Abstract
A multiplex real-time PCR method to simultaneously detect the stx1 and stx2 genes of Shiga toxin-producing Escherichia coli and a unique conserved single-nucleotide polymorphism in the E. coli O157:H7/H− uidA gene has been developed. There is more than 98.6% sensitivity and 100% specificity for all three gene targets based on a panel of 138 isolates. The PCR efficiencies were ≥1.89, and as few as 6 CFU/reaction could be detected.
Shiga toxin-producing Escherichia coli (STEC) strains are recognized as an important group of enteric pathogens capable of causing serious illnesses and death (15, 18, 20, 26). More than 100 E. coli serotypes may produce Shiga toxins (24). Several sporadic cases, large outbreaks, and illnesses worldwide have been associated with STEC organisms, primarily the E. coli O157:H7 serotype, making this an important class of food-borne pathogens (1, 7, 8, 9, 21, 22, 30, 33, 34, 35).
The Shiga toxins produced by STEC are generally considered the principal virulence characteristic responsible for serious illnesses associated with this organism. Therefore, the presence of these cytotoxins or their genes (stx1 and stx2) is the focus of many assays for STEC organisms. Due to the predominance of the O157:H7 STEC serotype associated with human illness, many methods also focus on the detection and identification of this serogroup. A highly conserved point mutation at position 93 of the uidA (β-glucuronidase) gene occurs in O157:H7 and nonmotile O157 strains, including atypical O157:H− clones implicated in German hemolytic-uremic syndrome outbreaks (11, 12, 13, 23). The detection of the stx1 and stx2 genes and the single-nucleotide polymorphism (SNP) at position 93 are the basis of a multiplex mismatch amplification mutation assay (6) and oligonucleotide probe hybridization tests (10).
Real-time PCR applications offer the advantages of being more sensitive and rapid by not requiring post-PCR procedures to detect amplification products used in conventional PCR-based procedures. Recent advances using minor-groove binder (MGB) modifications to significantly increase duplex stability have improved SNP detection in real-time PCR applications (19). The goal of this project was to develop an STEC multiplex real-time PCR method that can specifically detect stx1 and stx2 genes with a multiplex 5′ nuclease approach and simultaneously detect the presence of E. coli O157:H7/H− strains based on a unique and conserved SNP in the uidA gene by using a 3′ MGB probe. The real-time PCR MGB probe approach used here combines the advantages of a rapid PCR process similar to the mismatch amplification mutation assay without reducing PCR efficiency and the ability to optimize stringent hybridization requirements for SNP detection in one assay.
Primer pairs and internal fluorescent probes were selected and designed with Primer Express (Applied Biosystems, Foster City, Calif.) (Table 1) based on sequences submitted to GenBank. Sequence comparison and lineups were generated with the GCG program (Wisconsin Package, version 10.3; Accelrys Inc., San Diego, Calif.). Primers were synthesized by standard methods (IDT, Coralville, Iowa, and Sigma-Genosys, The Woodlands, Tex.). The stx1 probe (stx1P990) was 5′ end labeled with 6-carboxy-X-rhodamine (ROX) and 3′ end labeled with Black Hole Quencher (BHQ2) (Biosearch Technologies, Novato, Calif.); the stx2 probe (stx2P1249) was 5′ labeled with 6-carboxyfluorescein (FAM) and 3′ end labeled with BHQ1 (IDT). The uidA O157:H7/H− genotype MGB probe (uidAP266) was 5′ end labeled with 6-carboxy-4,7,2′,7′-tetrachloro-fluorescein (TET) and MGB nonfluorescence quencher moieties at the 3′ end (Applied Biosystems).
TABLE 1.
Primer and probe sequences used in a multiplex real-time PCR assay to detect stx1, stx2, and the position 93 uidA SNP of E. coli O157:H7/H−
| Primer or probea | GenBank accession no. | Sequence (5′→3′) | Tm (°C) |
|---|---|---|---|
| stx1F934 | M19473 | GTGGCATTAATACTGAATTGTCATCA | 58 |
| stx1R1042 | M19473 | GCGTAATCCCACGGACTCTTC | 60 |
| stx2F1218 | X07865 | GATGTTTATGGCGGTTTTATTTGC | 60 |
| stx2R1300 | X07865 | TGGAAAACTCAATTTTACCTTTAGCA | 58 |
| uidAF241 | AF305917 | CAGTCTGGATCGCGAAAACTG | 59 |
| uidAR383 | AF305917 | ACCAGACGTTGCCCACATAATT | 59 |
| stx1P990 | M19473 | Rox-TGATGAGTTTCCTTCTATGTGTCCGGCAGAT-BHQ2 | 69 |
| stx2P1249 | X07865 | 6FAM-TCTGTTAATGCAATGGCGGCGGATT-BHQ1 | 69 |
| uidAP266 | AF305917 | TET-ATTGAGCAGCGTTGG-MGB/NFQ | 66 |
Primer and probe names are composed of the name of the target gene, a letter indicating forward primer (F), reverse primer (R), or probe (P), and the 5′ base position of the oligonucleotide.
The four sets of optimal reaction conditions identified previously with the uidA O157:H7/H− genotype real-time PCR assay (36) were tested with a selected group of isolates in a multiplex format (Tables 2 and 3). The most stringent magnesium concentration (2 mM) and temperature (63°C for 25 s of annealing and extension) combination resulted in a false-negative result for stx2 with strain EDL 933. Among the other three sets of conditions tested, there were no false-negative or false-positive results for any of the test isolates. The set of conditions including 3 mM MgCl, 63°C for 25 s annealing and extension, and 0.025 μM uidA probe resulted in the lowest average end fluorescence, 2.684, for the non-O157:H7/H− isolates (C600, CFSAN$400, and ATCC 13337), and therefore these conditions were selected for testing additional strains.
TABLE 2.
Amplification conditions used for multiplex PCR of stx1, stx2, and the position 93 uidA SNP of E. coli O157:H7/H−
| Parameter seta | MgCl concn (mM) | Annealing and extension temp (°C) | Probe concn (μM)
|
||
|---|---|---|---|---|---|
| stx1 | stx2 | uidA | |||
| A | 3 | 63 | 0.1 | 0.1 | 0.025 |
| B | 4 | 63 | 0.1 | 0.1 | 0.025 |
| C | 2 | 61 | 0.1 | 0.1 | 0.025 |
| D | 2 | 63 | 0.1 | 0.1 | 0.05 |
For the Ct and end fluorescence values obtained with each set of conditions, see Table 3.
TABLE 3.
Ct and end fluorescence (dR) values for stx1, stx2, and the position 93 uidA SNP of E. coli O157:H7/H− with four amplification parameters setsa
| Gene and isolate | Genotype | Result obtained with amplification parameter set:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A
|
B
|
C
|
D
|
||||||
| Ct | dR | Ct | dR | Ct | dR | Ct | dR | ||
| stx1 | |||||||||
| EDL 933 | + | 20.14 | 131.115 | 19.59 | 182.974 | 20.89 | 112.544 | 22.36 | 76.967 |
| CFSAN$400 | + | 18.98 | 143.727 | 18.76 | 209.545 | 19.79 | 129.558 | 20.52 | 88.951 |
| ATCC 43889 | − | − | 0.112 | − | −1.916 | − | −0.572 | − | 0.216 |
| C600 | − | − | 0.938 | − | −2.059 | − | 1.171 | − | −2.317 |
| ATCC 13337 | − | − | 1.094 | − | 2.505 | − | 1.288 | − | −1.102 |
| Neg. control | − | − | 1.202 | − | −1.314 | − | 4.606 | − | −0.855 |
| stx2 | |||||||||
| EDL 933 | + | 18.8 | 235.545 | 18.46 | 336.727 | 19.76 | 149.934 | −b | 4.127b |
| CFSAN$400 | − | − | −1.075 | − | 0.806 | − | −0.580 | − | −2.229 |
| ATCC 43889 | + | 18.1 | 315.091 | 17.59 | 361.455 | 18.91 | 246.909 | 21.36 | 89.976 |
| C600 | − | − | 0.402 | − | −1.644 | − | 0.351 | − | 0.4498 |
| ATCC 13337 | − | − | −0.074 | − | −0.389 | − | −0.383 | − | 0.785 |
| Neg. control | − | − | −0.276 | − | 1.405 | − | 0.545 | − | −1.384 |
| uidA | |||||||||
| EDL 933 | + | 22.14 | 91.467 | 21.12 | 110.560 | 23.76 | 75.75 | 23.82 | 86.992 |
| CFSAN$400 | − | − | 2.185 | − | 2.313 | − | 1.606 | − | 1.633 |
| ATCC 43889 | + | 20.77 | 117.005 | 19.97 | 123.825 | 21.56 | 101.536 | 21.37 | 128.908 |
| C600 | − | − | 5.138 | − | 5.084 | − | 6.649 | − | 4.009 |
| ATCC 13337 | − | − | 0.728 | − | 2.201 | − | 0.450 | − | −0.760 |
| Neg. control | − | − | 0.660 | − | −1.343 | − | −0.143 | − | −0.210 |
Multiplex PCR conditions were as described in the text; for the conditions that correspond to parameter sets A, B, C, and D, see Table 2. +, positive; − and Neg., negative.
False-negative result.
However, under these conditions, STEC strains that were non-O157:H7/H− and stx2 positive had considerable spectral overlap between the adjacent FAM (stx2) and TET (uidA O157:H7/H− genotype) channels. Reducing the FAM (stx2) probe concentration to 0.025 μM resulted in no false-positive results in the TET (uidA O157:H7/H− serotype) or FAM (stx2) channels (Table 4). The end fluorescence values in the FAM (stx2) channel for stx2-positive isolates decreased, but the stx2 (FAM)-positive isolates remained positive even at the reduced probe concentration, with an average cycle threshold (Ct) value of 19.82, compared to 19.61 (n = 14). Real-time fluorogenic multiplex assays can be complicated by several variables related to the fluorescence emission spectra generated by each probe. While the introduction of dark quencher molecules, where fluorescence resonance energy transfer (FRET) is to the infrared rather than the UV spectrum, allows less nonspecific background fluorescence in multiplex assays, the emissions of the reporter dyes in adjacent channels must still be carefully optimized. In this multiplex format, the optimal probe concentration was reduced to 0.025 μM for the two probes (stx2 and uidA) located in adjacent channels (FAM and TET).
TABLE 4.
Effect of reduced stx2 (FAM) probe concentration on background end fluorescence (dR) in the FAM and TET channelsa
| Isolate | Result in channel with indicated stx2 probe concn (μM)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
stx2 (FAM)
|
uidA SNP (TET)
|
|||||||||
| Genotype | 0.1
|
0.025
|
Genotype | 0.1
|
0.025
|
|||||
| Ct | dR | Ct | dR | Ct | dR | Ct | dR | |||
| 13A71 | Pos. | 19.33 | 315.7 | 19.99 | 104.456 | Neg. | − | 33.897 | − | 28.3 |
| 13A74 | Pos. | 19.93 | 389.5 | 20.36 | 111.5 | Neg. | 24.79b | 44.9 | − | 24.042 |
| 13A75 | Pos. | 19.72 | 409 | 19.68 | 106.828 | Neg. | − | 31 | − | 18 |
| 13A76 | Pos. | 19.7 | 404.422 | 20.09 | 103.389 | Neg. | − | 35.238 | − | 22.571 |
| 13B47 | Pos. | 21.43 | 275.952 | 20.85 | 96.564 | Pos. | 22.91 | 82.714 | 22.31 | 77.714 |
| CDC 3493-88 | Pos. | 20.24 | 268.344 | 19.86 | 102 | Neg. | − | 42 | − | 12.993 |
| 13C06 | Pos. | 19.61 | 306.429 | 19.69 | 106.65 | Neg. | − | 34.571 | − | 33.9 |
| 13C10 | Pos. | 19.34 | 414.762 | 19.83 | 116.75 | Neg. | − | 35.022 | − | 23.25 |
| CFSAN$406 | Pos. | 16.81 | 366 | 17.63 | 104 | Neg. | 21.76b | 51.3 | − | 27 |
| CFSAN$407 | Pos. | 21.08 | 277.571 | 21.12 | 105.5 | Neg. | − | 34.182 | − | 26.3 |
| 3377-85 | Pos. | 19.14 | 344.25 | 19.18 | 114.381 | Neg. | − | 14.1 | − | 24.1 |
| 3493-88 | Pos. | 19.93 | 302.25 | 20.32 | 94.714 | Neg. | − | 23.75 | − | 7.560 |
| 3024-94 | Pos. | 19.76 | 445.133 | 19.93 | 119.286 | Neg. | − | 26 | − | 12.052 |
| EDL 933 | Pos. | 18.54 | 413 | 18.92 | 102.5 | Pos. | 22.91 | 68.091 | 23.24 | 70.286 |
Pos., positive; − and Neg., negative.
False-positive result due to spectral overlap from the stx2 (FAM) probe at 0.1 μM.
The optimized method utilized 10 mM Tris HCl (pH 8.3), 50 mM KCl (PCR Gold Buffer II; Applied Biosystems), 200 μM (each) dGTP, dCTP, dTTP, and dATP, 3.0 mM MgCl2, a 0.25 μM concentration of each primer (stx1F934, stx1R1042, stx2F1218, stx2R1300, uidAF241, and uidAR383) (Table 1), a 0.1 μM concentration of the stx1 probe (stx1P990ROX), a 0.025 μM concentration of the stx2 probe (stx2P1249FAM), a 0.025 μM concentration of the uidA O157:H7/H− serotype probe (uidAP266TET-MGB), 1.25 U of AmpliTaq Gold (Applied Biosystems), and 0.5 μl of sample template in a total volume of 25 μl. The amplification program included an initial polymerase activation step, 10 min at 94°C, and 40 cycles of 20 s at 94°C and 25 s at 63°C, performed on a Smart Cycler thermal cycler (Cepheid, Sunnyvale, Calif.). Fluorescence values were recorded in each round during the 25-s, 63°C annealing-extension step in the FAM, TET, and ROX channels. Ct values were based on primary curve analysis using manual threshold settings set at 15.0 fluorescence units, with default background subtraction.
The optimized method was tested with 138 isolates, which had various stx1, stx2, and uidA E. coli O157:H7/H− genotypes that had been previously determined. The assay specificity was 100% of this multiplex real-time PCR for all three targets with 138 isolates, and the assay sensitivity was 98.6, 100, and 100% for stx1, stx2, and uidA O157:H7/H− targets, respectively. All of the isolates except one produced the correct genotypic pattern with this real-time multiplex PCR method (Table 5). The one isolate that did not was CFSAN$407 (E. coli O15:H27), which gave a false-negative result for the stx1 (ROX) gene. Although this isolate did not cross the threshold, there appeared to be some amplification, which resulted in an end fluorescence value of 27.727 in the ROX channel. The appropriate stx1 genotype with a Ct value of 22.60 was achieved when the annealing-extension temperature was reduced to 60 from 63°C. In addition, sequencing of the stx1 gene from this strain revealed two mismatches with the probe and one mismatch with the reverse primer, perhaps contributing to the reduced reaction efficiency. The stx1 gene sequence of this strain had the greatest similarity with the stx1 variant GenBank sequences AY135685, AJ314839, and AJ314838 (2).
TABLE 5.
Ct values for 138 isolates representing different genotypes for stx1, stx2, and the uidA E. coli O157:H7/H− position 93 SNP
| Organism | Serotype (no. of isolates tested) | Genotypek
|
Avg Ct (SD)
|
||||
|---|---|---|---|---|---|---|---|
| stx1 | stx2 | uidA O157:H7/H− | stx1 | stx2 | uidA O157:H7/H− | ||
| EHEC | O157:H7 (39)a-g | + | + | + | 19.68 (0.548) | 18.98 (0.560) | 23.77 (1.248) |
| O157:H7 (1) | + | − | + | 19.41 | − | 22.21 | |
| O157:H7 (11)a,b,c,e,f | − | + | + | − | 19.71 (1.469) | 23.29 (1.017) | |
| O157:H7 (1) | − | − | + | − | − | 23.30 | |
| O157:H− (1)b | + | + | + | 18.63 | 20.85 | 22.31 | |
| O157:H− (1)f | − | + | + | − | 18.83 | 22.25 | |
| STEC | O68:H− (1)e | + | + | − | 19.43 | 19.11 | − |
| O48: (1) | 18.62 | 19.99 | − | ||||
| O45:H2 (1)g | 19.32 | 19.89 | − | ||||
| O137:H41 (3)g,h | 18.12 (0.125) | 19.96 (0.326) | − | ||||
| O111:H− (3)e,f | 19.47 (0.567) | 19.21 (0.480) | − | ||||
| O22:H8 (1)f | 17.87 | 17.63 | − | ||||
| O15:H27 (1)f | −i | 21.12 | − | ||||
| O4:H− (1)g | 18.92 | 19.18 | − | ||||
| O26:H11 (7)e,f,h | + | − | − | 19.43 (0.757) | − | − | |
| O26:H− (2)e | 20.45 (0.488) | − | − | ||||
| O45:H2 (1)g | 20.18 | − | − | ||||
| O85:H− (1)e | 19.86 | − | − | ||||
| O103:H2 (1)e | 19.82 | − | − | ||||
| O103:H6 (1)e | 19.46 | − | − | ||||
| O111:H11 (1)f | 18.89 | − | − | ||||
| O125:H− (2)f,g | 19.68 (0.092) | − | − | ||||
| O126:H27 (1)g | 18.66 | − | − | ||||
| O146:H21 (2)g | 19.63 (0.269) | − | − | ||||
| Strain with stx1 insert (1)a | 18.99 | − | − | ||||
| O14:H19 (1)g | − | + | − | − | 18.61 | − | |
| O28:H35 (1)h | − | 19.83 | − | ||||
| O48:H21 (1)h | − | 18.40 | − | ||||
| O55:H7 (1)f | − | 21.97 | − | ||||
| O104:H21 (4)d,g | − | 20.02 (0.247) | − | ||||
| O121:H19 (1)g | − | 18.99 | − | ||||
| O165:H25 (3)e | − | 20.00 (0.442) | − | ||||
| Strain with stx2 insert (1)g | − | 19.10 | − | ||||
| Nontoxigenic E. coli | Non-O157:H7 (2)g | − | − | − | − | − | − |
| O55:H7 (1) | − | − | − | ||||
| O157:H16 (3)a | − | − | − | ||||
| O157:H45 (1) | − | − | − | ||||
| Shigella dysenteriae | (1) | + | − | − | 21.39 | − | − |
| Hafnia alvei | (2) | − | − | − | − | − | − |
| Morganella morganii | (1) | − | − | − | − | − | − |
| Citrobacter freundii | (2) | − | − | − | − | − | − |
| Leclercia adecarboxylata | (1) | − | − | − | − | − | − |
| Shigella sonnei | (1)a | − | − | − | − | − | − |
| Shigella boydii | (1)a | − | − | − | − | − | − |
| Shigella flexneri | (1)a | − | − | − | − | − | − |
| Salmonella group 30 | (1) | − | − | − | − | − | − |
| Salmonella enterica serovar Lansing, group P | (1) | − | − | − | − | − | − |
| Klebsiella pneumoniae | (1) | − | − | − | − | − | − |
| Listeria monocytogenes | (1) | − | − | − | − | − | − |
| Listeria innocua | (1) | − | − | − | − | − | − |
| Listeria ivanovii | (1) | − | − | − | − | − | − |
| Listeria seeligeri | (1) | − | − | − | − | − | − |
| Listeria welshimeri | (1) | − | − | − | − | − | − |
| Vibrio cholerae | O1 Inaba (1) | − | − | − | − | − | − |
| Vibrio parahaemolyticus | O4 (1) | − | − | − | − | − | − |
| Vibrio vulnificus | (1) | − | − | − | − | − | − |
| Staphylococcus aureus | (1) | − | − | − | − | − | − |
| Rhodococcus equi | (1) | − | − | − | − | − | − |
| Lactobacillus sp. | (2) | − | − | − | − | − | −/PICK> |
| Salmonella enterica serovar Typhimurium | (1)a | − | − | − | − | − | − |
| Streptococcus pyogenes | (1) | − | − | − | − | − | − |
| Alcaligenes faecalis | (1) | − | − | − | − | − | − |
| Salmonella enterica serovar Choleraesuis | (1) | − | − | − | − | − | − |
| Yersinia enterocolitica | (2) | − | − | − | − | − | − |
| Enterobacter cloacae | (1) | − | − | − | − | − | − |
| Avgj | 19.51 (0.713) | 19.32 (0.906) | 23.56 (1.225) | ||||
Isolate received from the Washington State Department of Health.
Isolate received from the Oregon State Department of Health.
Isolate received from the King County Public Health Department.
Isolate received from the Montana Public Health Laboratory.
Isolate received from Phil Tarr, Children's Hospital Seattle, Wash.
Isolate received from Peter Feng, Center for Food Safety and Nutrition, Food and Drug Administration.
Isolate received from Joy Wells, Centers for Disease Control and Prevention.
Isolate received from Sharon Abbott, California Health Department.
False-negative result obtained under these reaction conditions.
Overall average Ct for positive isolates (n = 74, 76, and 54 for stx1, stx2, and uidA, respectively).
+, positive; −, negative.
The test panel included 52 E. coli O157:H7 and two E. coli O157:H− isolates, all of which were detected by the unique E. coli O157:H7/H− uidA position 93 component of this multiplex assay. A real-time PCR assay that targets the rfbE gene (lipopolysaccharide O side chain of E. coli O157) would not distinguish between E. coli O157:H7 and E. coli O157 with other H-flagellin antigens (14). The assay reported here did not detect E. coli O157 with other flagellin serotypes, including three E. coli O157:H16 isolates and an E. coli O157:H45 isolate. Other real-time PCR assays target the eaeO157 (γ-intimin) gene and detect E. coli O55:H7 and E. coli O55:NM strains in addition to E. coli O157:H7 and E. coli O157:H− strains (25, 32). This assay did not detect two strains of the closely related E. coli O55:H7/H− serotype.
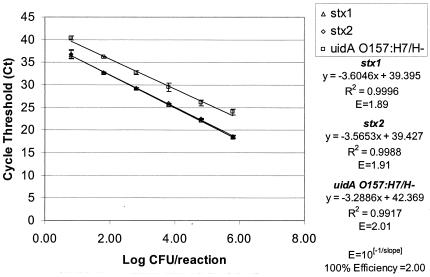
The sensitivity of the multiplex format was reliable, with as few as 6 CFU/reaction within 40 cycles for E. coli O157:H7 strain EDL 933. Serial dilutions of the EDL 933 template also demonstrated the potential quantitative ability of this multiplex real-time PCR application, with an average shift in Ct values of 3.66 for each 10-fold dilution (Fig. 1). The real-time PCR efficiency was calculated for each gene in the multiplex based on the slope of the lines using the formula 10−1/slope (5, 27, 28). The calculated efficiencies for each of the components of this multiplex were similar, with values of 1.89, 1.91, and 2.01 for the stx1, stx2, and uidA O157 genotypes, respectively. Optimal PCR efficiency would be equal to 2.00 and generate a slope of −3.32. The sensitivity for each component of the multiplex reaction can be compared based on the y intercept (5, 27, 28). In this case, the stx1 and stx2 genes had y intercepts of 39.395 and 39.427, respectively, while the uidA O157 genotype was slightly less sensitive, with a y intercept of 42.369.
FIG. 1.
Standard curve for the multiplex real-time PCR analysis for the stx1 and stx2 genes and the E. coli O157:H7/H− uidA SNP at position 93 tested with E. coli O157:H7 isolate EDL 933. The standard deviations are based on three PCR amplifications.
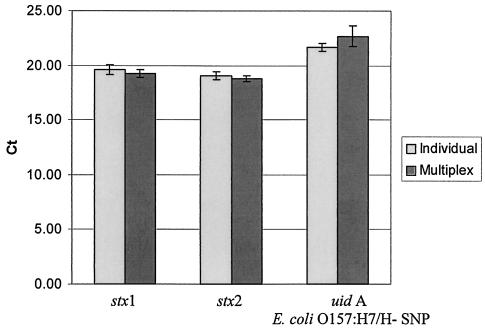
The average Ct values for each amplification product (stx1, stx2, and uidA O157 genes) were similar regardless of whether the amplifications were run individually or in the multiplex format (Fig. 2). The average Ct values for the individually run reactions were 19.62, 19.08, and 21.67 for the stx1, stx2, and uidA O157 genotypes, respectively, based on 14 replicates. When the real-time PCR was run in a multiplex format, the average Ct value shifted only to 19.23 for stx1, 18.81 for stx2, and 22.69 for uidA O157 genotype, also based on 14 replicates.
FIG. 2.
Average Ct values for amplifications of stx1, stx2, and the E. coli O157:H7/H− uidA SNP at position 93 for reactions run individually and in multiplex format for E. coli O157:H7 strain EDL 933, based on 14 replicates.
The specificity of the stx1 and stx2 assay components of this real-time multiplex PCR is attributed to the specificity of the primers and probes for sequences present only in STEC strains. In contrast, the primers in the uidA O157:H7/H− assay are designed to amplify a 143-bp fragment of the uidA gene that occurs in nearly all E. coli strains. The O157:H7/H− specificity is conferred by the specificity of an internal MGB probe for the conserved SNP at position 93. For use in 5′ nuclease assays such as this one, the MGB is attached to the 3′ end of the probe. Because the specificity of the uidA MGB probe needs to be more tightly controlled than that of the stx1 or stx2 probe, the optimization of the entire multiplex assay is more contingent on the uidA assay requirements than the stx1 or stx2 components. The similar slopes observed for all three multiplex assay components (stx1, stx2, and uidA) indicate that the PCR efficiencies for all three are generally equivalent even in a multiplex format. The Ct value lag of 3 and the increased y intercept of the uidA component relative to those of stx1 and stx2 could be attributed more to differences in the annealing efficiency of the internal probes than to the PCR efficiency.
Other real-time PCR STEC methods have focused primarily only on detection of the stx1 and stx2 genes (3, 4, 17, 29, 31, 32). Some real-time PCR assays may include additional components to identify EHEC by targeting the intimin (eaeA), enterohemolysin (E-hly), and O-antigen (rfbE) genes (14, 16, 25, 31, 32). This is the first multiplex real-time PCR method to specifically target the highly conserved SNP at position 93 of E. coli O157:H7/H− uidA and the stx1 and stx2 genes. Real-time PCR methods for detection of STEC and EHEC use a variety of fluorogenic detection approaches, including the use of SYBR green (Molecular Probes, Inc., Eugene, Oreg.) and melt curve analyses (17), 5′ nuclease assay probes (16, 25, 31, 32), FRET hybridization with melt curves to distinguish stx2 and stx2e (4, 29), and molecular beacons (3, 14). Several instrument platforms, including Light Cycler (4, 17, 29), Smart Cycler (3), ABI Prism (14, 25, 32), and the I-Cycler (16), have been used to support these assays. This multiplex assay uses fluorogenic probes in a 5′ nuclease assay format and was optimized on a Smart Cycler instrument. Overall, this multiplex real-time PCR method can be used for the rapid detection of all STEC strains and other Shiga toxin-producing bacteria by targeting the stx1, stx2, and variant genes as well as providing specific identification of the O157:H7/H− serotype, the predominant STEC serotype associated with human illness.
REFERENCES
- 1.Ahmed, S., and M. Donaghy. 1998. An outbreak of Escherichia coli O157:H7 in central Scotland, p. 59-65. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger, S. D., M. Boissinot, C. Manard, F. J. Picard, and M. G. Bergon. 2002. Rapid detection of Shiga toxin-producing bacteria by multiplex PCR with molecular beacons on the Smart Cycler. J. Clin. Microbiol. 40:1436-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellin, T., M. Pulz, A. Matussed, H. G. Hempen, and F. Gunzer. 2001. Rapid detection of enterohemorrhagic Escherichia coli by real-time PCR with fluorescent hybridization probes. J. Clin. Microbiol. 39:370-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. [DOI] [PubMed] [Google Scholar]
- 6.Cebula, T. A., W. L. Payne, and P. Feng. 1995. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J. Clin. Microbiol. 33:248-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1993. Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States, 1992-1993. Morbid. Mortal. Wkly. Rep. 42:258-263. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1996. Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice. Morbid. Mortal. Wkly. Rep. 45:44. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1997. Update outbreaks of Escherichia coli O157:H7 associated with eating alfalfa sprouts—Michigan and Virginia, June-July 1997. Morbid. Mortal. Wkly. Rep. 46:741-744. [PubMed] [Google Scholar]
- 10.Feng, P. 1993. Identification of Escherichia coli serotype O157:H7 by DNA probe specific for an allele of uidA gene. Mol. Cell. Probes 7:151-154. [DOI] [PubMed] [Google Scholar]
- 11.Feng, P., P. I. Fields, B. Swaminathan, and T. S. Whittam. 1996. Characterization of nonmotile Escherichia coli O157 and other serotypes by using an anti-flagellin monoclonal antibody. J. Clin. Microbiol. 34:2856-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, P., and K. A. Lampel. 1994. Genetic analysis of uidA gene expression in enterohemorrhagic Escherichia coli serotype O157:H7. Microbiology 140:2101-2107. [DOI] [PubMed] [Google Scholar]
- 13.Feng, P., K. A. Lampel, H. Karch, and T. S. Whittam. 1998. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J. Infect. Dis. 177:1750-1753. [DOI] [PubMed] [Google Scholar]
- 14.Fortin, N. Y., A. Mulchandani, and W. Chen. 2001. Use of real-time polymerase chain reaction and molecular beacons for the detection of Escherichia coli O157:H7. Anal. Biochem. 289:281-288. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 16.Ibekwe, A. M., P. M. Watt, C. M. Greive, V. K. Sharma, and S. R. Lyons. 2002. Multiplex fluorogenic real-time PCR for detection and quantification of Escherichia coli O157:H7 in dairy wastewater wetlands. Appl. Environ. Microbiol. 68:4853-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jothikumar, N., and M. W. Griffiths. 2002. Rapid detection of Escherichia coli O157:H7 with multiplex real-time PCR assays. Appl. Environ. Microbiol. 68:3169-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutyavin, I. V., I. A. Afonina, A. Mills, V. V. Gorn, E. A. Lukhtanov, E. S. Belousov, M. J. Singer, D. K. Walburger, S. G. Lokhov, A. A. Gall, R. Dempcy, M. W. Reed, R. B. Meyer, and J. Hedgpeth. 2000. 3′-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 28:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law, D. 2000. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J. Appl. Microbiol. 88:729-745. [DOI] [PubMed] [Google Scholar]
- 21.McGowan, K. L., E. Wickersham, and N. A. Strockbine. 1989. Escherichia coli O157:H7 from water. Lancet i:967-968. [DOI] [PubMed]
- 22.Michino, H., K. Araki, S. Minani, T. Nakayama, Y. Ejima, K. Hiroe, H. Tanaka, N. Fujita, S. Usami, M. Yonekawa, K. Sadamoto, S. Takaya, and N. Sakai. 1998. Recent outbreaks of infections caused by Escherichia coli O157:H7 in Japan, p. 73-81. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 23.Monday, S. R., T. S. Whittam, and P. C. H. Feng. 2001. Genetic and evolutionary analysis of mutations in the gusA gene that cause the absence of β-glucuronidase activity in Escherichia coli O157:H7. J. Infect. Dis. 184:918-921. [DOI] [PubMed] [Google Scholar]
- 24.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberst, R. D., M. P. Hays, L. K. Bohra, R. K. Phebus, C. T. Yamashiro, C. Paszako-Kolva, S. J. A. Flood, J. M. Sargeant, and J. R. Gillespie. 1998. PCR based DNA amplification and presumptive detection of Escherichia coli O157:H7 with an internal fluorogenic probe and the 5′ nuclease (TaqMan) assay. Appl. Environ. Microbiol. 64:3389-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed]
- 29.Reischl, U., M. T. Youssef, J. Kilwinski, N. Lehn, W. L. Zhang, H. Karch, and N. A. Strockbine. 2002. Real-time fluorescence PCR assays for detection and characterization of Shiga toxin, intimin and enterohemolysin genes from Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 40:2555-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B., McGee, J. G. Wells, B. R. Davis, R. J. Herbert, G. S. Olcott, L. M. Johnson, N. T. Hargett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype O157:H7. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 31.Sharma, V. K., E. A. Dean-Nystrom, and T. A. Casey. 1999. Semi-automated fluorogenic PCR assays (TaqMan) for rapid detection of Escherichia coli O157:H7 and other Shiga toxigenic E. coli. Mol. Cell. Probes 13:291-302. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, V. K. 2002. Detection and quantitation of enterohemorrhagic Escherichia coli O157, O111, O26 in beef and bovine feces by real-time polymerase chain reaction. J. Food Prot. 65:1371-1380. [DOI] [PubMed] [Google Scholar]
- 33.Strockbine, N. A., L. R. M. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swerdlow, D. L., B. A. Woodruff, R. C. Brady, P. M. Griffin, S. Tippen, H. D. Donnell, Jr., E. Geldreich, B. J. Payne, A. Neyer, J. G. Wells, K. D. Greene, M. Bright, N. Bean, and P. A. Blake. 1992. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann. Intern. Med. 117:812-819. [DOI] [PubMed] [Google Scholar]
- 35.Tilden, J., Jr., W. Young, A.-M. McNamara, C. Custer, B. Boesel, M. A. Lambert-Fair, J. Majkowski, D. Vugia, S. B. Werner, J. Hollingsworth, and J. G. Morris, Jr. 1996. A new route of transmission of Escherichia coli: infection from dry fermented salami. Am. J. Public Health 86:1142-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshitomi, K. J., K. C. Jinneman, and S. D. Weagant. Optimization of 3′-minor groove binder-DNA probe for the rapid detection of Escherichia coli O157:H7/H− using real-time PCR. Mol. Cell. Probes, in press. [DOI] [PubMed]