Abstract
Activity measurements by radioisotopic methods and cultural and molecular approaches were used in parallel to investigate the microbial biodiversity and its physiological potential in formation waters of the Samotlor high-temperature oil reservoir (Western Siberia, Russia). Sulfate reduction with rates not exceeding 20 nmol of H2S liter−1 day−1 occurred at 60 and 80°C. In upper horizons (AB, A, and B), methanogenesis (lithotrophic and/or acetoclastic) was detected only in wells in which sulfate reduction did not occur. In some of the wells from deeper (J) horizons, high-temperature sulfate reduction and methanogenesis occurred simultaneously, the rate of lithotrophic methanogenesis exceeding 80 nmol of CH4 liter−1 day−1. Enrichment cultures indicated the presence of diverse physiological groups representing aerobic and anaerobic thermophiles and hyperthermophiles; fermentative organotrophs were predominant. Phylogenetic analyses of 15 isolates identified representatives of the genera Thermotoga, Thermoanaerobacter, Geobacillus, Petrotoga, Thermosipho, and Thermococcus, the latter four being represented by new species. Except for Thermosipho, the isolates were members of genera recovered earlier from similar habitats. DNA obtained from three samples was hybridized with a set of oligonucleotide probes targeting selected microbial groups encompassing key genera of thermophilic bacteria and archaea. Oligonucleotide microchip analyses confirmed the cultural data but also revealed the presence of several groups of microorganisms that escaped cultivation, among them representatives of the Aquificales/Desulfurobacterium-Thermovibrio cluster and of the genera Desulfurococcus and Thermus, up to now unknown in this habitat. The unexpected presence of these organisms suggests that their distribution may be much wider than suspected.
The presence of microorganisms in subsurface environments has long been recognized. For a very long time, the mesophilic microbes that were recovered from the earth's crust were suspected to be surface contaminants. Increasing evidence of microbial populations thriving in deep geological formations has been given by recent key discoveries (39, 51, 53). Among these peculiar habitats, oil fields occurring at significant subsurface depth and, consequently, characterized by a high in situ temperature represent unique biotopes that constitute ecological niches providing suitable conditions to support thermophilic organisms (50). During the past decades, several approaches have been used (mainly individually) to study the microbial ecology of high-temperature oil reservoirs. Radioisotopic investigations of microbial sulfate reduction and methanogenesis were used to determine the rates of these processes in oil reservoirs of Kazakhstan, Western Siberia, and China (32, 34, 46). Enrichment and isolation experiments performed with samples collected from high-temperature oil fields revealed the presence of physiologically diverse microbial communities and led to the characterization of numerous bacterial and archaeal thermophiles (and hyperthermophiles), including both chemolithoautotrophic and chemoorganoheterotrophic strains. The resident communities include hydrocarbon-oxidizing bacteria (35), methanogens (36), sulfate reducers (7, 37), manganese and iron reducers (44, 49), and a variety of fermentative microorganisms (16, 27). Molecular methods based on reverse genome probing, hybridization with functional gene probes, 16S rDNA analysis, and immunological techniques have been applied mainly after primary enrichments to identify sulfate-reducing populations in oil field environments and to assess their physiological and genetic diversity (9, 21, 56-58). Up to now, only the recent paper by Orphan et al. (38) characterized microbial assemblages of high-temperature petroleum reservoirs by both culture-dependent and -independent approaches.
The majority of the studies described above were performed on offshore oil reservoirs, in which seawater is used as injection water for secondary oil recovery. Since anaerobic thermophiles can survive transportation in cold ocean water, their presence in these hot oil-bearing formations has been attributed to continuous inoculation of the reservoirs by low concentrations of hyperthermophiles contained in the injection water (50). On the other hand, isolation of hyperthermophilic species from continental oil fields of the Paris Basin suggested their indigenous origin (22).
A field expedition was organized to study the microbial ecology of a continental oil reservoir (Western Siberia, Russia) by using independent approaches. The Samotlor oil reservoir located in the middle of Eurasia is equally remote from the ocean and zones of hydrothermal activity. Samples of injection and formation waters were collected and investigated by chemical analyses, specific activity measurements, culture-based enrichments, 16S ribosomal DNA (rDNA) sequence analysis, and hybridization of native nucleic acids to a matrix array of oligonucleotide probes targeting 16S rRNA. The analysis of 16S rDNA recovered from one of the collected samples will be described elsewhere. Here we determined rates of sulfate reduction and lithotrophic and acetoclastic methanogenesis; enriched, isolated, and identified aerobic and strictly anaerobic thermophiles; and compared these results with those obtained by hybridization with probes targeting selected microbial groups encompassing key genera of thermophilic bacteria and archaea.
MATERIALS AND METHODS
Characteristics of sampling sites.
The Samotlor oil field (60°′N, 80°′E) is located in the middle Ob region in the vicinity of Nizhnevartovsk (Western Siberia, Russia). Geologically, this field is a typical representative of the largest oil fields of the world with respect to reservoir rock (sandstone), trap type (anticlinal), age of reservoir rock (mainly Lower Cretaceous and Upper Jurassic oil-bearing formations), average depth (between 1,700 and 2,450 m), and depositional environment (shelf or shallow marine) (10). Oil-producing wells studied in this work were located in an area of 6 km2, the distance between neighboring wells ranging from 0.2 to 0.5 km. The Samotlor oil field has been mostly exploited by flooding with a mixture of coproduced water and freshwater of the Vah river (about 30%) containing 0.5 to 3 mg of O2 liter−1. On the surface, the water-oil suspension produced by the well is separated within 2 to 3 h; after that, the coproduced water is mixed with water of the Vah river and again injected into the oil wells. The temperature of injection water varies from 4 to 40°C depending on the season. All wells of Cretaceous horizons (AB4-5, A7, B8, and B10) are flooded with injection water, whereas wells of the Jurassic horizons (J) contain only formation water.
Sample collection.
In June 1998, 17 samples of oil-water mixtures and one sample of injection water, taken directly from production wellheads and an injection well, respectively, were collected in sterile 0.5-liter serum bottles. The bottles were sealed with rubber stoppers and screw caps and transported at ambient temperature to the field laboratory, where they were immediately prepared for further analyses.
Analytical methods.
The chemical composition of waters was determined with a Biotronic ion chromatograph. Concentrations of lower fatty acids were determined in samples fixed with saturated KOH (2 ml/50 ml of sample). In the laboratory, 0.98 ml of each sample was acidified with 0.02 ml of 25% HCl and analyzed on a Chrom-41 chromatograph equipped with a flame ionization detector. The column (length, 2 m; diameter, 0.3 cm) was packed with Porapak Q (100 to 120 mesh). The evaporizer temperature was 180°C. The carrier gas was a mixture of N2 (95%) and CO2 (5%), fed at a rate of 70 ml min−1. Hydrogen sulfide was analyzed colorimetrically with dimethyl-p-phenylenediamine by the method described by Trüper and Schlegel (55). All values were determined to within 0.001 g liter−1.
Radioisotopic methods.
The formation water samples were transferred to 50-ml sterile flasks and sealed with rubber stoppers. Two hundred microliters of a radioisotope solution was added to each flask. The rates of lithotrophic and acetoclastic methanogenesis were estimated by the addition of NaH14CO3 (0.2 ml; 3.0 MBq; Isotope, Saint Petersburg, Russia) and 14CH3COONa (0.2 ml; 0.5 MBq; Isotope), respectively. Sulfate reduction rates were determined by the addition of Na235SO4 (0.2 ml; 1.6 MBq; FEI, Moscow, Russia). After 24 h of incubation at 60°C (AB, A, and B horizons) or 80°C (J horizons), samples were inactivated by adding 0.5 ml of 10 M NaOH.
The radioactive methane formed during the incubation was determined by a modification of the method of Belyaev and Ivanov (8). Methane was transferred from the flask by an air current over 1 h at a flow rate of 100 to 120 ml min−1. Although the presence of NaOH prevented labeled CO2 from leaving the solution, traces of it were trapped in saturated NaOH solution. For methane combustion, the gas flow was directed to a quartz tube filled with Co oxide-bearing silica gel. The temperature of the tube was 800 to 900°C. The combustion product, 14CO2, was trapped in scintillation liquid mixed with β-phenylethylamine (Fluka, Buchs, Switzerland) and methanol at a ratio of 3:1:1. Determination of S−2 radioactivity was performed by a modification of the method of Ivanov and Terebkova (20). An aliquot volume (20 ml) of each sample was placed in a 250-ml flask, and Na2S (0.5 mM) was added as a carrier. The flask was heated to 70°C under a flow of Ar (100%; 100 ml min−1) and with constant mixing. Ten to 20 ml of 10% H3PO4 was slowly added to the flask to a final pH of 3.0. H2S was distilled into a trap containing Lipoluma scintillation liquid, β-phenylethylamine, and methanol mixed at a ratio of 3:1:1 Radioactivity was measured in a RackBeta liquid scintillation counter (LKB Wallac, Turku, Finland). All experiments were run in two parallel sets, the results differing on average by 12%.
Enrichment cultures.
Thermophilic microorganisms of different physiological groups were enriched by inoculation of selective media with samples of formation or injection water. Aerobic cultivation was performed in 250-ml flasks; anaerobic cultivation was carried out in 15-ml Hungate tubes or 50-ml serum bottles. If not specified otherwise, the headspace in anaerobic cultures was filled with a mixture of N2 and CO2 (80:20, atmospheric pressure). Aerobic hydrocarbon-oxidizing bacteria were cultivated in medium P (31) containing 4% (vol/vol) hexadecane. Aerobic organotrophs were enriched in distilled water containing Bacto tryptone (5.0 g liter−1), yeast extract (2.5 g liter−1), and glucose (1.0 g liter−1; pH 7.0). Anaerobic organotrophs were cultivated in a medium consisting of basic mineral solution (29) and organic substrates: peptone or starch (4 and 10 g liter−1, respectively), with or without elemental sulfur (10 g liter−1) or sodium thiosulfate (2 g liter−1). Nitrate-reducing bacteria were enriched at 60°C in Adkins medium (1), with sodium acetate (2 g liter−1) as the energy substrate and with sodium nitrate (0.85 g liter−1) as the electron acceptor. For the cultivation of sulfate-reducing prokaryotes, the medium described by Postgate (40) with sodium lactate (4 g liter−1) or sodium acetate (1.5 g liter−1) were used. Methanogens were enriched in LBPM medium (61) with acetate (2 g liter−1) or H2 plus CO2 (80:20; atmospheric pressure) as the growth substrate. Cultures were incubated either at 60°C immediately after inoculation or at 70 and 85°C after transportation to the laboratory.
Isolation and identification of thermophilic isolates.
For isolation, selected enrichments were subjected to serial dilutions with corresponding liquid medium or were transferred by streaking onto media solidified with 1% (wt/vol) Phytagel (Sigma, Saint Quentin Fallavier, France) or 2% Difco agar.
Phylogenetic positions of thermophilic isolates were determined by analyzing their partial or complete 16S rDNA sequence. Extraction of genomic DNA from cultures of the isolates, PCR-mediated amplification of 16S rDNA, and sequencing of the purified PCR products were carried out as described by Rainey et al. (42). For Archaea, the primers used for DNA amplification and sequencing reactions were those described by Barns et al. (5). The reaction products were electrophoresed with a model 373A automatic DNA sequencer (Applied Biosystems, Foster City, Calif.). The 16S rDNA sequences were manually aligned with published sequences of the DSMZ database with the ae2 editor (28), and sequences were retrieved from EMBL.
DNA extraction from samples of injection and formation waters.
Samples of injection and formation waters (approximate volumes of 10 to 20 liters) were concentrated under a nitrogen pressure of 5 to 6 atm with an Amicon stirred-cell device (Millipore Corp., Bedford, Mass.) with 0.22-μm-pore cellulose filters (Schleicher & Schuell, Dassel, Germany). The filters were then stored at −20°C. For the isolation of DNA, filters frozen in liquid nitrogen were ground to a dust-like state in a porcelain mortar containing 15 ml of TE (Tris-EDTA) buffer (pH 8.0). The following reagents were added successively to the ground material: lysozyme (100 μg ml−1), followed by incubation at 37oC for 30 min; and proteinase K (100 μg ml−1) and sodium dodecyl sulfate (SDS; 0.5%), followed by incubation at 54°C for 1 to 2 h. After being mixed on a shaker, SDS was added to a final concentration of 2%. Deproteinization was performed by shaking with a mixture of phenol and chloroform (5:1), supplemented with 1 M NaCl and heated to 60°C. After centrifugation, the water phase was once again deproteinized on ice with a chloroform-isopropanol (24:1) mixture. After centrifugation, 2 volumes of cold 96% ethanol were added to the water phase for DNA precipitation. DNA was collected by centrifugation, washed with 70% and then 96% ethanol, dried, and resuspended in TE buffer (pH 8.0). The quality of DNA was checked spectrophotometrically with a Pye Unicam SP 1800 spectrophotometer.
Microchip analyses.
The following strains were used as sources of reference nucleic acids: Thermococcus celer DSM 2476T, Thermococcus litoralis DSM 5474T, Pyrococcus furiosus DSM 3638T, Desulfurococcus amylolyticus DSM 3822T, Methanobacterium thermoformicicum DSM 3720T, Thermotoga maritima strain MP (100% DNA-DNA hybridization with the species type strain; V. A. Svetlichny, personal communication), Thermotoga subterranea DSM 9912T, Petrotoga mobilis DSM 10674T, Thermoanaerobacter siderophilus DSM 12299T, Thermoanaerobacter kivui DSM 2030T, Desulfotomaculum kuznetsovii DSM 6115T, and Geobacillus (formerly Bacillus) stearothermophilus DSM 22T. Reference strains were cultivated in the media recommended by DSMZ. DNA of reference strains was isolated as described above for natural samples. 16S rDNA was amplified by PCR with the following primers: S-D-Bact-0011-a-S-17 (5′-GTTTGATCCTGGCTCAG-3′) for Bacteria (2) and Arch9F (5′-CYGGTTGATCCYGCCRGA-3′) for Archaea (our modification) as the forward primers and the universal primer traditionally called S-D-Bact-1492-a-A-21 (5′-ACGGYTACCTTGTTACGACTT-3′) as the reverse primer for both domains (2). In vitro transcripts were obtained by using amplificates and forward primers possessing the T7 promoter on the 5′ end for T7 transcription. In vitro transcription was performed with the MEGAshortscript T7 kit (Ambion, Austin, Tex.) according to the manufacturer's recommendations. In vitro transcripts of 16S rRNA genes were fragmented and labeled by Texas red-sulfonyl chloride mixed isomers (Molecular Probes, Eugene, Oreg.) as described previously (41). Specific oligonucleotide probes used in this study (Table 1) were either published earlier or designed specially for this work by using two versions of original software (52; Y. P. Lysov, unpublished). The specificity of the probes was additionally checked by using the Check_Probe RDP (28; http://rdp.cme.msu.edu/html/) and GenBank BLAST (3; http://www.ncbi.nlm.nih.gov/BLAST/) facilities. The detailed procedure for microchip fabrication was described previously (15, 17, 60). Hybridization was performed at room temperature for 14 h in 0.5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) (48). The volume of the hybridization chamber was 30 μl, and the concentration of DNA varied from 0.1 to 1 pmol/ml. The hybridization mixture was replaced with fresh 0.5× SSPE hybridization buffer (48) directly before the measurement of hybridization signals. Fluorescence was monitored at 30 and 42°C. The exposure times were 0.5 and 1 s. Then, the signals were analyzed by using the MATLAB toolbox (60).
TABLE 1.
16S rRNA-targeted oligonucleotide probes used in microchip analyses
| Probe no. | Target organism(s) | Probe sequence | Designation of probea | Source or reference |
|---|---|---|---|---|
| 4 | Methanobacterium [other than strains reacting with Mb(II)Mbt 1285] | 5′-CGAACTACGACCTGGTTTAG-3′ | Mb(I) 643 | This work |
| 5 | M. thermoautotrophicum, M. wolfei, M. thermaggregans, Methanothermobacter | 5′-CCCGGCCTCAAGCCTAATAG-3′ | Mb(II)Mbt 1285 | This work |
| 6 | Thermococcus + Pyrococcus | 5′-ACTTACGGGCCCGGCTACGG-3′ | TcPc 579 | This work |
| 21 | Geotoga | 5′-AGTTTCATGCGCACTGACAC-3′ | Got 625 | This work |
| 25 | Petrotoga | 5′-CTGTAGTGTGTCCACTACCC-3′ | Pet 454 | This work |
| 27 | Petrotoga | 5′-GCCCAGTAGACCAACTTCTT-3′ | Pet 725 | This work |
| 30 | Thermococcus | 5′-CCAGTACCTCAGGCCTAT-3′ | Tc 179 | This work |
| 39 | Thermodesulfobacterium | 5′-TAACCTATGACTTGTCCAGC-3′ | Tdb 588 | This work |
| 44 | Desulfurococcus | 5′-TCGACCCGACCCAAGGGGCC-3′ | Dco 1440 | This work |
| 46 | Desulfotomaculum (D. geothermicum, D. thermobenzoicum, D. australicum) | 5′-GCGGACCCATCCATTAGCGG-3′ | Dfm(I) 218 | This work |
| 48 | Desulfotomaculum (D. ruminis, D. nigrificans) | 5′-AAGTCCACCAGTATCAGAGG-3′ | Dfm(II) 634 | This work |
| 50 | Thermoanaerobacter | 5′-CTCGCTACTACCTGCATGTC-3′ | Tab 993 | This work |
| 52 | Thermus (T. thermophilus, T. flavus, T. aquaticus, but not T. filiformis and T. scotoductus) | 5′-GGGTTTCGTCCCGGGTTC-3′ | S-G-Thus-0438-a-A-18 | 18 |
| 53 | Geobacillus | 5′-GGGTGTGACCCCTCTAAC-3′ | S-*-Tbcil-0832-a-A-18 | 18 |
| 54 | Thermotoga + Thermosipho | 5′-GTTCCGTCTCCCTCTACC-3′ | S-*-Ttoga-0660-a-A-18 | 18 |
| 55 | Aquificales + Desulfurobacterium and Thermovibrio species | 5′-TCGCGCAACGCTCGGGACC-3′ | S-O-Hydr-0540-a-A-19 | 18 |
| 58 | Methanobacterium (all species) + Methanothermobacter | 5′-CCACCCCGTTAAGAGTG-3′ | MbMbt 408 | This work |
The designations of the probes published earlier (17) are according to the Oligonucleotide Probe Database nomenclature (2). The designations of the probes designed in the present work include generic abbreviations adopted in the Ribosomal Database Project (27) and the target position in E. coli numbering.
RESULTS
Physicochemical characteristics of the Samotlor oil field.
The depths of the sampled petroleum horizons ranged from 1,799 to 2,427 m, with in situ temperatures between 60 and 85°C. The pH and salinity of formation waters varied from 5.95 to 7.7 and from 5.66 to 52.31 g liter−1, respectively. Bicarbonates were present in all samples, while sulfates were low to virtually absent. Hydrogen sulfide was never detected by the method applied, its concentration thus being below 1 mg liter−1. The physicochemical characteristics of formation waters and of injection water are given in Table 2. The oil from the Jurassic horizon after degassing had a specific weight of 855 kg/m3. The oil contained silica gel resin (6.7%), asphaltenes (1.9%), paraffins (3.1%), and sulfur (0.9%). The gas contained methane (67.02%), ethane (5.63%), propane (10.75%), higher homologues (14.14%), N2 (1.92%), and CO2 (0.54%).
TABLE 2.
Characteristics of the samples collected in Samotlor oil reservoir, Western Siberia
| Well no. | Horizon | Depth (m) | Temp (°C) | pH | Salinity (g liter−1) | Amt (g liter−1) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Cl− | SO4−2 | HCO3− | Total Fe | K+ + Na+ | Acetate | ||||||
| 656 | Injection water | 16 | 7.3 | 17.98 | 0.20 | 0.06 | 10.63 | 0 | 0.56 | NDa | 6.53 | ND | |
| 692 | AB4-5 | 1,900 | 60 | 7.50 | 12.05 | 0.28 | 0 | 6.91 | 0.016 | 0.51 | ND | 4.35 | ND |
| 13044 | AB4-5 | 1,799 | 60 | 7.30 | 7.73 | 0.38 | 0.13 | 4.24 | 0.025 | 0.66 | 0.011 | 2.31 | 0.008 |
| 31079 | AB4-5 | 1,800 | 60 | 7.45 | 50.19 | 0.82 | 0 | 29.78 | <0.001 | 0.88 | ND | 18.71 | 0.005 |
| 35363 | AB4-5 | 1,801 | 60 | 7.23 | 10.54 | 0.36 | 0.25 | 8.60 | 0.008 | 1.33 | ND | 5.17 | 0.002 |
| 642 | A7 | 1,850 | 60 | 7.35 | 20.51 | 0.48 | 0.03 | 12.05 | <0.001 | 0.55 | ND | 7.40 | 0.007 |
| 39636 | B8 | 2,090 | 70 | 7.35 | 9.10 | 0.40 | 0.05 | 5.36 | 0.023 | 0.27 | 0.002 | 3.02 | 0.012 |
| 4642 | B8 | 2,090 | 71 | 7.55 | 8.77 | 0.34 | 0.06 | 5.02 | 0.018 | 0.43 | 0.005 | 2.92 | 0 |
| 4640b | B8 | 2,150 | 71 | 7.18 | 6.71 | 0.20 | 0.02 | 3.90 | 0.028 | 0.23 | 0.002 | 2.34 | 0.01 |
| 10318 | B8 | 2,132 | 71 | 7.40 | 5.66 | 0.18 | 0.02 | 3.24 | 0.023 | 0.27 | 0.002 | 1.95 | 0 |
| 6151 | B8 | 2,220 | 72 | 7.00 | 18.11 | 0.34 | 0.25 | 9.83 | 0.008 | 1.59 | 0.005 | 6.10 | 0.007 |
| 706b | B10 | 2,300 | 72 | 7.00 | 39.78 | 0.80 | 0.06 | 23.43 | 0 | 0.97 | ND | 14.52 | ND |
| 714 | J | 2,299 | 84 | 6.80 | 30.50 | 1.04 | 0.25 | 17.87 | 0.011 | 1.04 | 0.070 | 10.30 | 0.462 |
| 735b | J | 2,287 | 84 | 7.70 | 14.32 | 0.24 | 0.07 | 8.09 | 0 | 0.71 | 0.07 | 5.14 | 0.30 |
| 757 | J | 2,384 | 84 | 7.25 | 32.85 | 1.00 | 0.32 | 19.32 | 0.014 | 1.04 | 0.074 | 11.17 | 0.544 |
| 767 | J | 2,427 | 85 | 5.95 | 26.66 | 1.00 | 0.39 | 15.97 | 0.012 | 0.60 | 0.003 | 8.70 | 0 |
| 739 | J | 2,350 | 84 | 7.30 | 24.80 | 0.781 | 0 | 14.89 | 0 | 0.27 | ND | 8.86 | ND |
| 12597 | J | 2,350 | 84 | 7.30 | 52.31 | 0.80 | 0 | 31.02 | <0.001 | 0.93 | ND | 19.55 | ND |
ND, not determined.
Rates of anaerobic microbial activities.
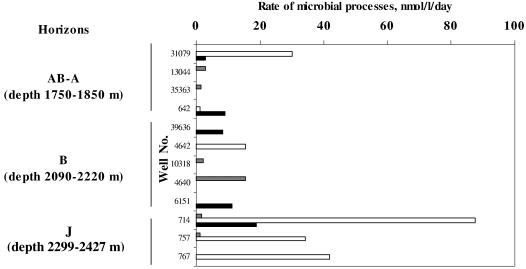
The processes of sulfate reduction and lithotrophic and/or acetoclastic methanogenesis were detected by radioisotopic methods in all horizons studied (Fig. 1). The rate of sulfate reduction, both at 60 and 80°C, was low, not exceeding 20 nmol of H2S liter−1 day−1. In the upper (AB and B) horizons, methanogenesis occurred mostly in the wells with a low sulfate concentration, where the process of sulfate reduction was not observed (Table 2 and Fig. 1). In wells of the Jurassic horizon, high-temperature sulfate reduction and methanogenesis occurred simultaneously. In these wells, the rate of lithotrophic methanogenesis exceeded 80 nmol of CH4 liter−1 day−1. High-temperature acetoclastic methanogenesis was detected in well 714, its rate being comparable with that of the lithotrophic process (18.5 nmol of CH4 liter−1 day−1).
FIG. 1.
Rates of sulfate reduction (shaded bars) and lithotrophic (open bars) and acetoclastic (solid bars) methanogenesis in water samples of the Samotlor oil reservoir measured at 60°C (for horizons AB, A, and B) and 80°C (for horizon J).
Enrichment and identification of thermophilic isolates.
Application of cultivation methods to samples of injection and formation waters from oil-bearing horizons of the Samotlor oil reservoir revealed the presence of various groups of thermophilic prokaryotes (Table 3). Some of the successful enrichments were used to isolate the dominant culturable organisms. Surprisingly, most of the identified isolates represented new species.
TABLE 3.
Enrichment of thermophilic microorganisms from Samotlor oil well samples
| Sample | Growth ofa:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aerobic organotrophs (60°C) | Hydrocarbon oxidizers (60°C) | Denitrifiers (60°C) | Anaerobic organotrophs
|
Sulfate reducers growing (60°C) on
|
Methanogens growing (60°C) on
|
|||||
| 60°C | 70°C | 85°C | Lactate | Acetate | H2+CO2 | Acetate | ||||
| Injection water | + | + | + | + | ND | ND | + | + | − | − |
| 692 | − | − | − | + | + | + | − | − | − | − |
| 13044 | − | − | − | + | + | + | − | − | + | − |
| 31079 | + | − | − | + | ND | ND | − | − | + | − |
| 35363 | + | + | − | + | ND | ND | − | − | − | − |
| 39636 | + | + | − | + | + | + | + | + | + | + |
| 10149 | − | − | − | + | ND | ND | − | − | − | − |
| 4640b | + | − | − | + | ND | ND | − | − | − | − |
| 4642 | + | − | − | + | ND | ND | + | − | − | − |
| 10318 | + | − | − | + | ND | ND | − | − | − | − |
| 6151 | − | − | − | + | ND | + | − | − | − | − |
| 714 | − | − | − | + | ND | − | − | − | + | − |
| 735b | − | − | − | + | + | − | − | − | + | − |
| 739 | − | − | − | + | + | + | − | − | − | − |
| 757 | − | − | − | + | ND | + | − | − | − | − |
| 767 | − | − | − | + | ND | ND | − | − | − | − |
| 769 | − | − | − | + | ND | ND | − | − | + | − |
+, growth; −, no growth; ND, not determined.
(i) Aerobic organotrophs.
Aerobic organotrophs, including hydrocarbon utilizers, were enriched at 60°C from injection water and formation waters from upper horizons. No aerobic organotrophic cultures were obtained either at higher temperatures or from Jurassic horizons. Positive enrichments consisted exclusively of spore-forming rod-shaped organisms. One of the isolates, strain Sam, exhibited a close phylogenetic relatedness to thermophilic bacilli of group 5 (4, 35, 43). This motile neutrophilic organism grew aerobically, utilizing crude oil, hydrocarbons, carbohydrates, lower fatty acids, and alcohols. Anaerobic growth with acetate and nitrate accompanied by formation of N2 was also observed. Due to its unique 16S rDNA sequence and fatty acid composition, strain Sam was described as a new species of the genus Geobacillus (35).
(ii) Nitrate reducers.
Nitrate reducers were enriched only at 60°C from injection water. Positive cultures consisted of spore-forming bacilli. No attempts were made to isolate representatives of the denitrifying populations. However, since they were mainly composed of spore-forming bacilli, we can reasonably hypothesize that at least some of them represent the denitrifying members of the genus Geobacillus.
(iii) Anaerobic organotrophs.
Anaerobic organotrophs proved to be the most widespread group, detected in virtually all samples studied. They included saccharolytic and peptolytic rod-shape or coccoid organisms, among them moderate and extreme thermophiles and hyperthermophiles. At 60 and 70°C, only rod-shaped organisms differing in cell size, motility, and the presence of sheaths were detected both on starch and peptone in the presence and absence of electron acceptors (sulfur and thiosulfate). Anaerobic organotrophic hyperthermophiles growing at 85°C on peptone with sulfur or thiosulfate were found in six samples of eight tested. Morphologically, they were regular and irregular cocci, except for sample 12597, in which cocci were mixed with small sheathed rods.
(a) Thermotogales.
Several strains of extremely thermophilic sheathed rod-shaped organotrophic anaerobes were isolated and identified. Cells of strain M12597 were small rods (0.2 by 3 to 0.2 by 4 μm), enriched in starch-thiosulfate medium at 85°C. Analysis of partial 16S rDNA sequence (500 nucleotides) showed 100% similarity to Thermotoga maritima. Strains SL30, SL31, and 39636, the cells of which were short, thick rods, were enriched in peptone-yeast extract medium at 70°C. Their partial 16S rDNA sequence (500 nucleotides) indicated that they belonged to the same species and exhibited the highest levels of similarity to members of the genus Thermosipho (96.4% with T. africanus and 96.3% with T. japonicus). These isolates were further shown to represent a new species of the genus Thermosipho (24).
Five motile, strictly anaerobic thermophilic bacteria designated SL24, SL25, SL27, SL29, and SL32 were enriched from peptone-yeast extract medium at 60°C. Based upon partial 16S rDNA sequences (500 nucleotides), strains SL25, SL27, SL29, and SL32 were identical. Strains SL24 and SL25 were phylogenetically most closely related to Petrotoga miotherma (99.4 and 98.9% similarity, respectively). However, several phenotypic properties and DNA-DNA hybridization experiments indicated that both of the strains SL24 and SL25 merit the rank of a new species (25).
(b) Thermoanaerobacter spp.
Two nonmotile rod-shape unsheathed strains, designated SL26 and SL28, were enriched in peptone-yeast extract medium at 70°C. Their partial 16S rDNA sequences (500 nucleotides) were identical and shared 100% similarity with Thermoanaerobacter acetoethylicus. Strain M739 possessed unsheathed rod-shape motile cells with terminal spores. It grew at 70°C on starch medium, either supplemented with sulfur or thiosulfate or without sulfur compounds. The partial 16S rDNA sequence of strain M739 also placed it in the genus Thermoanaerobacter. It exhibited 98.3% similarity to Thermoanaerobacter brockii subsp. brockii and T. brockii subsp. finnii.
(c) Thermococcus spp.
Three strains (MM739, MM39636, and MM642) were isolated from cultures obtained in peptone-sulfur medium at 85°C. Analysis of partial sequences (500 nucleotides) of their 16S rDNA revealed that they were 100% similar and belonged to the archaeal genus Thermococcus. The complete 16S rRNA sequence of isolate MM739 indicated that its closest relatives were Thermococcus litoralis, T. aggregans, T. fumicolans, and T. alcaliphilus (range of sequence similarity, 97.2 to 98.8%). This isolate was further fully characterized and was shown to represent a new species named T. sibiricus (30).
(iv) Sulfate reducers.
Spore-forming rods resembling Desulfotomaculum were enriched at 60°C only from the injection water and from two samples of formation water. No sulfate reducers were obtained at higher temperatures.
(v) Methanogens.
Lithotrophic rod-shape to filamentous cells that fluoresced under UV light and morphologically resembled members of Methanobacteriales were obtained from several Cretaceous and Jurassic samples at 60°C. Enrichments of acetate-utilizing methanogens were obtained only from sample 39636 and were composed of thin curved rods. No methanogens were enriched at 80°C.
Microchip investigation.
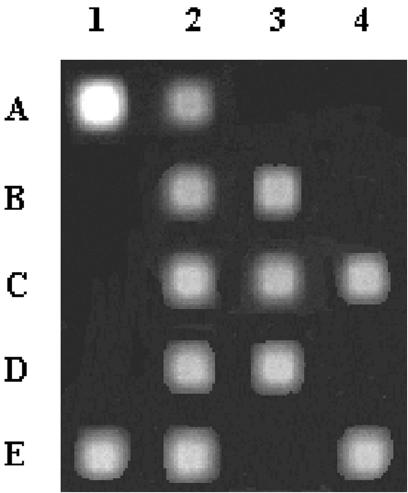
Specificity of oligonucleotide probes was tested by hybridization with in vitro transcripts of 16S rDNA genes of reference strains. The 17 probes that gave strong signals with the target strains and no signal with other reference strains (Table 1) were considered reliable and were used in further analyses. Oligonucleotide microchips were then applied to samples of injection water and of Cretaceous and Jurassic horizons for comparative evaluation of their microbial composition. These analyses confirmed generally the presence of the organisms identified or detected by culture-based methods (Table 4; Fig. 2). However, although members of the genus Geobacillus were isolated only from injection water, their specific probe gave a positive signal only with sample 757 from Jurassic horizon. Interestingly, organisms that had not been identified among the isolated strains were also detected. Thus, positive signals were recorded when samples were hybridized with probes targeting the genera Desulfurococcus, Thermus, Geotoga, Thermodesulfobacterium, and the Aquificales/Desulfurobacterium-Thermovibrio cluster. Mesophilic Methanobacteriales spp. targeted with probe 4 were not detected.
TABLE 4.
Microorganisms detected in Samotlor water samples by the microchip method
| Targeted organism(s) | No. of probes hybridizing with DNA of sample
|
Identification by culture-based methodsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13044 | 757 | Injection water | ||||||||
| Thermococcus + Pyrococcus | 6, 30 | 6, 30 | —b | + | ||||||
| Desulfurococcus | 44 | — | — | − | ||||||
| Methanobacterium + Methanothermobacter | 5, 58 | 5, 58 | 5, 58 | +c | ||||||
| Thermotoga + Thermosipho | 54 | 54 | 54 | + | ||||||
| Geotoga | — | 21 | — | − | ||||||
| Petrotoga | 25, 27 | — | — | + | ||||||
| Thermoanaerobacter | 50 | 50 | 50 | + | ||||||
| Desulfotomaculum I | 46 | 46 | 46 | +c | ||||||
| Desulfotomaculum II | 48 | 48 | — | +c | ||||||
| Thermodesulfobacterium | 39 | — | — | − | ||||||
| Aquificales + Desulfurobacterium + Thermovibrio | 55 | 55 | 55 | −d | ||||||
| Geobacillus | — | 53 | — | + | ||||||
| Thermus | — | 52 | 52 | − | ||||||
+, identification successful; −, identification failed.
—, hybridization at background level.
Morphotypes similar to known representatives of these genera were observed in enrichment cultures.
No cultivation attempts were made for these organisms.
FIG. 2.
Hybridization of Texas red-labeled 16S rRNAs to the microchip. The microchip with immobilized probes was hybridized to in vitro-transcribed 16S rRNAs obtained from formation water of well 757. Specific oligonucleotide probes were loaded on the microchip as follows: A1, probe 54 (Thermotoga and Thermosipho); A2, probe 21 (Geotoga); A3 and A4, probes 25 and 27, respectively (Petrotoga); B1, probe 4 (Methanobacterium); B2, probe 5 (Methanobacterium); B3, probe 58 (Methanobacterium plus Methanothermobacter); C1, probe 39 (Thermodesulfobacterium); C2, probe 50 (Thermoanaerobacter); C3, probe 6 (Thermococcus and Pyrococcus); C4, probe 30 (Thermococcus); D1, probe 44 (Desulfurococcus); D2 and D3, probes 46 and 48, respectively (Desulfotomaculum); E1, probe 52 (Thermus); E2; probe 55 (Aquificales and Desulfurobacterium-Thermovibrio) and E4, probe 53 (Geobacillus). B4, D4, and E3 were empty gel elements.
DISCUSSION
The different approaches used to investigate the Samotlor reservoir microbial ecosystem result in different data sets, which have to be cross-checked for a valid description of the populations within their natural habitat. Thermophilic methanogenesis and sulfate reduction were detected by radioisotopic experiments in formation waters of all horizons studied. The rates of these microbial processes were in accordance with the data obtained previously in Mykhpay and Talinskoe high-temperature oil fields, also located in Western Siberia (32, 47), and in two high-temperature oil reservoirs in China (34). Low rates of sulfate reduction are consistent with the low concentrations of sulfates contained in the formation waters. For comparison, in the sulfate-rich Romashkino oil reservoir, they exceeded 5 μmol of H2S liter−1 day−1 (33). In the upper horizons of the Samotlor oil reservoir, sulfate concentration was inversely related to the mineralization of water (Table 2). The extraction of sulfates from the oil-bearing rocks by the injected river water was suggested to explain this relationship (46). In the upper horizons, methanogenesis was active at a low sulfate reduction rate, indicating a competition between the two processes for energy substrates. In the nonflooded Jurassic horizons, rates of sulfate reduction and methanogenesis were not related to either the concentration of sulfate or the rate of the competing process. At moderate temperature (60°C), sulfate reduction and lithotrophic methanogenesis were well supported by cultivation and molecular data. Such reliability was not attained for high-temperature Jurassic horizons, although the presence of microorganisms (Archaeoglobus spp. and Methanothermococcus spp.) capable of these reactions in high-temperature terrestrial and marine oil reservoirs has been well documented (6, 22, 50, 59). In our experiments, methane formation from acetate occurred at 60°C, although the only enrichment obtained at this temperature could not be maintained. It is possible that thermophilic methane production from acetate was the result of interspecies hydrogen transfer, as was previously shown for thermophilic assemblages associated with other Western Siberia oil wells (14).
Anaerobic organotrophs are widespread in high-temperature petroleum reservoirs (27, 38, 54). It is therefore not surprising that they were enriched from all of the Samotlor samples. These phylogenetically diverse microorganisms were represented mainly by sheathed bacteria (Thermotogales) and irregular to regular cocci (Thermococcales), which dominated at 60 and 85°C, respectively. It has been proposed that the wide distribution of Thermotogales and Thermococcales in oil reservoirs could be related to their ability to survive under starvation conditions (low concentrations of organic substrates) (54). We previously showed that seven of the eight reservoirs' heterotrophic isolates (T. maritima strain M12597; Thermoanaerobacter acetoethylicus strains SL26 and SL28; T. brockii strain M739; and Thermococcus sp. strains MM739, MM39636, and MM642) were capable of lithotrophic growth with molecular hydrogen and ferric iron (49). This provided further evidence of the importance of the iron cycle in deep reservoirs, but it might also suggest that the capacity for lithotrophic iron reduction is a possible survival strategy for anaerobic organotrophs in formation waters with low concentrations of organic matter.
Except for species of the genus Petrotoga, which have been isolated up to now only from oil-bearing formations (13), the thermophilic isolates identified in this study belong to genera that are not restricted to this peculiar environment. Indeed, although they have been previously isolated and/or detected in high-temperature petroleum systems worldwide, species of Thermococcus, Thermotoga, Thermosipho, Thermoanaerobacter, and Geobacillus are also known to thrive in marine and/or terrestrial geothermally heated areas (26, 35). Interestingly, most of the strains isolated from the Samotlor reservoir represented new species. This indicates that despite recent culture isolation efforts (27), our perception of the microbial diversity of petroleum reservoir remains rudimentary.
In order to confirm the results obtained with culture-based methods and investigate more deeply the microbial diversity of the Samotlor oil reservoir, microchip analysis was applied to some samples. Several observations demonstrate that the results of microchip analyses deserve confidence: first, the microchip analysis confirmed the presence of all of the genera retrieved by cultivation; second, identical results were obtained when two different probes were used to target the same organisms. However, an unexpected mismatch between the cultivation and molecular data sets was found. It remains unclear why geobacilli were detected in the Jurassic sample but not in the injection water from which they were isolated. Because they were also cultivated from 6 of the 10 Cretaceous formation water samples, it is plausible that these organisms reside in discrete microhabitats within the reservoir. Application of the molecular method also revealed the presence of organisms common to oil reservoirs (i.e., Thermodesulfobacterium and Geotoga) (12, 27) that had nevertheless escaped our cultivation and isolation efforts. However, the culture conditions used were not optimal for the growth of these organisms. Indeed, no hydrogen was provided to promote the growth of hydrogen-dependent sulfate reducers, and the lowest incubation temperature was higher than or corresponded to the maximum temperature for growth of Geotoga species. Unexpectedly, organisms affiliated with the genus Desulfurococcus were detected in sample 13044, organisms belonging to the genus Thermus were detected in samples 757 and injection water, and members of Aquificales/Desulfurobacterium-Thermovibrio cluster were found in all three of the samples studied. Although specific media used to enrich for aerobic organotrophs and heterotrophic sulfur reducers could have allowed the growth of Thermus and Desulfurococcus species, representatives of these genera were not isolated. Since Thermus and Desulfurococcus species share common physiological and nutritional characteristics with, respectively, the fast-growing species of Geobacillus (aerobes often able to grow anaerobically with nitrate as terminal electron acceptor) and Thermococcus (sulfur-dependent anaerobes), we suggest that they could have been overgrown in our cultures or that they are not dominant components of the subsurface community. Members of Aquificales and species of the genera Desulfurobacterium and Thermovibrio are, respectively, thermophilic microaerophilic chemolithoautotrophs that use molecular hydrogen and strictly anaerobic chemolithoautotrophs that reduce sulfur and nitrogen compounds (19, 23, 45). These organisms were not obtained in laboratory cultures, probably because of inadequacy of our culture media. With the exception of Thermus, some strains of which have been isolated from anthropogenic environments (11), the known habitats of the bacteria mentioned above were, up to now, restricted to natural hydrothermal areas such as deep sea hydrothermal vents and terrestrial hot springs (19, 23, 26, 45). Their presence in an oil reservoir environment extends the known ecological habitat of these groups of organisms. Further work, including direct probing of rRNA and selective isolation of viable cultures, is needed to further investigate whether these organisms play a significant role in high-temperature oil reservoirs.
Our cross-evaluation of biogeochemical data, culture-dependent techniques, and microchip analysis in the investigation of a high-temperature reservoir confirms that most of the thermophilic organisms in oil reservoirs are metabolically adapted to active participation in the energy and carbon cycles, as previously suggested by Orphan et al. (38). However, isolation of a number of novel species and molecular detection of several groups new for the oil fields demonstrate that our current knowledge of the microbial diversity in this habitat is still insufficient to draw an overall picture. Additional molecular, culture-based, and geochemical analyses are still necessary in order to understand the distribution, function, interactions, and ecological significance of deep subsurface bacteria.
It is noteworthy that there are significant similarities between the thermophilic population described here and those that were found in marine hydrothermal areas. The Samotlor oil field, as well as all oil fields of the Middle Ob region, occurs in an area that, in the late Jurassic and early Cretaceous, was intermediate between a vast alluvial deltaic plain and a marine basin where prodelta deposits accumulated extensively (10). The existence of thermophiles, including marine organisms, in this deep location, thousands of kilometers from all volcanic areas and hundreds of kilometers from the nearest ocean, suggests that they were deposited with the original sediment and survived over geological time.
Acknowledgments
This work was supported by INTAS (grant 96-1341), the Russian Ministry of Industry, Science and Technology (project “New Hyperthermophiles”), and the Program “Biodiversity” of the Russian Federation.
Footnotes
This paper is dedicated to the memory of A. D. Mirzabekov.
REFERENCES
- 1.Adkins, J. P., L. A. Cornell, and R. S. Tanner. 1992. Microbial composition of carbonate petroleum reservoir fluids. Geomicrobiol. J. 10:87-97. [Google Scholar]
- 2.Alm, E. W., D. B. Oerther, N. Larsen, D. A. Stahl, and L. Raskin. 1996. The Oligonucleotide Probe Database. Appl. Environ. Microbiol. 62:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PCI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash, C., A. E. Farrow, S. Wallbanks, and M. D. Collins. 1991. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett. Appl. Microbiol. 13:202-206. [Google Scholar]
- 5.Barns, S. M., R. E. Fundyga, M. W. Jeffries, and N. R. Pace. 1994. Remarkable archaeal diversity in a Yellowstone National Park hot spring environment. Proc. Natl. Acad. Sci. USA 91:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeder, J., R. K. Nilsen, J. T. Rosnes, T. Torsvik, and T. Lien. 1994. Archaeoglobus fulgidus isolated from hot North Sea oil field waters. Appl. Environ. Microbiol. 60:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeder, J., T. Torsvik, and T. Lien. 1995. Thermodesulforhabdus norvegicus gen. nov., sp. nov., a novel thermophilic sulfate-reducing bacterium from oil field water. Arch. Microbiol. 164:331-336. [PubMed] [Google Scholar]
- 8.Belyaev, S. S., and M. V. Ivanov. 1975. Radioisotopic method for determination of the intensity of bacterial methanogenesis. Mikrobiologiya 44:166-168.125843 [Google Scholar]
- 9.Christensen, B., T. Torsvik, and T. Lien. 1992. Immunomagnetically captured thermophilic sulfate-reducing bacteria from North Sea oil field waters. Appl. Environ. Microbiol. 58:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, J. W., O. W. Girard, Jr., J. Peterson, and J. Rachlin. 1978. Petroleum geology of Western Siberian basin and Samotlor oil field. Oil Gas J. 8:311-328. [Google Scholar]
- 11.Da Costa, M. S., M. Fernanda Nobre, and F. A. Rainey. 2001. Genus I. Thermus, p. 404-414. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer Verlag, New York, N.Y.
- 12.Davey, M. E., B. J. MacGregor, and D. A. Stahl. 2001. Genus III. Geotoga, p. 377-381. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer Verlag, New York, N.Y.
- 13.Davey, M. E., B. J. MacGregor, and D. A. Stahl. 2001. Genus IV. Petrotoga, p. 382-385. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer Verlag, New York, N.Y.
- 14.Davydova-Charakhchjan, I. A., V. G. Kuznetsova, L. L. Mityushina, and S. S. Belyaev. 1992. Methanogenic bacilli from the oil reservoirs of Tataria and Western Siberia. Mikrobiologiya 61:202-208. [Google Scholar]
- 15.Drobyshev, A., N. Mologina, V. Shik, D. Pobedimskaya, G. Yershov, and A. Mirzabekov. 1997. Sequence analysis by hybridization with oligonucleotide microchip: identification of beta-thalassemia mutations. Gene 25:45-52. [DOI] [PubMed] [Google Scholar]
- 16.Grassia, G. C., K. M. McLean, P. Glénat, J. Bauld, and A. Sheehy. 1996. A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol. Ecol. 21:47-58. [Google Scholar]
- 17.Guschin, D. Y., B. K. Mobarry, D. Proudnikov, D. A. Stahl, B. E. Rittmann, and A. D. Mirzabekov. 1997. Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Appl. Environ. Microbiol. 63:2397-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmsen, H. J. M., D. Prieur, and C. Jeanthon. 1997. Group-specific 16S rRNA-targeted oligonucleotide probes to identify thermophilic bacteria in marine hydrothermal vents. Appl. Environ. Microbiol. 63:4061-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber, H., S. Diller, C. Horn, and R. Rachel. 2002. Thermovibrio ruber gen. nov., sp. nov., an extremely thermophilic, chemolithoautotrophic, nitrate-reducing bacterium that forms a deep branch within the phylum Aquificae. Int. J. Syst. Evol. Microbiol. 52:1859-1865. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov, M. V., and L. S. Terebkova. 1959. Microbial processes of H2S formation in Lake Solenoe. Mikrobiologiya 28:251-256. [Google Scholar]
- 21.Leu, J. Y., C. P. McGovern-Traa, A. J. R. Porter, W. J. Harris, and W. A. Hamilton. 1998. Identification and phylogenetic analysis of thermophilic sulfate-reducing bacteria in oil field samples by 16S rDNA gene cloning and sequencing. Anaerobe 4:165-174. [DOI] [PubMed] [Google Scholar]
- 22.L'Haridon, S., A.-L. Reysenbach, P. Glénat, D. Prieur, and C. Jeanthon. 1995. Hot subterranean biosphere in a continental oil reservoir. Nature 337:223-224. [PubMed] [Google Scholar]
- 23.L'Haridon, S., V. Cilia, P. Messner, G. Raguénès, A. Gambacorta, U. W. Sleytr, D. Prieur, and C. Jeanthon. 1998. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulfur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 48:701-711. [DOI] [PubMed] [Google Scholar]
- 24.L'Haridon, S., M. L. Miroshnichenko, H. Hippe, M.-L. Fardeau, E. A. Bonch-Osmolovskaya, E. Stackebrandt, and C. Jeanthon. 2001. Thermosipho geolei sp. nov., a thermophilic bacterium isolated from a continental petroleum reservoir of Western Siberia. Int. J. Syst. Evol. Microbiol. 51:1327-1334. [DOI] [PubMed] [Google Scholar]
- 25.L'Haridon, S., M. L. Miroshnichenko, H. Hippe, M.-L. Fardeau, E. Bonch-Osmolovskaya, E. Stackebrandt, and C. Jeanthon. 2002. Petrotoga olearia and Petrotoga siberica sp. nov., two thermophilic bacteria isolated from a continental petroleum reservoir in Western Siberia. Int. J. Syst. Evol. Microbiol. 52:1715-1722. [DOI] [PubMed] [Google Scholar]
- 26.Lowe, S. E., M. K. Jain, and J. G. Zeikus. 1993. Biology, ecology, and biotechnological applications of anaerobic bacteria adapted to environmental stresses in temperature, pH, or substrates. Microbiol. Rev. 57:451-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magot, M., B. Ollivier, and B. K. C. Patel. 2000. Microbiology of petroleum reservoirs. Antonie Leeuwenhoek 77:103-116. [DOI] [PubMed] [Google Scholar]
- 28.Maidak, B. L., G. J. Olsen, N. Larsen, N. Overbeek, M. J. McCaughey, and C. R. Woese. 1997. The RDP (Ribosomal Database Project). Nucleic Acids Res. 25:109-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miroshnichenko, M. L., G. M. Gongadze, F. A. Rainey, A. S. Kostukova, A. M. Lysenko, N. A. Chernyh, and E. A. Bonch-Osmolovskaya. 1998. Thermococcus gorgonarius sp. nov. and Thermococcus pacificus sp. nov.: heterotrophic extremely thermophilic archaea from New Zealand submarine hot vents. Int. J. Syst. Bacteriol. 48:23-29. [DOI] [PubMed] [Google Scholar]
- 30.Miroshnichenko, M. L., H. Hippe, E. Stackebrandt, N. A. Kostrikina, N. A. Chernyh, C. Jeanthon, T. N. Nazina, S. S. Belyaev, and E. A. Bonch-Osmolovskaya. 2001. Isolation and characterization of Thermococcus sibiricus sp. nov. from a Western Siberia high temperature oil reservoir. Extremophiles 5:85-91. [DOI] [PubMed] [Google Scholar]
- 31.Nazina, T. N., E. P. Rozanova, and S. I. Kuznetsov. 1985. Microbial oil transformation processes accompanied by methane and hydrogen-sulfide formation. Geomicrobiol. J. 4:103-130. [Google Scholar]
- 32.Nazina, T. N., A. E. Ivanova, I. A. Borzenkov, S. S. Belyaev, and M. V. Ivanov. 1995. Occurrence and geochemical activity of microorganisms in high-temperature, water-flooded oil fields of Kazakhstan and Western Siberia. Geomicrobiol. J. 13:181-192. [Google Scholar]
- 33.Nazina, T. N., A. E. Ivanova, G. F. Kandaurova, R. R. Ibatullin, S. S. Belyaev, and M. V. Ivanov. 1998. Microbiological investigation of the carbonate collector of the Romashkinskoe oil field: background study before testing a biotechnology for the enhancement of oil recovery. Mikrobiologiya 67:701-709. [Google Scholar]
- 34.Nazina, T. N., Y.-F. Xue, X.-Y. Wang, S. S. Belyaev, and M. V. Ivanov. 2000. Microorganisms of the high-temperature Liaohe oil field of China and their potential for MEOR. Resour. Environ. Biotechnol. 3:109-120. [Google Scholar]
- 35.Nazina, T. N., T. P. Tourova, A. B. Poltaraus, E. V. Novikova, A. A. Grigoriyan, A. E. Ivanova, V. V. Petrunyaka, G. A. Osipov, S. S. Belyaev, and M. V. Ivanov. 2001. Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermooleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 51:433-446. [DOI] [PubMed] [Google Scholar]
- 36.Nilsen, R. K., and T. Torsvik. 1996. Methanococcus thermolithotrophicus isolated from North Sea oil field reservoir water. Appl. Environ. Microbiol. 62:728-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsen, R. K., T. Torsvik, and T. Lien. 1996. Desulfotomaculum thermocisternum sp. nov., a sulfate reducer isolated from a hot North Sea oil reservoir. Int. J. Syst. Bacteriol. 46:397-402. [Google Scholar]
- 38.Orphan, V. J., L. T. Taylor, D. Hafenbradl, and E. F. Delong. 2000. Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl. Environ. Microbiol. 66:700-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkes, R. J., B. A. Cragg, S. J. Bale, J. M. Getliff, K. Goodman, P. A. Rochelle, J. C. Fry, A. J. Weightman, and S. J. Harvey. 1994. Deep bacterial biosphere in Pacific Ocean sediments. Nature 371:410-413. [Google Scholar]
- 40.Postgate, J. R. 1984. The sulfate-reducing bacteria, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 41.Proudnikov, D., and A. D. Mirzabekov. 1996. Chemical method of DNA and RNA fluorescent labeling. Nucleic Acids Res. 24:4535-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 43.Rainey, F. A., D. Fritze, and E. Stackebrandt. 1994. The phylogenetic diversity of thermophilic members of the genus Bacillus as revealed by 16S rDNA analysis. FEMS Microbiol. Lett. 115:205-212. [DOI] [PubMed] [Google Scholar]
- 44.Rees, G. N., G. S. Grassia, A. J. Sheehy, P. P. Dwivedi, and B. K. C. Patel. 1995. Desulfacinum infernum gen. nov., sp. nov., a thermophilic sulfate-reducing bacterium from a petroleum reservoir. Int. J. Syst. Bacteriol. 45:85-89. [Google Scholar]
- 45.Reysenbach, A.-L. 2001. Order I Aquificales ord. nov., p. 359. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer Verlag, New York, N.Y.
- 46.Rozanova, E. P., V. N. Bykov, A. L. Baldina, and T. A. Kosogorova. 1976. Biogenous elements and sulfate reduction in watered oil carbonate layer. Mikrobiologiya 45:318-322. [PubMed] [Google Scholar]
- 47.Rozanova, E. P., A. S. Savvichev, Y. M. Miller, and M. V. Ivanov. 1997. Microbial processes in a West Siberian oil field flooded with waters containing a complex of organic compounds. Mikrobiologiya 66:852-859. [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Slobodkin, A. I., C. Jeanthon, S. L'Haridon, T. N. Nazina, M. L. Miroshnichenko, and E. A. Bonch-Osmolovskaya. 1999. Dissimilatory reduction of Fe(III) by thermophilic bacteria and archaea in deep subsurface petroleum reservoirs of Western Siberia. Curr. Microbiol. 39:99-102. [DOI] [PubMed] [Google Scholar]
- 50.Stetter, K. O., R. Huber, E. Blöchl, M. Kurr, R. D. Eden, M. Fielder, H. Cash, and I. Vance. 1993. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 365:743-745. [Google Scholar]
- 51.Stevens, T. O., and J. P. McKinley. 1995. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270:450-454. [Google Scholar]
- 52.Subbotina, I. V., N. A. Chernyh, I. V. Kublanov, T. G. Sokolova, E. A. Bonch-Osmolovskaya, and A. V. Lebedinsky. 2003. Oligonucleotide probes for the detection of representatives of the genus Thermoanaerobacter. Mikrobiologiya 72:374-382. [PubMed] [Google Scholar]
- 53.Szewzyk, U., R. Szewzyk, and T. A. Stenström. 1994. Thermophilic, anaerobic bacteria isolated from a deep borehole in granite in Sweden. Proc. Natl. Acad. Sci. USA 91:1810-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahata, Y., M. Nishijima, T. Hoaki, and T. Maruyama. 2000. Distribution and physiological characteristics of hyperthermophiles in the Kubiki oil reservoir in Niigata, Japan. Appl. Environ. Microbiol. 66:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trüper, H. G., and H. G. Schlegel. 1964. Sulfur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Leeuwenhoek 30:321-323. [DOI] [PubMed] [Google Scholar]
- 56.Voordouw, G., S. M. Armstrong, M. F. Reimer, B. Fouts, A. J. Telang, Y. Shen, and D. Gevertz. 1996. Characterization of 16S rRNA genes from oil field microbial communities indicates the presence of a variety of sulfate-reducing, fermentative, and sulfide-oxidizing bacteria. Appl. Environ. Microbiol. 62:1623-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voordouw, G., V. Niviere, F. G. Ferris, P. M. Fedorak, and D. W. S. Westlake. 1990. Distribution of hydrogenase genes in Desulfovibrio spp. and their use in the identification of species from the oil field environment. Appl. Environ. Microbiol. 56:3748-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voordouw, G., J. K. Voordouw, T. R. Jack, J. Foght, P. M. Fedorak, and D. W. S. Westlake. 1992. Identification of distinct communities of sulfate-reducing bacteria in oil fields by reverse sample genome probing. Appl. Environ. Microbiol. 58:3542-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitman, W. B., and C. Jeanthon. 2002. Methanococcales. In M. Dworkin (ed.), The prokaryotes, an electronic resource for the microbial community. [Online.] Springer-Verlag, New York, N.Y. http://link.springer.de.
- 60.Yershov, G., V. Barsky, A. Belgovsky, E. Kirillov, E. Kreindlin, I. Ivanov, S. Parinov, D. Guschin, A. Drobishev, S. Dubiley, and A. Mirzabekov. 1996. DNA analysis and diagnostics on oligonucleotide microchips. Proc. Natl. Acad. Sci. USA 93:4913-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeikus, J. G., P. J. Weimer, D. R. Nelson, and L. Daniels. 1975. Bacterial methanogenesis: acetate as a methane precursor in pure culture. Arch. Microbiol. 104:129-134. [Google Scholar]