pressure versus heat. Furthermore, we investigated the effect of demineralization and remineralization of the B. subtilis spores on their resistance to hydrostatic pressure.
Preparation of spore suspensions.
B. subtilis IFO13722 was obtained from the Institute of Fermentation, Osaka (Osaka, Japan). After growth in nutrient broth (Eiken Chemical Co., Ltd., Tokyo, Japan) for 24 h at 30°C, the stationary-phase culture of B. subtilis was incubated on a soil infusion agar plate (6), which consisted of nutrient agar (Eiken Chemical Co., Ltd., Tokyo, Japan) and soil extract, at 30, 37, or 44°C for 10 days. The spores were harvested by scraping with a sterile glass microscope slide with sterile distilled water and were washed three times by centrifugation (4,000 × g, 5°C, 30 min) and resuspension (N spores).
The N spores were titrated with 0.033 N HCl until the pH had stabilized at a value of 4.0 after about 5 h at room temperature. In this procedure, minerals in the N spores were replaced with protons present in the acid medium (5). After this procedure, the spores were washed three times by centrifugation and resuspension in distilled water. The H spores were immersed in the 10 mM acetate solutions (acetates of calcium, magnesium, potassium, and manganese [pH 6 to 7]) for 5 h at room temperature to form remineralized spores (31). After this incubation, the spores were washed three times by centrifugation and resuspension in distilled water. The spore suspensions (N, H, Ca, Mg, K, and Mn spores) were stored at 4°C until use. The dry weight of the spores in each suspension was obtained after drying at 105°C.
Minerals (Ca2+, Mg2+, Mn2+, and K+) in the prepared spores were assayed by use of an inductively coupled plasma mass spectrometer, PMS2000 (Yokogawa Analytical Systems, Inc., Tokyo, Japan). Before measurement, the spores were completely solubilized with concentrated nitric acid and 30% hydrogen peroxide (Nacalai Tesque, Inc., Kyoto, Japan) and heating and then diluted with 0.05 N nitric acid.
Heat treatment and HHP treatment.
Each spore suspension was adjusted with distilled water to a concentration of 107 CFU/ml. A sample (0.5 ml) of each spore suspension was mixed with 4.5 ml of sterilized distilled water in a screw-cap glass tube that had been preheated in a thermostatic bath at 85°C. After heat treatment for 10 to 30 min, the spore suspensions were immediately cooled in a water bath.
A sterile screw-cap plastic tube (Greiner Labortechnik Co., Ltd., Frickenhausen, Germany) was used for HHP treatments. A sample (1.5 ml) of each spore suspension, previously adjusted with distilled water to 106 CFU/ml, was sealed into the plastic tubes. The plastic tubes containing the samples were pressurized by using a prototype pressurization apparatus (12) to 100, 200, or 300 MPa at 55°C for 30 min. After decompression, the sample tubes were cooled in a water-ice bath.
After these treatments, surviving spores were enumerated by a colony count method using nutrient agar plates. The plates were incubated for 24 to 48 h at 30°C.
Statistical analysis.
Assay of mineral content and treatments with heat and HHP were repeated in triplicate, and the data are presented as arithmetic means. The data in the figures are presented as the logarithm to the base 10 of the inactivation achieved (N/N0, in which N was the number of survivors and N0 was the initial number) at each treatment. Fisher's least significant difference test was employed to determine statistically significant differences (P < 0.05). Data were analyzed by using StatView-J 4.11 software (Abacus Concepts, Inc., Berkeley, Calif.).
Heat resistance of Bacillus spores.
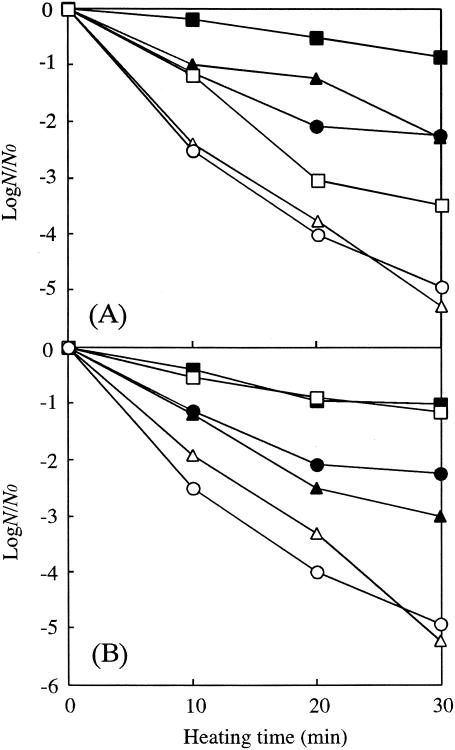
The N spores sporulated at 44°C had the highest heat resistance among the spores sporulated at 30, 37, and 44°C (Fig. 1A). The survival of H spores decreased approximately 2 to 2.5 log cycles after treatment at 85°C for 30 min compared to N spores for each sporulation temperature. Similar results were observed in other studies; that is, the heat resistance of Bacillus spores was decreased by demineralization procedures (1, 5, 19, 27).
FIG. 1.
Heat resistance of B. subtilis N spores (solid symbols) and H spores (open symbols) sporulated at 30°C (triangles), 37°C (circles), and 44°C (squares) (A) and N spores (•), H spores (○), and remineralized spores (Ca spores [▪], Mg spores[□], Mn spores [▴], and K spores [▵]) (B) prepared from N spores sporulated at 37°C. Heat treatments were performed at 85°C. Least significant difference are 0.174 (A) and 0.216 (B).
There have been many studies to investigate the effect of sporulation temperature on the heat resistance of bacterial spores. Most authors concluded that the heat resistance of bacterial spores increased with increasing sporulation temperature (3, 10, 20, 21). Lechowich and Ordal (17) reported that higher sporulation temperatures resulted in more minerals in the spores. Cazemier et al. (7) observed increased heat resistance for Bacillus spores sporulated on medium supplemented with Ca2+, Mg2+, Mn2+, Fe2+, and K+ compared to those sporulated on medium supplemented with Mn2+ only. In the present study, however, no direct correlation between heat resistance and mineral content of the N spores could be seen by comparing the data in Table 1 and Fig. 1A. Merry et al. (20) found that core water content was lower in B. subtilis spores prepared at higher temperatures, and such spores were more resistant to wet heat than those prepared at lower temperatures. Khoury et al. (16) observed decreased heat resistance for spores of a B. subtilis mutant that is missing a heat shock-induced protein. In our studies, demineralization of spores caused decreased heat resistance for all sporulation temperatures, and the relationships between sporulation temperature and heat resistance were the same for both H spores and N spores. These results support the idea that not only minerals but also other factors, such as protoplast dehydration and thermal adaptation, might influence the heat resistance of N spores sporulated at different temperatures.
TABLE 1.
Mineral content of B. subtilis N spores sporulated at 30, 37, and 44°C; H spores; and remineralized spores
| Spore type | Sporulation temp (°C) | Spore mineral content (μmol/mg [dry wt] of spores)a
|
|||
|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Mn2+ | K+ | ||
| N | 30 | 0.108* | 0.011* | 0.001*‡ | 0.041* |
| 37 | 1.101† | 0.128† | 0.005*† | 0.253† | |
| 44 | 0.544‡ | 0.070‡ | 0.005† | 0.149‡ | |
| H | 30 | 0.056* | 0.003* | 0.000‡ | 0.005§ |
| 37 | 0.055* | 0.004* | 0.000‡ | 0.018*§ | |
| 44 | 0.050* | 0.002* | 0.001†‡ | 0.017§ | |
| Remineralized | |||||
| Cab | 1.319§ | 0.004* | 0.000‡ | 0.013*§ | |
| Mg | 0.100* | 0.301§ | 0.000‡ | 0.003§ | |
| Mn | 0.059* | 0.005* | 0.076§ | 0.023*§ | |
| K | 0.050* | 0.003* | 0.000‡ | 0.282† | |
Mean values in the same column that are not followed by the same symbol are significantly different (P < 0.05).
The spores sporulated at 37°C were used for preparation of remineralized spores.
Figure 1B shows the effect of remineralization on the heat resistance of B. subtilis spores sporulated at 37°C. Remineralization resulted in increased heat resistance relative to H spores, and the resistance of Ca and Mg spores in particular became higher than that of the N spores. Some researchers used ion exchange for the remineralization of H spores with various types of cation to investigate the effect of the mineral composition of bacterial spores on germination and heat resistance (1, 2, 5, 19, 31, 36). These researchers concluded that remineralized Bacillus spores, except for those remineralized with Na+, were more resistant to heat than H spores. Our results also demonstrate increased heat resistance after the remineralization procedure.
HHP resistance of Bacillus spores.
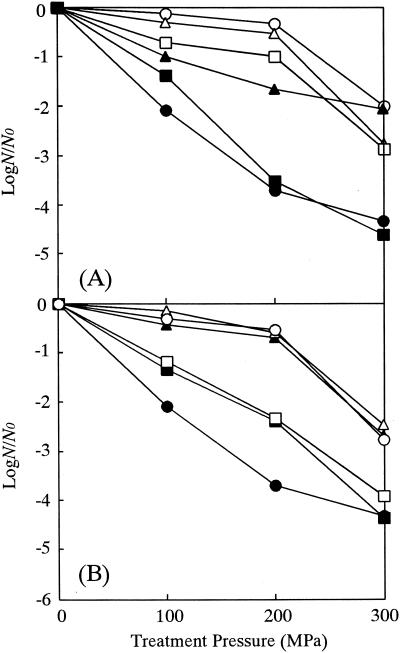
Figure 2A shows the HHP resistance of N spores and H spores. The N spores sporulated at 30°C had the highest HHP resistance among the N spores sporulated at three different temperatures. Raso et al. (28), working with Bacillus cereus spores, found that a lower sporulation temperature suppressed the initiation of germination of the spores. The inactivation of bacterial spores by hydrostatic pressure, unlike the inactivation of vegetative bacteria, occurs in two stages (9, 32). High pressure first causes germination of the spores and then inactivates the germinated spores. Therefore, the spores that sporulated at 30°C are considered to have germinated less readily under high pressure and thus acquired their higher HHP resistance.
FIG. 2.
HHP resistance of B. subtilis N spores (solid symbols) and the H spores (open symbols) sporulated at 30°C (triangles), 37°C (circles), and 44°C (squares) (A) and N spores (•), H spores (○), and remineralized spores (Ca spores [▪], Mg spores [□], Mn spores [▴], and K spores [▵]) (B) prepared from N spores sporulated at 37°C. HHP treatments were performed at 55°C for 30 min. The least significant differences are 0.232 (A) and 0.270 (B).
The HHP resistance of H spores was higher than that of the N spores. It was observed that there was only a 1-log-cycle or less decrease in survival of the H spores after an HHP treatment of 200 MPa and there was a 2- or 3-log-cycle decrease after an HHP treatment of 300 MPa at 55°C for 30 min. Thus, a reduction in the mineral content of B. subtilis spores increased their HHP resistance.
Bender and Marquis (4) reported that H spores of Bacillus megaterium did not germinate appreciably at 493 atm (49 MPa), and less than 10% germination occurred even at 1,020 atm (102 MPa). In our study, the higher HHP resistance of H spores of B. subtilis might also have been caused by the suppression of germination. In other words, the lower mineral content of the H spores could have inhibited pressure-induced germination. By the same reasoning, the N spores sporulated at 30 and 44°C might have acquired higher HHP resistance than those sporulated at 37°C, because their mineral contents were approximately 90 and 50% lower, respectively (Table 1).
HHP resistance decreased when H spores were remineralized with Ca2+ or Mg2+, whereas remineralization with K+ or Mn2+ had no effect (Fig. 2B). Bender and Marquis (4) investigated the ability of hydrostatic pressure to induce germination of several salt forms of B. megaterium spores. For treatments at 493 atm (49 MPa), they found that the hierarchy of resistance of the various salt forms to pressure-induced germination was H > K > Ca = Mg > native. This hierarchy is in agreement with our results on the inactivation of ion-exchanged B. subtilis spores, as shown in Fig. 2B.
Three receptors for known germinants and two receptors with as-yet-undiscovered ligands are said to participate in the first stage of nutritional germination of B. subtilis spores (33). Upon ligand binding, these receptors trigger the release of the spore core's large store of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), along with its associated divalent cations, predominantly Ca2+ (25, 26). In the second stage, DPA release triggers the activation of cortex-lytic enzymes, which hydrolyze spore peptidoglycan (33). There are two cortex-lytic enzymes in B. subtilis, CwlJ and SleB, and either one of these proteins was sufficient for spore germination (13, 22). Paidhungat et al. (24) investigated pressure-induced germination by using mutant spores lacking germinant receptors and suggested that a pressure of 100 MPa activated these receptors and subsequent hydrolyzation by cortex-lytic enzymes. However, the activation of CwlJ during spore germination is caused directly by the Ca2+-DPA released from the spore core (23). We concluded that the Ca2+ deficiency of the H spores prevented CwlJ activation and inhibited germination, leading to higher HHP resistance, whereas Ca2+-DPA release was normal in Ca spores, triggering normal germination and leading to lower HHP resistance. However, further investigation is required to elucidate the mechanisms of inactivation by hydrostatic pressure, because the Mg spores also had reduced resistance to HHP.
Acknowledgments
We are indebted to Midori Watanabe, the Center of Advanced Instrumental Analysis, Kyushu University, for the measurement of mineral contents.
REFERENCES
- 1.Alderton, G., and N. Snell. 1963. Base exchange and heat resistance in bacterial spores. Biochem. Biophys. Res. Commun. 10:139-143. [DOI] [PubMed] [Google Scholar]
- 2.Ando, Y., and T. Tsuzuki. 1983. Mechanism of chemical manipulation of the heat resistance of Clostridium perfringens spores. J. Appl. Bacteriol. 54:197-202. [DOI] [PubMed] [Google Scholar]
- 3.Beaman, T. C., and P. Gerhardt. 1986. Heat resistance of bacterial spores correlated with protoplast dehydration, mineralization, and thermal adaptation. Appl. Environ. Microbiol. 52:1242-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, G. R., and R. E. Marquis. 1982. Sensitivity of various salt forms of Bacillus megaterium spores to the germinating action of hydrostatic pressure. Can. J. Microbiol. 28:643-649. [Google Scholar]
- 5.Bender, G. R., and R. E. Marquis. 1985. Spore heat resistance and specific mineralization. Appl. Environ. Microbiol. 50:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry, M. R., Jr., and J. G. Brandshaw. 1980. Heating characteristics of condensed cream of celery soup in a steritort: heat penetration and spore count reduction. J. Food Sci. 45:869-879. [Google Scholar]
- 7.Cazemier, A. E., S. F. M. Wagenaars, and P. F. ter Steeg. 2001. Effect of sporulation and recovery medium on the heat resistance and amount of injury of spores from spoilage bacilli. J. Appl. Microbiol. 90:761-770. [DOI] [PubMed] [Google Scholar]
- 8.Cheftel, J. C. 1992. Effects of high hydrostatic pressure on food constituents: an overview, p. 195-209. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure biotechnology. Colloque INSERM/John Libbey and Co., Ltd., London, United Kingdom.
- 9.Clouston, J. G., and P. A. Wills. 1969. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J. Bacteriol. 97:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condon, S., M. Bayarte, and F. J. Sala. 1992. Influence of the sporulation temperature upon the heat resistance of Bacillus subtilis. J. Appl. Bacteriol. 73:251-256. [DOI] [PubMed] [Google Scholar]
- 11.Gould, G. W. 1970. Germination and the problem of dormancy. J. Appl. Bacteriol. 33:34-49. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa, I., T. Kanno, K. Yoshiyama, and Y. Fujio. 1994. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J. Food Sci. 59:164-167. [Google Scholar]
- 13.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 180:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, F. H., and C. E. Zobell. 1949. The retardation of thermal disinfection of Bacillus subtilis spores by hydrostatic pressure. J. Bacteriol. 57:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joslyn, L. J. 1991. Sterilization by heat, p. 495-526. In S. S. Block (ed.), Disinfection, sterilization, and preservation. Lea & Febiger, London, United Kingdom.
- 16.Khoury, P. H., M. W. Qoronfleh, U. N. Streips, and R. A. Slepecky. 1990. Altered heat resistance in spores and vegetative cells of a mutant from Bacillus subtilis. Curr. Microbiol. 21:249-253. [Google Scholar]
- 17.Lechowich, R. J., and Z. J. Ordal. 1962. The influence of the sporulation temperature on the heat resistance and chemical composition of bacterial spores. Can. J. Microbiol. 8:287-295. [DOI] [PubMed] [Google Scholar]
- 18.Mallidis, C. G., and D. Drizou. 1991. Effect of simultaneous application of heat and pressure on the survival of bacterial spores. J. Appl. Bacteriol. 71:285-288. [DOI] [PubMed] [Google Scholar]
- 19.Marquis, R. E., and G. R. Bender. 1985. Mineralization and heat resistance of bacterial spores. J. Bacteriol. 161:789-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merry, E., P. C. Genest, M. E. Gilmore, S. Little, D. L. Popham, A. Driks, and P. Setlow. 2002. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 92:1105-1115. [DOI] [PubMed] [Google Scholar]
- 21.Michels, M. J. M., and E. F. Visser. 1976. Occurrence and thermoresistance of spores of psychrophilic and psychrotrophic aerobic sporeformers in soil and foods. J. Appl. Bacteriol. 41:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama, R., A. Hattori, S. Miyata, S. Kudoh, and S. Makino. 1996. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J. Bacteriol. 178:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat, M., B. Setlow, W. B. Daniels, D. Hoover, E. Papafragkou, and P. Setlow. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 68:3172-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palop, A., P. Mañas, and S. Condón. 1999. Sporulation temperature and heat resistance of Bacillus spores: a review. J. Food Safety 19:57-72. [Google Scholar]
- 28.Raso, J., G. Barbosa-Cánovas, and B. G. Swanson. 1998. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J. Appl. Microbiol. 85:17-24. [DOI] [PubMed] [Google Scholar]
- 29.Raso, J., M. M. Góngora-Nieto, G. V. Barbosa-Cánovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, C. M., and D. G. Hoover. 1996. Sensitivity of Bacillus coagulans spores to combinations of high hydrostatic pressure, heat, acidity and nisin. J. Appl. Bacteriol. 81:363-368. [Google Scholar]
- 31.Rode, L. J., and J. W. Foster. 1966. Influence of exchangeable ions on germinability of bacterial spores. J. Bacteriol. 91:1582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sale, A. J. H., G. W. Gould, and W. A. Hamilton. 1970. Inactivation of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:323-334. [DOI] [PubMed] [Google Scholar]
- 33.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timson, W. J., and A. J. Short. 1965. Resistance of microorganisms to hydrostatic pressure. Biotechnol. Bioeng. 7:139-159. [Google Scholar]
- 35.Wuytack, E. Y., and C. W. Michiels. 2001. A study of the effects of high pressure and heat on Bacillus subtilis spores at low pH. Int. J. Food Microbiol. 64:333-341. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki, K., Y. Kawai, N. Inoue, and H. Shinano. 1997. Influence of sporulation medium and divalent ions on the heat resistance of Alicyclobacillus acidoterrestris spores. Lett. Appl. Microbiol. 25:153-156. [DOI] [PubMed] [Google Scholar]