Abstract
A plasmid marker rescue system based on restoration of the nptII gene was established in Streptococcus gordonii to study the transfer of bacterial and transgenic plant DNA by transformation. In vitro studies revealed that the marker rescue efficiency depends on the type of donor DNA. Plasmid and chromosomal DNA of bacteria as well as DNA of transgenic potatoes were transferred with efficiencies ranging from 8.1 × 10−6 to 5.8 × 10−7 transformants per nptII gene. Using a 792-bp amplification product of nptII the efficiency was strongly decreased (9.8 × 10−9). In blood sausage, marker rescue using plasmid DNA was detectable (7.9 × 10−10), whereas in milk heat-inactivated horse serum (HHS) had to be added to obtain an efficiency of 2.7 × 10−11. No marker rescue was detected in extracts of transgenic potatoes despite addition of HHS. In vivo transformation of S. gordonii LTH 5597 was studied in monoassociated rats by using plasmid DNA. No marker rescue could be detected in vivo, although transformation was detected in the presence of saliva and fecal samples supplemented with HHS. It was also shown that plasmid DNA persists in rat saliva permitting transformation for up to 6 h of incubation. It is suggested that the lack of marker rescue is due to the absence of competence-stimulating factors such as serum proteins in rat saliva.
The use of genetically modified (GM) crops in food and feed production needs to undergo an assessment of the undesired effects that may occur, especially those affecting the health of a consumer that may arise from the transfer of recombinant DNA to bacteria in association with food and the digestive tract (2, 15, 31). The only known way by which bacteria can acquire plant DNA is by transformation, and it was shown in several studies that the DNA from GM crops can be transferred to soil bacteria by this mechanism (9, 10, 16, 21, 35). In these studies, homology between donor and recipient DNA was a prerequisite for the transfer event. Such regions of homology between DNA sequences of GM crops and bacteria may also facilitate the cotransfer of nonhomologous foreign DNA (11, 16).
Foods contain high amounts of RNA and DNA (20), leading to a roughly estimated dietary daily intake of 0.1 to 1 g per person (13). This DNA in food may persist for long periods of time (7, 32, 44, 45). The oral cavity and intestinal tract are densely populated with bacteria that may become transformed by this DNA. In vitro studies have shown that plant DNA is largely degraded during its passage through the human gastrointestinal tract (26). Nevertheless, in vivo studies revealed that plant-associated DNA from soybean leaves may persist for days in the intestines of mice (18). Reports about the competence development of bacteria inhabiting the digestive tract are, however, rare and focused on in vitro studies with oral bacteria (47). Many oral streptococci are capable of natural transformation, which has been best studied in Streptococcus gordonii (8). Recently, it has been shown that S. gordonii can be transformed by plasmid DNA or via chromosomal integration of plasmid DNA at increased frequency in the presence of human saliva (29, 30). These authors incubated plasmid DNA with human saliva or exposed the DNA to it in vivo and observed that the nucleic acid keeps its capability to transform oral streptococci in vitro. Up to now no data are available on the in vivo development of competence and transformation of bacteria inhabiting the digestive tract. To perform a risk assessment in accordance with the recommendation of the Food and Agriculture Organization (FAO) and World Health Organization (WHO) (1), these data as well as those on the persistence of DNA are needed to estimate the likelihood of an in vivo transfer of recombinant DNA to bacteria in the food chain.
In general, once a transformation event has taken place a further propagation of the acquired genetic information is facilitated via the more efficient mechanisms of horizontal gene transfer, conjugation, and transduction. Thus, transformation of bacteria in food and in the mouth can well contribute to the distribution of acquired traits to intestinal bacteria. In the food, either contaminating intestinal bacteria may become transformed directly or food-specific bacteria may spread the DNA acquired by transformation in food to bacteria of the digestive tract upon ingestion. Not only was Bacillus subtilis as a food-associated bacterium shown to develop natural competence during growth in foods (7, 49), but also the intestinal bacterium Escherichia coli was shown to have the ability of being transformed in foods (3, 4).
In this work we studied the transfer of recombinant DNA to the oral bacterium S. gordonii by using the marker rescue system based on the restoration of the kanamycin resistance gene nptII (22). The natural transformation of S. gordonii is controlled by a peptide-dependent quorum-sensing system (8, 17), and it was shown that serum which can be naturally contained in foods stimulates transformation (19, 37). S. gordonii strain TIGR, containing a plasmid-encoded marker rescue system, was used as a recipient to detect transformation in vitro, in foods, and in gnotobiotic rats by using bacterial and transgenic plant DNA as donor DNA.
MATERIALS AND METHODS
Bacterial strains, plant materials, and growth conditions.
In this study the following strains were used: Streptococcus gordonii TIGR (46), S. gordonii LTH 5597 harboring pMK110 with deleted nptII (22), S. gordonii LTH 5743 harboring pMK111 (a derivative of pMK110 with intact nptII), B. subtilis LTH 5466 harboring pMK110 (22), E. coli DH5α containing pMR2 (10) or pSR8-30 (14), and E. coli Sure (Stratagene). Solanum tuberosum cv. Apriori (AVEBE, Foxhole, The Netherlands) containing intact nptII in the genome was used as GM plant material. B. subtilis and E. coli were grown at 37°C in Luria-Bertani medium (40). S. gordonii was grown anaerobically at 37°C in brain-heart infusion (BHI) broth (Merck). Solidified media contained 1.5% (wt/vol) agar. Selective media contained 10 μg of chloramphenicol (Sigma)/ml and 100 μg of ampicillin (Roth)/ml for E. coli, 10 μg of erythromycin (Sigma)/ml and 1,000 μg of kanamycin (Sigma)/ml for S. gordonii, or 10 μg of erythromycin (Sigma)/ml for B. subtilis.
Foodstuffs.
Ultra-high temperature (UHT)-treated milk with a fat content of 3.5% was used. Blood sausage (100 g) obtained from a local grocery market was homogenized in 100 ml of saline peptone (0.85% NaCl, 0.1% peptone). One part of the homogenate was heat treated at 56°C for 30 min (H1), whereas the other part remained untreated (H2). Potato extract (PE) was prepared by homogenizing 43 g of ground Apriori potatoes in 50 ml of saline peptone (see above) and autoclaved. The mixture was centrifuged (3,000 × g, 4°C, 6 min), and the supernatant was used as extract.
DNA isolations.
Chromosomal or plasmid DNA from E. coli, B. subtilis, and plant DNA from potato tubers was isolated as described previously (22). Plasmid DNA from streptococci was extracted by using the QIAprep Spin Miniprep kit (Qiagen) according to the manufacturer's instructions with the following modification: cells of a 10-ml culture at the end of the exponential growth phase were harvested by centrifugation (4000 × g, 8 min, 4°C) and washed with 1 ml of 10 mM Tris-HCl (pH 8.0). The cell pellet was resuspended in 250 μl of STE buffer (20% sucrose, 10 mM Tris-HCl [pH 8.0], 10 mM EDTA, 50 mM NaCl) containing lysozyme (5 mg/ml) and mutanolysin (500 U/ml), and the suspension was incubated at 37°C for 15 min. Concentrations of DNA solutions were determined as described previously (22).
Transformation of S. gordonii.
An overnight culture (approximately 109 CFU/ml) was diluted 1:1,000 in fresh BHI broth supplemented with 10% (vol/vol) heat-inactivated (56°C, 30 min) horse serum (HHS). After incubation at 37°C for 4 h, the donor DNA was added, and the suspension was further incubated for 1 h before plating.
Marker rescue experiments in vitro and in foods.
The marker rescue experiments were based on the transformation protocol using S. gordonii LTH 5597 as a recipient. Plasmids pMR2 and pSR8-30 and chromosomal DNA of bacterial (E. coli Sure) and plant (Apriori potato) origin, all containing the intact nptII, were used as donor DNA. In addition, the 792-bp DNA fragment of nptII was used. It was generated by PCR amplification using primers Eonpt2 and Sonpt2 and chromosomal DNA of E. coli Sure as template DNA as described previously (22). DNA was added at concentrations below the saturation level. Total counts or counts of transformants in BHI media containing erythromycin (10 μg/ml) or kanamycin (1,000 μg/ml) were determined. Verification of the restoration of nptII was performed by amplification of the 792-bp fragment of nptII and digestion with NcoI as described previously (22). The detection limit of marker rescue transformation was the reciprocal value of the total recipient cells in the transformation assay.
Three marker rescue experiments were performed with foods. In trial I, overnight cultures of S. gordonii LTH 5597 grown in UHT milk (5 × 108 CFU/ml) and BHI medium (7 × 108 CFU/ml) were diluted 1:1,000 in milk and BHI medium, respectively, without or with supplementation of 10% (vol/vol) of the HHS. After incubation at 37°C for 4 h, DNA of pMR2 was added to a final concentration of 2.3 μg/ml. The mixtures were further incubated for 1 h before microbial counting. In trial II, both heated and nonheated homogenates of the blood sausage (H1, H2) were inoculated with an overnight culture of S. gordonii LTH 5597 grown in BHI medium, yielding a final count of 3 × 106 CFU/ml. Homogenates with 10% HHS or without supplementation were incubated at 37°C for 4 h. DNA of plasmid pMR2 was added to a final concentration of 6 μg/ml, and the mixture was further incubated for 1 h before microbial counting. In trial III, the PE was inoculated with strain LTH 5597 as described in trial II. Both PE without HHS and PE with 10% HHS were incubated at 37°C for 4 h and then supplemented with DNA of pMR2 or the Apriori potato to final concentrations of 9 or 30 μg of DNA/ml, respectively. The mixtures were further incubated for 1 h before microbial counting.
Animals, diets, inoculations, and experimental design.
Ten germfree rats of the inbred strain Fisher 344/Rehbrücke (12-week-old male and female rats, with body weights of 255 ± 19 g [mean ± standard error]) were used. The rats had free access to diet and drinking water, and coprophagy was not prevented. One milliliter of an overnight culture (ca. 1.8 × 109 CFU/ml) of S. gordonii LTH 5597 was given orally for the three following days. From the first day of colonization up to 6 days the donor DNA (pMR2) was administered with the drinking water (200 μg/rat/day) to animals 1 to 6. The drinking water contained 0.1 mM Tris-HCl (pH 8.0) and 5% sucrose, which increased the colonization of S. gordonii in the mouth as found in preliminary studies (data not shown). To ensure the maintenance of pMK110 in the recipient, lincomycin (10 mg/kg of body weight) was given with the drinking water from the beginning of inoculation. After 6 days of DNA administration, the DNA was administered directly into the mouth of rats with the following daily dose: animals 1 and 4 received 200 μg for the remaining 3 days; animals 2 and 3 received 70, 100, and 200 μg for the 7th, 8th, and 9th day, respectively; animals 5 and 6 received 200 μg for the 7th day and 100 μg for the remaining 2 days. In the control, animals 7 to 10 were associated with S. gordonii LTH 5597 and received the same drinking water as described above but without plasmid DNA. Animal experiments were performed in accordance with permit 32/48-3560-0/3 from the Ministry of Agriculture, Environment Protection and Regional Development of the state of Brandenburg, Germany.
Before association, fecal samples were taken directly from the anus to ensure the germfree condition in the rats. From the second day of colonization, fecal and saliva samples were collected daily. From the 7th day of association, additional saliva samples from the animals 1 and 4 were taken 1, 2, 4, and 6 h after oral administration of the donor plasmid. From animals 2, 3, 5, and 6, saliva samples were taken after 90 min. To obtain saliva samples, the oral cavities of rats were rubbed with sterile cotton swabs. Swabs were soaked with 10 μl (males) and 5 μl (females) of saliva and vigorously agitated in 1 ml of BHI medium for 15 min to recover the bacterial cells (corresponding to 1 and 0.5% pure saliva in saliva samples, respectively). After 10 days the rats were killed by application of pentobarbital intraperitoneally (i.p.), and samples were taken from the various gut sections. All samples were subjected to microbial analysis.
Controls for animal studies.
For control I, four female germfree rats were associated with S. gordonii LTH 5597 (2 rats) or S. gordonii LTH 5743 (2 rats) and received the drinking water as described above. Saliva and fecal samples were taken daily for up to 5 days, and cell counts were determined on selective agar plates. For control II, saliva samples obtained from male germfree rats (sampling see above) with and without the addition of 10% (vol/vol) HHS were inoculated with S. gordonii LTH 5597 to a final count of 2.8 × 105 CFU/ml. The mixtures were incubated at 37°C for 4 h. DNA of pMR2 was added to a final concentration of 28 μg/ml, and the mixture was further incubated for 1 h before microbial counting. For control III, an aliquot (200 μl) of saliva and fecal sample taken at day 8 from animals 1, 4, and 6 was mixed with 1 volume of BHI medium supplemented with 10% (vol/vol) of heat-inactivated (56°C, 30 min) fetal calf serum and incubated at 37°C for 4 h. DNA of pMR2 was added to a final concentration of 25 μg/ml, and the mixture was further incubated for 1 h before microbial counting. For control IV, samples (0.5 g) of the contents of the stomach, jejunum, cecum, and colon obtained after killing animal 1 were mixed with 1 ml of saline peptone. One aliquot was spiked with plasmid DNA to a final concentration of 190 μg/ml. The mixtures were immediately centrifuged (200 × g, 4°C, 12 min) to remove large particles, and DNA was isolated as described below in “Stability of DNA” for the saliva samples. Aliquots were serially diluted and subjected to PCR amplification of the 792-bp nptII fragment. For control V, 1 ml of the drinking water was supplemented with 25 μg of DNA of pMR2 and incubated in an isolator for 24 h, and serial dilutions were subjected to agarose gel electrophoresis (6 μl).
Stability of DNA.
Five saliva samples from germfree rats were spiked with DNA of pMR2 (final concentration of 200 μg/ml) and incubated at 37°C for 0.2, 30, 60, 120, and 360 min. In the control, the saliva was replaced by 0.1 mM Tris-HCl (pH 8.0). After incubation, the mixtures were treated once with 1 volume of phenol-chloroform-isoamyl alcohol (25:24:1) and once with 1 volume of chloroform. The DNA was precipitated with ethanol, washed with 70% (vol/vol) ice-cold ethanol, and resuspended in 50 μl of 0.1 mM Tris-HCl (pH 8.0). Aliquots (40 μl) were used in marker rescue experiments with S. gordonii LTH 5597 using BHI medium supplemented with 10% (vol/vol) HHS.
RESULTS
In vitro marker rescue transformation.
Plasmid pMK110 containing the nptII-based marker rescue system was isolated from B. subtilis LTH 5466 and introduced into S. gordonii TIGR via natural transformation, resulting in strain S. gordonii LTH 5597. This strain was used as a recipient to investigate in vitro the effects of different types of donor DNA on the efficiency of marker rescue by natural transformation. Competent cells of S. gordonii were incubated with plasmid and chromosomal DNAs of bacterial and/or plant origin, all harboring the intact nptII, as well as with amplified DNA fragments of intact nptII. As shown in Table 1, high marker rescue efficiencies (approximately 10−6 to 10−5 transformants per nptII gene) were obtained when plasmids isolated from E. coli and bacterial chromosomal DNA of E. coli Sure were used. With genomic plant DNA and PCR fragments as donor DNA, the efficiency was below that obtained with DNA of plasmid pSR8-30 by approximately 1 and 3 orders of magnitude, respectively. No transformants were obtained when the final concentration of plant DNA was decreased by approximately one-half (13 μg/ml).
TABLE 1.
Marker rescue transformation of S. gordonii LTH 5597 with different types of donor DNA containing intact nptII genea
| Transforming DNA | Size of donor DNA (bp) | Final DNA concn (μg/ml) | No. of nptII genes per ml | No. of recipients (CFU/ml) | No. of transformants (CFU/ml) | Transformation frequency | No. of transformants per nptII gene |
|---|---|---|---|---|---|---|---|
| Plasmid pSR8-30 | 7,800 | 6.6 ± 2.5 | (7.9 ± 3.0) × 1011 | (2.3 ± 0.5) × 108 | (6.2 ± 2.2) × 106 | (2.5 ± 1.1) × 10−2 | (8.1 ± 0.4) × 10−6 |
| Plasmid pMR2 | 5,786 | 4.4 ± 0.9 | (7.0 ± 1.4) × 1011 | (1.9 ± 0.7) × 108 | (3.3 ± 0.9) × 106 | (2.4 ± 1.1) × 10−2 | (5.2 ± 1.5) × 10−6 |
| Chromosomal DNA of E. coli Sure | 4.6 × 106 | 3.2 ± 1.1 | (6.2 ± 2.2) × 108 | (1.4 ± 0.2) × 108 | (1.3 ± 0.6) × 103 | (5.4 ± 0.6) × 10−6 | (1.8 ± 0.6) × 10−6 |
| Amplified nptII fragment obtained from E. coli Sure | 792 | 13.1 ± 6.9 | (1.5 ± 0.8) × 1013 | (1.9 ± 1.6) × 108 | (1.2 ± 0.4) × 105 | (2.1 ± 0.8) × 10−3 | (9.8 ± 1.7) × 10−9 |
| Chromosomal DNA of the Apriori potato | 3.4 × 109 | 27.7 ± 2.7 | (3.0 ± 0.3) × 107 | (3.2 ± 0.9) × 108 | (2.3 ± 0.2) × 101 | (7.3 ± 1.9) × 10−8 | (5.8 ± 1.7) × 10−7 |
| 13 | 1.4 × 107 | 3.0 × 109 | 0 | BDb | BDb |
Data in columns 3 to 8 are means ± standard errors for triplicate experiments.
BD, below the limit of detection.
Transformation of S. gordonii in foods.
To investigate the transformation of S. gordonii LTH 5597 in foods, marker rescue experiments with milk, blood sausage, and cooked PE were performed. In trial I, the efficiency of marker rescue using pMR2 as donor DNA in UHT milk and BHI medium (control) with or without the HHS was investigated. The total counts in milk and BHI medium were ca. 108 CFU/ml. With addition of HHS, the transformation frequency in milk (10−7) was much lower than the control (5.6 × 10−2), corresponding to marker rescue efficiencies of 2.7 × 10−11 and 8.8 × 10−6 transformants per nptII gene, respectively. Without the addition of HHS no transformants were detected in milk, whereas in the control the transformation frequency and corresponding marker rescue efficiency were 8.0 × 10−4 and 8 × 10−7 (transformants per nptII gene), respectively.
Marker rescue experiments in the homogenates of blood sausage (trial II) were performed with DNA of pMR2. Marker rescue was detected in both heated and nonheated homogenates (H1, H2) with efficiencies of 3.0 × 10−9 and 7.9 × 10−10, respectively. The addition of 10% HHS to both homogenates did not enhance the efficiency of transformation (data not shown). In the experiments with the potato extracts (trial III) containing DNA of pMR2 or the transgenic potato, no marker rescue was detected with or without HHS supplementation of the extracts. In this food matrix the growth of S. gordonii was reduced (1.2 × 107 CFU/ml).
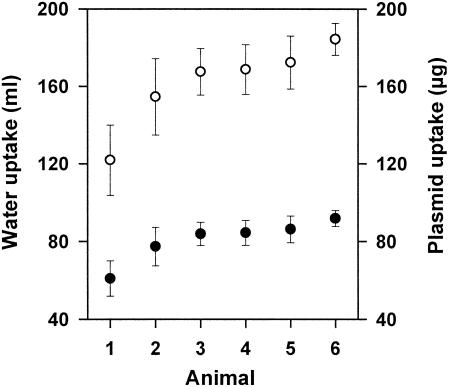
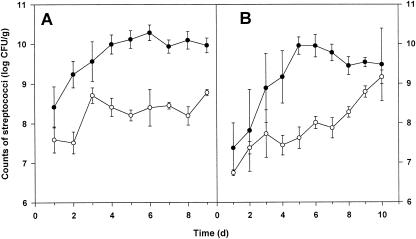
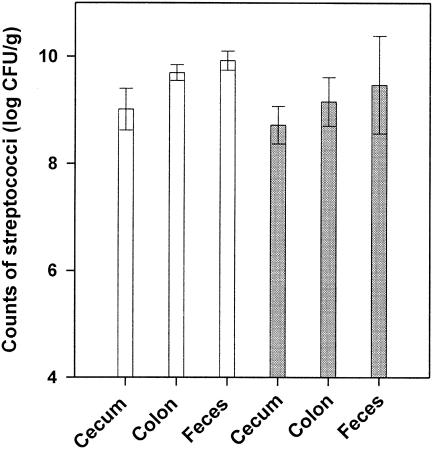
In vivo marker rescue experiments. In vivo transformation of S. gordonii was investigated by using gnotobiotic rats associated with the marker rescue recipient strain LTH 5597. The donor DNA (pMR2) was administered to the gnotobiotic animals in the drinking water for the first 6 days. The daily water uptake was determined for animals 1 to 6, and the mean values are depicted in Fig. 1. Calculation of the resulting DNA uptake revealed that the daily uptake of plasmid DNA ranged from 122 to 184 μg. Determination of the cell counts of S. gordonii LTH 5597 in the saliva and fecal samples showed that the recipient colonized the mouth and gastrointestinal tract of the animals (Fig. 2A and B). Within the first 6 days saliva and fecal samples were taken daily from all animals and investigated for the presence of kanamycin-resistant cells. Neither marker rescue transformants nor spontaneously resistant cells were detected. The absence of transformants might have been caused by failing colonization or decreased competitiveness of S. gordonii upon marker restoration and by rapid digestion of the donor DNA. Therefore, from days 7 to 9 the plasmid DNA was daily administered directly into the mouth of animals 1 to 6. Analysis of saliva samples taken after 1 to 6 h upon DNA administration and of the fecal samples taken daily revealed no transformants. Investigation of the killed animals showed high streptococcal counts in the cecum and colon, comparable to amounts in the feces (Fig. 3). Again, no transformants were detected in these sections. Samples of the stomach, duodenum, and jejunum were not analyzed because no detectable colonization (<103 CFU/ml) was observed in preliminary studies.
FIG. 1.
Average daily uptake of drinking water (closed symbols) and plasmid DNA (open symbols) for the animals in days 1 to 6.
FIG. 2.
Counts of S. gordonii LTH 5597 in fecal (closed symbols) and saliva (open symbols) samples of animals 1 to 6 receiving the plasmid DNA (A) and of the control animals (7 to 10) receiving no DNA (B). Data are expressed as mean log10 CFU per gram of sample (± standard error of the mean).
FIG. 3.
Counts of S. gordonii LTH 5597 in different compartments of the gastrointestinal tracts of the gnotobiotic rats 1 to 6 (white bars) and 7 to 10 (grey bars). Data are mean log10 CFU per gram of sample (± standard error of the mean).
Several control experiments were performed to confirm the missing marker rescue. To investigate the colonization ability of the S. gordonii strain after marker rescue, association experiments (control I) were performed with S. gordonii LTH 5597 and LTH 5743, which contains plasmid pMK111 with intact nptII. The mean counts (± standard error) of strain LTH 5743, i.e., 6.7 × 108 (± 4.2 × 108) CFU/ml in saliva and 1.1 × 1010 (± 1.4 × 1010) CFU/ml in feces, were comparable to those obtained for the recipient strain LTH 5597, indicating similar colonization rates for both strains under the selective pressure of lincomycin. In control II, the growth and competence development of S. gordonii LTH 5597 in BHI medium supplemented with 1% saliva of germfree rats were investigated. Similar cell counts (ca. 1.8 × 109 CFU/ml) were obtained with and without supplementation of HHS. Marker rescue was detected exclusively in the presence of HHS (2.5 × 10−9 transformants per nptII). In control III, saliva and fecal samples freshly taken from the gnotobiotic rats were mixed with BHI medium supplemented with 10% (vol/vol) heat-inactivated fetal calf serum and DNA of pMR2. Marker rescue was detected in saliva as well as feces with the same efficiency as observed in vitro (approximately 106 transformants CFU/ml).
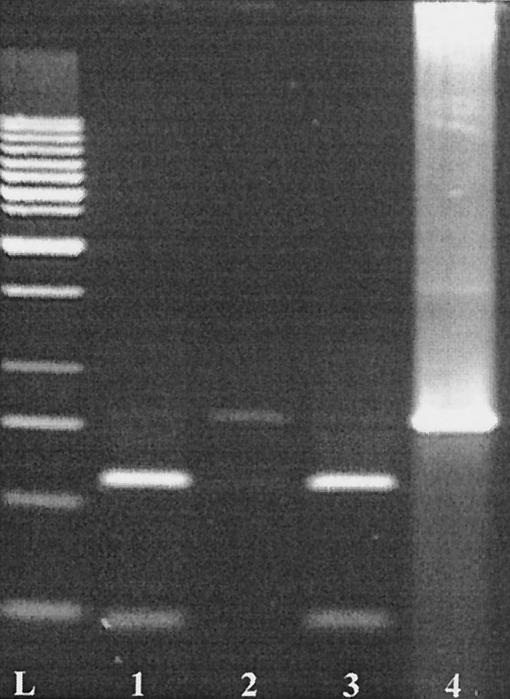
In control IV, the contents of different gut sections of animal 1 were searched for the presence of donor DNA. DNA was isolated from stomach, jejunum, cecum, and colon samples and subjected to PCR. The 792-bp fragment of nptII was amplified with the DNA obtained from the stomach, cecum, and colon samples (faint bands on the gel) as well as from all samples spiked with DNA of pMR2. In contrast to the spiked samples, the amplified fragments could not be digested with NcoI (Fig. 4), indicating that these fragments originated from plasmid pMK110 of S. gordonii LTH 5597 and not from the donor DNA. Finally, the stability of plasmid DNA in the drinking water under the conditions prevailing in the isolators was confirmed for 24 h (control V, data not shown). The DNA remained detectable by agarose gel electrophoresis up to a dilution of 10−2.
FIG. 4.
Verification of the origin of the 792-bp amplification fragments of nptII generated with the DNA isolated from colon samples. The fragments were digested with NcoI. Lane 1, colon sample of animal 1 spiked with DNA of plasmid pMR2 (donor DNA); lane 2, colon sample of animal 1; lane 3, fragment amplified with DNA of E. coli Sure; Lane 4, nondigested fragment of nptII. L, 1-kb DNA ladder (MBI Fermentas).
Stability of DNA in saliva of germfree rats.
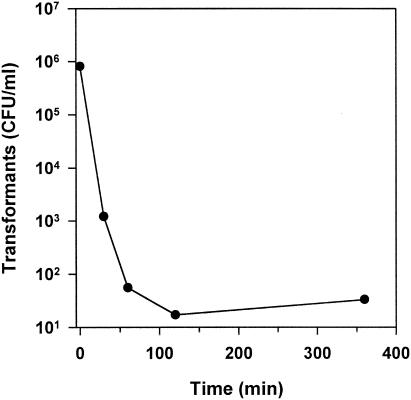
The persistence of DNA (pMR2) in the saliva of germfree rats was investigated by its ability to transform competent cells of S. gordonii. As depicted in Fig. 5, the number of transformants decreased considerably within the first 120 min of incubation. However, after 6 h of incubation of the plasmid DNA in the saliva, transformants were still detectable. Remarkably, after 0.2 min of incubation only 8.1 × 105 transformants per ml were obtained, whereas in the control (without saliva) the counts of transformants were 1.6 × 107. This indicated that a very short incubation of the plasmid DNA in saliva resulted in a reduction of the transformation efficiency.
FIG. 5.
Transformation of S. gordonii LTH 5597 with the reisolated pMR2 DNA which had been exposed to the saliva of germfree rats.
DISCUSSION
In this study we have shown by marker rescue transformation that bacterial and transgenic plant DNA are transferred to S. gordonii under laboratory conditions (in vitro) as well as in complex ecosystems such as foods. The organism is involved in the formation of oral biofilms (24) but may also occur in foods as contaminant due to poor hygiene or even deliberate addition of saliva to start spontaneous fermentation processes, e.g., production of maize beer in South America (33, 42). The efficiency of marker rescue in vitro was highest with plasmid and bacterial chromosomal DNA and decreased by 3 orders of magnitude with the 792-bp PCR fragment (Table 1). As binding and uptake of DNA in S. gordonii is not sequence specific (41), the difference in efficiency may be caused by the physical characteristics of the donor DNA. This observation is consistent with the dependence of the efficiency of transformation in Streptococcus pneumoniae on the size of DNA (34); the mechanism of transformation in this species is highly similar to that of S. gordonii (8, 43). The observation of similar marker rescue efficiencies of plasmid and chromosomal DNA (Table 1) is furthermore consistent with the findings that these DNA molecules use the same binding and uptake pathway (5) and that covalently closed circular, open circular, or linear DNA are taken up with similar entry rates (28). It is remarkable that marker rescue using transgenic potato DNA occurs with an efficiency reduced only by approximately 1 order of magnitude compared to that observed with plasmid DNA (Table 1). The size of the plant genome exceeds that of the plasmid pMR2 approximately 106-fold, and thus, the likelihood of uptake of intact nptII sequences for marker restoration should be strongly decreased due to the competing nonhomologous DNA. Using a plasmid marker rescue system in Streptococcus mutans, Lindler and Macrina (23) observed that the increase in the amount of competing DNA does not linearly correlate with the decrease of recovery of transformants, and also De Vries et al. (9) determined that the efficiencies of nptII restoration for plasmid and transgenic plant DNA in the soil bacteria Acinetobacter sp. BD413 and Pseudomonas stutzeri were the same. On the other hand, DNA of the transgenic potato was not transferred to the food-associated B. subtilis, although amplified fragments thereof were capable of transforming the recipient carrying the marker rescue system (22).
Beside the well-studied quorum-sensing regulation of competence development (17), chemical and environmental factors such as peptides, CaCl2, pH, and temperature were described as affecting transformation in S. gordonii (8). Serum from horses, calves, and swine as well as serum albumin were found to stimulate the transformation (12, 25, 37, 38). Foods constitute a huge diversity of ecological niches not only as final products but also in the process of their production. Therefore, we included in our studies foods which may provide conditions that permit transformation. Milk contains approximately 6% serum (48), and a German blood sausage contains approximately 80% blood (corresponding to 56% serum). Transformation of S. gordonii LTH 5597 was observed in the blood sausage, whereas in milk the addition of HHS was required. Calculation of the serum albumin content according to Belitz and Grosch (6) revealed that milk, blood sausage, and BHI medium plus 10% HHS contain 0.03, 2.8, and 0.35% albumin, respectively. Since the presence of 0.2% serum albumin was found to be sufficient to stimulate transformation of S. gordonii (12, 36), the missing detection of transformation may have been caused by the low serum albumin content in milk. Furthermore, it can be assumed that the combined actions of ecological factors, food ingredients, and the matrix itself affect growth, competence development, and DNA uptake. This assumption is consistent with our observation that marker rescue transformation in the foods occurs with less efficiency (approximately 104- to 105-fold decreased) than in the in vitro experiments with BHI medium plus 10% HHS and plasmid DNA (Table 1). As the intact nptII is contained in the genome of the Apriori potato, we included this food matrix in our studies. However, despite addition of exogenous donor DNA no marker rescue transformation was observed. This observation is in agreement with our findings that S. gordonii LTH 5597 did not grow in this food matrix. It was not intended to broaden the experiments by using competent cells, as we have previously shown that competent cells take up free DNA from the food matrix (3, 7).
We initiated in vivo experiments with monoassociated rats, as our marker rescue system constitutes an efficient tool to detect the rare event of in vivo transformation. The system is based on the recombinational repair of nptII and does not require recircularization of the donor DNA as it is needed in transformation with nonhomologous plasmid DNA (5). The system is plasmid encoded, leading to increased transformation efficiencies as suggested by Lindler and Macrina (23) for S. mutans, since the homologous target DNA is provided in high copy number in comparison to chromosomal markers. Highest transformation frequencies (ca. 10−2) were obtained in vitro (Table 1), and therefore, we adopted these basic conditions for the animal model. However, no marker rescue transformation was observed in vivo when plasmid DNA was used. One reason may be the lack of availability of donor DNA in the compartments of the digestive tract, although a continuous supply was ensured by daily administration of high amounts of DNA. We found that plasmid DNA was rapidly degraded in the saliva of rats but marker rescue transformation with the partially degraded donor DNA was still detectable for up to 6 h (Fig. 5). This result is consistent with that of Mercer et al. (29, 30), who observed the degradation of plasmid DNA in human saliva. In the distal sections of the rats' gastrointestinal tracts we did not detect the donor DNA by PCR. Apparently, the DNA is rapidly degraded in vitro as shown by Maturin and Curtiss (27), who used the intestinal contents of gnotobiotic and conventional rats. Another reason for the lack of marker rescue transformation in vivo may be that S. gordonii does not develop competence in the animal model. This assumption is supported by the results of the control experiments II and III, showing that the efficient transformation of S. gordonii LTH 5597 in the presence of rat saliva or feces requires the addition of serum as stimulating factor. On the other hand, we observed (unpublished results) that strain LTH 5597 develops competence during growth in BHI medium in the presence of 10% human saliva without HHS, and also Mercer et al. (30) reported that filter-sterilized human saliva induces competence in S. gordonii. Thus, S. gordonii LTH 5597 has the potential to develop competence in vivo, at least in the mouth of the animal.
To detect the rare event of transformation, our in vivo studies were designed to take worst-case conditions into account. For that purpose the animals were monoassociated with S. gordonii to achieve defined conditions with regard to potential recipients in the oral microbiota. In addition, up to 200 μg of plasmid DNA were administered daily to the animals, corresponding to 3.2 × 1013 intact genes of nptII, an amount that is present in approximately 222 kg of transgenic potato tubers. This weight is far beyond the daily energy and nutrient requirements, as only ca. 49 g (7.1 × 109 nptII genes) of potato tubers can be taken up maximally by a rat with 263 g of body weight. Despite application of the worst-case conditions, no transformation of S. gordonii was detectable in vivo. The meaning of this result for assessing the likelihood of transfer of transgenic plant DNA to S. gordonii in the human digestive tract is rather limited for numerous reasons. For example, no data about the competence development of S. gordonii in the human oral cavity are available. Thus, the animal model has limitations in its value to calculate transfer rates meaningful for humans. In addition, the human exposure to intact potato DNA can hardly be estimated for the following reason. The average consumption of potatoes by Austrians has been calculated to be 55.8 kg per capita per year (20). Potatoes are, however, eaten only after being processed, and thus, the DNA is subjected to degradation depending on the degree of processing involved. For example, in fried potatoes the degradation went so far that no DNA fragments of >100 bp were detectable by PCR (unpublished results). Finally, it has to be considered that during transformation each incoming DNA fragment has to be rescued by homologous recombination or, as recently demonstrated for S. pneumoniae, by homology-directed illegitimate recombination (39).
In conclusion, an in vivo transfer of recombinant DNA to S. gordonii has not been detected under the experimental conditions but cannot be excluded and may show up when the experiments are performed on a large scale. To take into consideration the whole food chain, the key event of the transfer under these conditions is the experimentally proven transformation in the food matrix. The transformed bacteria can then spread the acquired genetic information to other components of the flora inhabiting the digestive tract. Thus, our results confirm the need for a case-by-case consideration in risk assessment of genetically modified organisms.
Acknowledgments
We thank E. Focken, M. Kranz, C. Lis, M. Urbich, and B. Gruhl for excellent technical assistance and I. Grüner and U. Lehman for excellent animal care. We are indebted to K. Düring and D. Falkenburg, W. Wackernagel, and C. Alpert and G. Corthier for providing pSR8-30, pMR2, and S. gordonii TIGR, respectively. We thank AVEBE for supporting our work by providing the transgenic potato Apriori. We are thankful to S. Schön for construction of primers Eonpt2 and Sonpt2.
This work was partly supported by EU grant QLK1-1999-00527.
REFERENCES
- 1.Anonymous. 1995. Application of risk analysis to food standards issues, p. 1-39. Report of the joint FAO/WHO expert consultation. WHO/FNU/FOS/95.3. [Online.] http://www.who.int/fsf/Documents/applira.pdf.
- 2.Anonymous. 2001. Review of the work by international organizations on the evaluation of the safety and nutrition aspects of foods derived from biotechnology, p. 1-12. Codex alimentarius commission of joint FAO/WHO CX/FBT/01/3. [Online.] http://www.who.int/fsf/GMfood/Task_Force2001/bt01_03e.pdf.
- 3.Bauer, F., C. Hertel, and W. P. Hammes. 1999. Transformation of Escherichia coli in foodstuffs. Syst. Appl. Microbiol. 22:161-168. [DOI] [PubMed] [Google Scholar]
- 4.Baur, B., K. Hanselmann, W. Schlimme, and B. Jenni. 1996. Genetic transformation in freshwater: Escherichia coli is able to develop natural competence. Appl. Environ. Microbiol. 62:3673-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behnke, D. 1981. Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol. Gen. Genet. 182:490-497. [DOI] [PubMed] [Google Scholar]
- 6.Belitz, H. D., and W. Grosch. 1999. Food chemistry, 2nd ed. Springer-Verlag, Berlin, Germany.
- 7.Bräutigam, M., C. Hertel, and W. P. Hammes. 1997. Evidence for natural transformation of Bacillus subtilis in foodstuffs. FEMS Microbiol. Lett. 155:93-98. [DOI] [PubMed] [Google Scholar]
- 8.Cvitkovitch, D. G. 2001. Genetic competence and transformation in oral streptococci. Crit. Rev. Oral Biol. Med. 12:217-243. [DOI] [PubMed] [Google Scholar]
- 9.De Vries, J., P. Meier, and W. Wackernagel. 2001. The natural transformation of the soil bacteria Pseudomonas stutzeri and Acinetobacter sp. by transgenic plant DNA strictly depends on homologous sequences in the recipient cells. FEMS Microbiol. Lett. 195:211-215. [DOI] [PubMed] [Google Scholar]
- 10.De Vries, J., and W. Wackernagel. 1998. Detection of nptII (kanamycin resistance) genes in genomes of transgenic plants by marker rescue transformation. Mol. Gen. Genet. 257:606-613. [DOI] [PubMed] [Google Scholar]
- 11.De Vries, J., and W. Wackernagel. 2002. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc. Natl. Acad. Sci. USA 99:2094-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrzanski, W. T., and H. Osowiecki. 1967. Isolation and some properties of the competence factor from group H streptococcus strain challis. J. Gen. Microbiol. 48:299-304. [DOI] [PubMed] [Google Scholar]
- 13.Doerfler, W., and R. Schubbert. 1997. Fremde DNA im Säugersystem. Dtsch. Ärztebl. 94:51-52. [Google Scholar]
- 14.Düring, K. 1994. A plant transformation vector with a minimal T-DNA. Transgenic Res. 3:138-140. [DOI] [PubMed] [Google Scholar]
- 15.Gasson, M. J. 2000. Gene transfer from genetically modified food. Curr. Opin. Biotech. 11:505-508. [DOI] [PubMed] [Google Scholar]
- 16.Gebhard, F., and K. Smalla. 1998. Transformation of Acinetobacter sp. strain BD413 by transgenic sugar beet DNA. Appl. Environ. Microbiol. 64:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havarstein, L. S., and D. A. Morrison. 1999. Quorum sensing and peptide pheromones in streptococcal competence for genetic transformation, p. 9-26. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. American Society for Microbiology, Washington, D.C.
- 18.Hohlweg, U., and W. Doerfler. 2001. On the fate of plant or other foreign genes upon the uptake in food or after intramuscular injection in mice. Mol. Genet. Genomics 265:225-233. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss, R. D., and H. Ephrussi-Taylor. 1951. Use of serum albumin as source of serum factor in pneumococcal transformation. Fed. Proc. 10:200.
- 20.Jonas, D. A., I. Elmadfa, K.-H. Engel, K. J. Heller, G. Kozianowski, A. König, D. Müller, J. F. Narbonne, W. Wackernagel, and J. Kleiner. 2001. Safety considerations of DNA in food. Ann. Nutr. Metab. 45:235-254. [DOI] [PubMed] [Google Scholar]
- 21.Kay, E., T. M. Vogel, F. Bertolla, R. Nalin, and P. Simonet. 2002. In situ transfer of antibiotic resistance genes from transgenic (transplastomic) tobacco plants to bacteria. Appl. Environ. Microbiol. 68:3345-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharazmi, M., W. P. Hammes, and C. Hertel. 2002. Construction of a marker rescue system in Bacillus subtilis for detection of horizontal gene transfer in food. Syst. Appl. Microbiol. 25:471-477. [DOI] [PubMed] [Google Scholar]
- 23.Lindler, L. E., and F. L. Macrina. 1986. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J. Bacteriol. 166:658-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macrina, F. L., C. L. Keeler, Jr., K. R. Jones, and P. H. Wood. 1980. Molecular characterization of unique deletion mutants of the streptococcal plasmid pAMβ1. Plasmid 4:8-16. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Orue, S. M., A. G. O'Donnell, J. Arino, T. Netherwood, H. J. Gilbert, and J. C. Mathers. 2002. Degradation of transgenic DNA from genetically modified soya and maize in human intestinal simulations. Br. J. Nutr. 87:533-542. [DOI] [PubMed] [Google Scholar]
- 27.Maturin, L., and R. Curtiss. 1977. Degradation of DNA by nucleases in intestinal tract of rats. Science 196:216-218. [DOI] [PubMed] [Google Scholar]
- 28.Mejean, V., and J. P. Claverys. 1993. DNA processing during entry in transformation of Streptococcus pneumoniae. J. Biol. Chem. 268:5594-5599. [PubMed] [Google Scholar]
- 29.Mercer, D. K., K. P. Scott, C. M. Melville, L. A. Glover, and H. J. Flint. 2001. Transformation of an oral bacterium via chromosomal integration of free DNA in the presence of human saliva. FEMS Microbiol. Lett. 200:163-167. [DOI] [PubMed] [Google Scholar]
- 30.Mercer, D. K., K. P. Scott, W. A. Bruce-Johnson, L. A. Glover, and H. J. Flint. 1999. Fate of free DNA and transformation of the oral bacterium Streptococcus gordonii DL1 by plasmid DNA in human saliva. Appl. Environ. Microbiol. 65:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz, P. L. J., and J. P. Nap. 1997. A transgene-centred approach to the biosafety of transgenic plants: overview of selection and reporter genes. Acta Bot. Neerl. 46:25-50. [Google Scholar]
- 32.Meyer, R., F. Chardonnens, P. Hübner, and J. Lüthy. 1996. Polymerase chain reaction (PCR) in the quality and safety assurance of food: detection of soya in processed meat products. Z. Lebensm. Unters. Forsch. 203:339-344. [DOI] [PubMed] [Google Scholar]
- 33.Morris, C. 1979. Maize beer in the economics, politics, and religion of the Inca Empire, p. 21-34. In C. F. Gastineau, W. J. Darby, and T. B. Turner (ed.), Fermented food beverages in nutrition. Academic Press, Inc., New York, N.Y.
- 34.Morrison, D. A., and W. R. Guild. 1972. Transformation and deoxyribonucleic acid size: Extent of degradation on entry varies with size of donor. 112:1157-1168. [DOI] [PMC free article] [PubMed]
- 35.Nielsen, K. M., J. D. van Elsas, and K. Smalla. 2000. Transformation of Acinetobacter sp. strain BD413 (pFG4ΔnptII) with transgenic plant DNA in soil microcosms and effects of kanamycin on selection of transformants. Appl. Environ. Microbiol. 66:1237-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osowiecki, H., and W. T. Dobrzanski. 1968. Synthetic medium for development of competence and transformation in Streptococcus challis. Pathol. Microbiol. 31:226-332. [DOI] [PubMed] [Google Scholar]
- 37.Pakula, R., and W. Walczak. 1963. On the nature of competence of transformable streptococci. J. Gen. Microbiol. 31:125-133. [DOI] [PubMed] [Google Scholar]
- 38.Pozzi, G., R. A. Musmanno, P. M. J. Lievens, M. R. Oggioni, P. Plevani, and R. Manganelli. 1990. Method and parameters for genetic transformation of Streptococcus sanguis challis. Res. Microbiol. 141:659-670. [DOI] [PubMed] [Google Scholar]
- 39.Prudhomme, M., V. Libante, and J. P. Claverys. 2002. Homologous recombination at the border: insertion-deletions and the trapping of foreign DNA in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 19:2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Smith, H. O., D. B. Danner, and R. A. Deich. 1981. Genetic transformation. Annu. Rev. Biochem. 50:41-68. [DOI] [PubMed] [Google Scholar]
- 42.Steinkraus, K. H. 1979. Nutritionally significant indigenous foods involving an alcoholic fermentation, p. 35-59. In C. F. Gastineau, W. J. Darby, and T. B. Turner (ed.), Fermented food beverages in nutrition, Academic Press, Inc., New York, N.Y.
- 43.Stewart, G. J., and C. A. Carlson. 1986. The biology of natural transformation. Annu. Rev. Microbiol. 40:211-235. [DOI] [PubMed] [Google Scholar]
- 44.Straub, J. A., C. Hertel, and W. P. Hammes. 1999. Limits of a PCR-based detection method for genetically modified soya beans in wheat bread production. Z. Lebensm. Unters. Forsch. A 208:77-82. [Google Scholar]
- 45.Straub, J. A., C. Hertel, and W. P. Hammes. 1999. The fate of recombinant DNA in thermally treated fermented sausages. Eur. Food Res. Technol. 210:62-67. [Google Scholar]
- 46.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 47.Thomson, J. A. 2001. Horizontal transfer of DNA from GM crops to bacteria and to mammalian cells. J. Food Sci. 66:188-193. [Google Scholar]
- 48.Walstra, P., T. J. Geurts, A. Noomen, A. Jellema, and M. A. J. S. van Boekel. 1999. Dairy Technology. Marcel Dekker, Inc., New York, N.Y.
- 49.Zenz, K. I., H. Neve, A. Geis, and K. J. Heller. 1998. Bacillus subtilis develops competence for uptake of plasmid DNA when growing in milk products. Syst. Appl. Microbiol. 21:28-32. [DOI] [PubMed] [Google Scholar]