Abstract
Three point mutations on the Npb allele of the purine nucleoside phosphorylase locus in the mouse have been recovered by male germ cell mutagenesis. The mutants were backcrossed, 12–14 generations, and are designated in increasing order of severity of enzyme deficiency and phenotype: B6-NPE, Met-87 → Lys; B6-NPF, Ala-228 → Thr; and B6-NPG, Trp-16 → Arg. A marked decline in total cell numbers per thymus occurs between 2 and 3 months for the more severe B6-NPF and B6-NPG mutants (35% and 52%, respectively) and by 8 months for the less severe B6-NPE mutation. The thymocyte population is thereafter characterized by a 3- or 8-fold expanded precursor, CD4−CD8− double-negative population and 15% or 55% reduced CD4+CD8+ double-positive cells for the B6-NPF and B6-NPG strains, respectively. Spleen lymphocyte Thy-1+ cells are reduced by 50% and spleen lymphocyte response to T cell mitogen and interleukin 2 is reduced by 80%. Increases of thymocyte dGTP pools of 5- and 2.5-fold for B6-NPF and B6-NPG mutants, respectively, are observed. The purine nucleoside phosphorylase-deficient mouse exhibits age-dependent progressive perturbations in thymocyte differentiation, reduced numbers of thymocytes, and reduced splenic T cell numbers and response. The progressive T cell deficit is similar to the human disorder.
Keywords: T cell immunodeficiency, mouse model
Purine nucleoside phosphorylase (PNP) catalyzes the reversible phosphorolysis of inosine and guanosine and their deoxyribose counterparts to hypoxanthine and guanine and ribose- or deoxyribose-1-phosphate. PNP deficiency was first described in man in association with an autosomal recessive form of cellular immunodeficiency (1) in which patients generally present with recurrent infections and severe lymphopenia within the first 2 years. PNP-deficient patients have reduced T cell numbers and function, whereas B cell function is preserved in most cases (2–5). The thymus is absent on a chest x-ray, which showed only a vague cortex and poorly formed Hassall’s corpuscles (6). Autoimmune hemolytic anemia, autoimmune neutropenia, and systemic lupus erythematosus (5, 7, 8) and B cell lymphomas (9) have been observed in PNP-deficient patients. Presenting features in PNP deficiency have also included neurologic abnormalities. These encompass spastic tetraparesis and diplegia, developmental delay with motor involvement and hypotonia, behavioral abnormalities, and variable mental retardation (2, 5, 7, 10–12).
In PNP deficiency, the nucleoside substrates accumulate, and there is a corresponding decrease in uric acid (2–4). There is no evidence for mammalian kinases capable of phosphorylating the PNP ribonucleoside substrates inosine or guanosine (13). Deoxyguanosine, however, may be converted to dGMP by deoxyguanosine kinase and/or deoxycytidine kinase (13–16). Minimal increases in dGTP are found in erythrocytes and peripheral blood lymphocytes of PNP-deficient patients (4, 10, 17–19) compared with the marked increase in dATP of adenosine deaminase-deficient patients (12). dGTP has been proposed nonetheless to interfere with ribonucleotide reductase and indirectly impair DNA synthesis.
Interpretation of the selective toxicity of deoxyguanosine to lymphoid cells has been based upon their relatively high deoxyguanosine kinase activity (20, 21). Further, the specific T cell rather than B cell impairment characteristic of human PNP deficiency has been attributed to the protective effect of relatively high dNMP nucleotidase activity in B cells as compared with T cells (22). The net result would be the accumulation of dGTP in T but not B cells. Unstimulated human thymocytes do accumulate dGTP but not tonsillar T cells or peripheral blood lymphocytes (23). In vitro studies have indicated T suppressor cell function is sensitive to deoxyguanosine toxicity, whereas helper activity is not (24).
We previously described the recovery of two PNP-deficient mutants from the progeny of mutagen-treated male mice (25). Five PNP mutants were recovered in total, representing three distinct mutations. We report here the molecular characterization of these mutants and describe their physiological consequence. The present findings reveal an age-dependent progressive deficit in thymocyte numbers, a disruption of an early event in T cell differentiation, and decreased spleen leukocyte response in PNP-deficient mice. A significant but minimal accumulation of dGTP in thymocytes was characteristic of the more severely deficient mutants.
MATERIALS AND METHODS
Mutant Production and Congenic Strain Development.
Blood samples from the first-generation offspring of mutagen-treated male mice were screened by quantitative enzyme assay and isoelectric focusing (25), through a cooperative mutant screening program (26). Putative carrier mutant mice were mated to C57BL/6J and congenic strains developed for the B6-NPF and B6-NPE variants, now 12–14 generations backcrossed for this study. The B6-NPG mutation, also successively backcrossed 14 generations, was rescued from a DBA/2J lineage and is presently at the equivalent of a third-generation C57BL/6J backcross. Carrier status at each generation was determined by quantitative PNP assay with erythrocytes. Mice were maintained on autoclaved bedding and in cages equipped with filter caps (25).
PNP and Deoxyguanosine Kinase Assays.
Enzyme assays were performed on cell homogenates using [14C]inosine or [3H]deoxyguanosine as described (25, 27).
Fluorescence-Activated Cell Sorter (FACS) Analysis.
Single cell suspensions of thymocytes and spleen leukocytes from mutant and C57BL/6J mice were prepared in Dulbecco’s phosphate-buffered saline containing fetal calf serum (5%). Cells were incubated with antibodies for 20 min on ice and washed twice. The antibodies used were from Cedarlane Laboratories: fluorescein isothiocyanate-conjugated Thy 1.2 and CD4, phycoerythrin-conjugated CD8, and biotin-tagged CD3 for use with streptavidin PerCP (Becton Dickinson), and fluorescein isothiocyanate-conjugated IA (Boehringer Mannheim). Analyses were done on a FACScan equipped with an argon laser using lysys ii software (Becton Dickinson).
T Cell Stimulation.
Thymocytes or spleen leukocytes were cultured in the presence or absence of concanavalin A (con A; Pharmacia) and human recombinant interleukin 2 (IL-2; Amersham) at a density of 0.5 × 106 cells per 0.2 ml for 48 hr followed by a pulse, 3 hr for thymocytes or 1.5 hr for spleen leukocytes, with 0.4 μCi [3H]thymidine (5 Ci/mmol; Amersham; 1 Ci = 37 GBq). Cells were harvested and washed on glass fiber filters essentially as described (30) with a cell harvester (PHD, Cambridge, MA).
Total thymocyte and spleen leukocyte counts were determined by quantitative preparation of cells as described above. Cell counts were determined with a Coulter counter (model ZB1).
Cellular dGTP and dCTP Analyses.
Thymocyte lysates were prepared as described previously for HPLC analysis of nucleotides (27). The enzymatic assay for dNTPs employed synthetic oligonucleotides as templates for dGTP (5′-TTTCTTTCTTTCTTTCTTTCGGCGGTGGAGGCGG) or dCTP (5′-TTTGTTTGTTTGTTTGTTTGGGCGGTGGAGGCGG) and the universal primer (5′-CCGCCTCCACCGCC), as described by Sherman and Fyfe (28) with modifications (29). The 0.050-ml reaction contained 0.5 units of Sequenase 2.0 and the supplied buffer (United States Biochemical): 6 μM [3H]dATP, 8.4 Ci/mmol (Amersham), 10 μM template and 10 μM universal primer, and 0.005–0.010 ml of extracted lysate. Standards contained 2–10 pmol of dGTP or dCTP, respectively.
Sequencing and Molecular Analysis.
Primers flanking the PNP coding region, (5′Npnc1, dAGCGAAAGGGCAGGAATTCG; 3′Npnc1, dAAGGGTAAGCTTCTCTTTCC) were used to produce 1035-bp PCR products from cDNA generated from each of five mutant strains as described (30). Products were subcloned into pBluescript and sequenced (30, 31).
The codon changes were confirmed by PCR–restriction fragment length polymorphism analysis. The nucleotide substitution at position 260 for B6-NPE was confirmed by NdeI digestion of an independently amplified 1035-bp PCR product from cDNA which gave two fragments for C57BL/6J DNA of 298 and 737 bp, but B6-NPE DNA was refractory to digestion. Genomic DNA from B6-NPF, B6-NPH, B6-NPI, and C57BL/6J was amplified using the primers previously described (5′Npc2, dGGTTTGGAGCTCGTTTTCCGTGC; 3′Npnc2, dAGCAGCGGAGCTCTCCATTGC), which give 800-bp products that contain 381 bp of exon. The substitution at nucleotide 682 in these mutants was verified by a MaeIII restriction site present in the mutant strains and absent in C57BL/6J. The nucleotide substitution for the B6-NPG mutant at base pair 46 was verified by amplification of a 191-bp product from cDNA using primer 5′Npnc1 and 3′Npc150 (dGTAGTCAAAGATCTGAGCCTCC). The B6-NPG product contains an Fnu4HI site, resulting in 104- and 87-bp fragments not present in C57BL/6J DNA.
RESULTS
Molecular Analysis of Mutants.
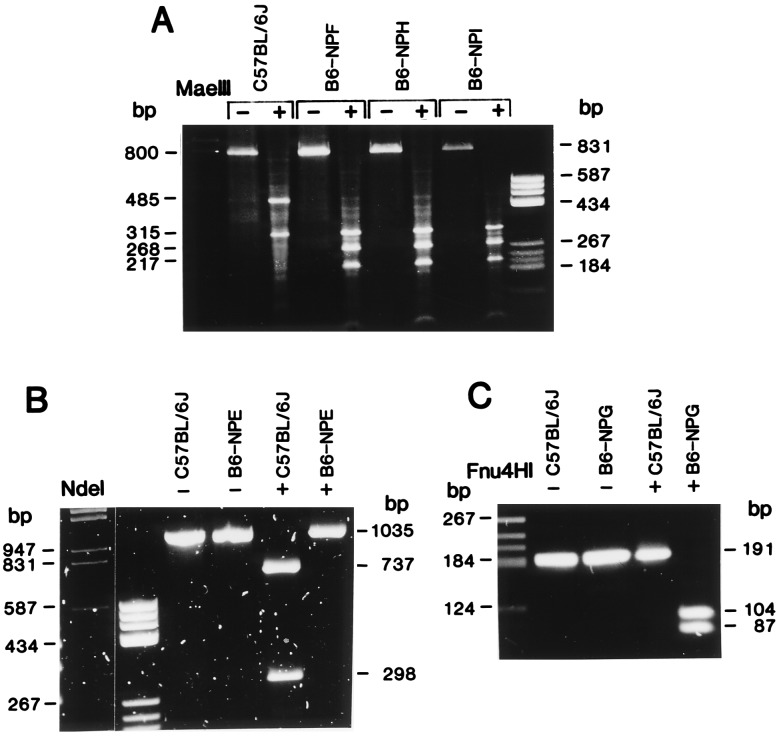
Among the progeny of a C57BL/6J × C3H/HeHa cross in which the male was mutagen-treated (26), five heterozygous mutant mice were identified by quantitative enzyme assay or isoelectric focusing and histochemical staining for PNP. The overall frequency of mutations at the PNP locus was approximately 5 per 2500 test progeny. All of the mutations were on the C57BL/6J allele, which we previously designated Npb (30, 31). cDNA sequence analysis revealed that three of the mutations were identical, B6-NPF, B6-NPI, and B6-NPH, presumably arising from a single founder male (Fig. 1A). Thus three unique mutations were recovered on the Npb allele, and these mutations have been designated B6-NPE, B6-NPF, and B6-NPG, in increasing order of severity (Table 1). The nucleotide substitutions and the corresponding amino acid changes for each of the three unique mutations are given in Table 1, and their verification by reverse transcription–PCR restriction analysis is shown in Fig. 1.
Figure 1.
PCR–restriction fragment length polymorphism analyses of nucleotide substitutions found in PNP-deficient mice. (A) Genomic DNA isolated from brain was amplified with 5′Npc2 and 3′Npnc2 primers to produce a fragment of approximately 800 bp containing 391 bp of exon. C57BL/6J, expressing the Npb allele, has a single MaeIII restriction site within an intron, resulting in 485- and 315-bp fragments. The G → A substitution at nucleotide 682 in the B6-NPF, B6-NPI, and B6-NPH variant strains produces an additional restriction site at base pair 678, resulting in digestion of the 485-bp fragment to 268- and 217-bp fragments. (B) C57BL/6J and B6-NPE cDNAs were amplified with 5′Npnc1 and 3′Npnc2 primers, producing a 1035-bp product. C57BL/6J contains a single NdeI site at nucleotide 257, resulting in 737- and 298-bp fragments. B6-NPE has lost this site as a result of the T → A substitution at nucleotide 260. (C) C57BL/6J and B6-NPG cDNAs were amplified with 5′Npnc1 and 3′Npc150 primers, producing a 191-bp fragment. B6-NPG contains a single Fnu4HI site at base pair 46 due to the T → C substitution at nucleotide 46, resulting in 104- and 87-bp fragments. This site is absent in C57BL/6J, resulting in an undigested 191-bp band.
Table 1.
Molecular characterization of three PNP mutants
| Strain | Allele | Erythrocytic PNP activity* | Mutation site | Codon change | Residue | Amino acid substitution | Restriction site |
|---|---|---|---|---|---|---|---|
| C57BL/6J | Npb | 41.0 ± 3.1 | |||||
| B6-NPE | Npb-m260 | 1.89 ± 0.11 | 260 | ATG to AAG | 87 | Met → Lys | NdeI (−) |
| B6-NPF | Npb-m682 | 0.58 ± 0.05 | 682 | GCA to ACA | 228 | Ala → Thr | MaeIII (+) |
| B6-NPG | Npb-m46 | 0.42 ± 0.20 | 46 | TGG to CGG | 16 | Trp → Arg | Fnu4HI (+) |
The mutation sites are given relative to the initiation of translation. Each mutation resulted in the loss (−) or gain (+) of a restriction enzyme site.
PNP activity is expressed in nanomoles per minute per milligram of protein as the mean ± SD for three to eight mice.
Thymocyte Numbers and Subset Analysis in PNP Deficiency.
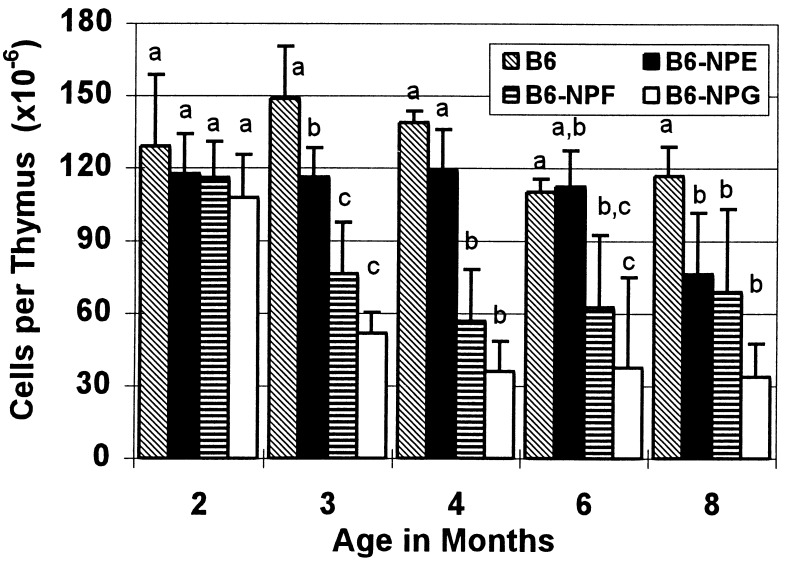
To eliminate other mutations and produce congenic stocks, the three unique mutations were backcrossed 12–14 generations before the homozygous PNP-deficient mice used in this study were produced. Gross pathology of the mice at 6–8 weeks was normal, and all three mutants exhibited a normal lifespan to greater than 16 months. Assessment of total cell numbers in the thymus revealed a marked and progressive decline in mice homozygous for the two severe mutations between 2 and 3 months, whereas the more leaky mutation, B6-NPE, did not show a decline in thymocytes until 8 months of age (Fig. 2). Cell numbers per thymus, which were essentially normal at 2 months, declined to 41% and 26% of those of control mice by 4 months for B6-NPF and B6-NPG mice, respectively.
Figure 2.
Number of cells per thymus in PNP-deficient mice as a function of age. Total thymocyte numbers are the mean ± SD as determined from three or four male mice, and a comparison was made for each month by the Student–Newman–Keuls test at P = 0.05, where the letters indicate significant differences among strains.
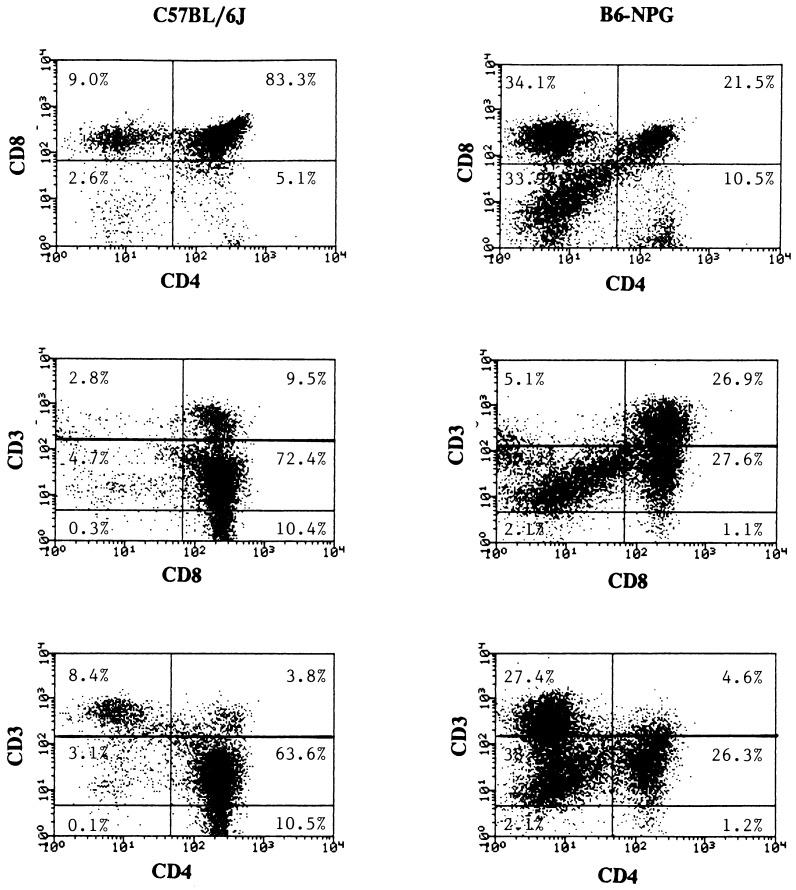
Analysis of thymocyte subpopulations in 8- to 10-month-old PNP-deficient mice was carried out by flow cytometry using the surface markers CD4, CD8, CD3, and Thy-1. A minor decrease in the number of Thy-1+ cells of the B6-NPG mutant was apparent (Table 2), and Thy-1 fluorescence exhibits a broadening of the peak for the positive population. The CD4−CD8− T cell precursor population is 8.3-fold increased in the more severe B6-NPG mutant, a trend which is evident but less pronounced in the other mutants (Table 2 and Fig. 3). A representative analysis for B6-NPG (Fig. 3) shows that this change is due to a decrease in the CD4+CD+CD3lo/int double- positive cells and an increase in the pre-T population of double-negative CD4−CD8− cells. The relative proportion of CD3 expression was essentially normal among the four CD4CD8 subsets. Compared with the background strain, C57BL/6J, there are significant decreases in the CD4+CD8+ fraction, 16% and 57% for B6-NPF and B6-NPG mice, respectively. Examination of these parameters at an earlier window of 5–6 months for B6-NPG revealed the same pattern with less severity: increased CD4−CD8− (366%) and decreased CD4+CD8+ (71%) relative to C57BL/6J.
Table 2.
Phenotype and number of thymocytes in PNP-deficient mice
| Surface Antigen | C57BL/6J (n = 10) | B6-NPE (n = 4) | B6-NPF (n = 4) | B6-NPG (n = 3) |
|---|---|---|---|---|
| Thy-1+ | 99.8 ± 0.3a | 99.8 ± 0.4a | 98.7 ± 1.4a | 93.7 ± 4.2b |
| CD4−CD8− | 2.9 ± 0.6a | 3.9 ± 2.3a | 8.7 ± 3.6a | 24.0 ± 9.8b |
| CD4+CD8+ | 88.0 ± 2.8a | 86.1 ± 4.0ab | 75.9 ± 7.7b | 42.6 ± 18.4c |
| CD4+CD8− | 6.3 ± 1.9a | 7.0 ± 2.9a | 9.0 ± 1.4a | 23.6 ± 9.2b |
| CD4−CD8+ | 2.8 ± 1.0a | 3.0 ± 1.1a | 6.3 ± 3.0b | 9.8 ± 1.4c |
Results are the mean ± SD for the number of mice given in parenthesis between 8 and 10 months of age. Multiple comparisons among means were made using the Student–Newmann–Keuls test (P = 0.05).
Letters indicate significant differences (P = 0.05) among strains for each surface antigen subtype.
Figure 3.
Flow cytometric analysis of thymocyte phenotype in control and B6-NPG PNP-deficient mice. Fluorescence-activated cell sorter analysis of CD3, CD4, and CD8 expression in thymocytes from control and B6-NPG PNP-deficient mice at 8 months. The gates for CD3 were as follows: CD3−, CD3lo and CD3int, CD3hi.
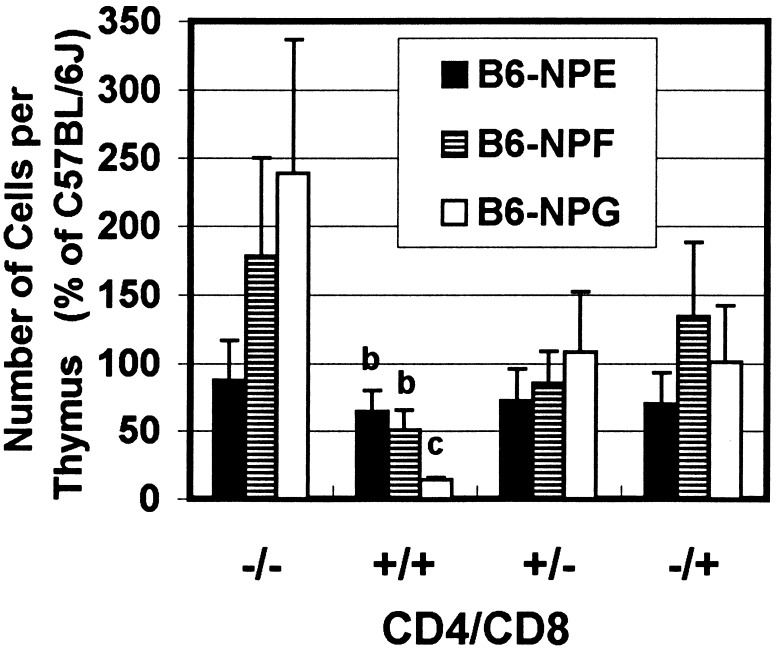
Combining the subset distribution pattern and the total cell counts per thymus reveals an absolute increase in the CD4−CD8− double-negative population for B6-NPF and B6-NPG mice (Fig. 4). There is a corresponding decrease in double-positive cells that is statistically significantly (P = 0.05) for all three mutants. For B6-NPG mice, the number of double-positive cells was only 14% of that for controls. An essentially constant and near normal proportion of single-positive CD4+CD8− and CD4−CD8+ thymocytes is preserved in all three mutant strains.
Figure 4.
Numbers of CD4/CD8 cell subsets per thymus in PNP-deficient mice as compared with C57BL/6J mice. From the total number of cells per thymus (Fig. 2) and the relative CD4/CD8 subset distribution (Table 2), the relative number of cells in each CD4/CD8 subset was determined as percentage of that for C57BL/6J. The cell numbers are reported as the mean ± SD. Letters indicate significant difference (P = 0.05) among strains and from the C57BL/6J control (group a).
Decreased Numbers and Response of Spleen T Cells in PNP Deficiency.
The proportion of B cells (IA+) in the spleen leukocyte population was equivalent for B6-NPE and B6-NPG, or marginally greater for B6-NPF than that of the background strain (Table 3). The proportion of Thy-1+ cells was significantly decreased for the B6-NPF and B6-NPG mutations to approximately 50% of the proportion for the control (Table 3). No significant differences were noted for total spleen leukocyte numbers between control and mutant animals at the various ages examined in Fig. 2 (data not shown). Single-positive CD4 or CD8 spleen leukocytes were present in normal proportions for B6-NPE mice. In contrast, there were changes for the other two mutations with the number of CD4−CD8+ cells being 57% and 41% of the number for control mice for B6-NPF and B6-NPG mice, respectively (Table 3).
Table 3.
Phenotype and number of spleen leucocytes for PNP-deficient mice
| Surface Antigen | C57BL/6J (n = 9) | B6-NPE (n = 4) | B6-NPF (n = 4) | B6-NPG (n = 3) |
|---|---|---|---|---|
| IA+ | 44.4 ± 7.7a | 49.5 ± 4.2a | 61.4 ± 3.5b | 47.9 ± 9.0a |
| Thy-1+ | 49.7 ± 11.5a | 45.3 ± 11.0ab | 24.4 ± 12.8b | 25.9 ± 13.9b |
| CD4−CD8− | 55.2 ± 9.5a | 59.6 ± 3.3a | 72.0 ± 2.1b | 56.7 ± 8.5a |
| CD4+CD8+ | 0.4 ± 0.2a | 0.2 ± 0.2a | 0.3 ± 0.1a | 0.5 ± 0.3a |
| CD4+CD8− | 30.2 ± 6.1a | 26.7 ± 0.5ab | 19.6 ± 3.1b | 37.0 ± 7.0a |
| CD4−CD8+ | 14.3 ± 3.8a | 13.6 ± 2.7a | 8.2 ± 3.7b | 5.8 ± 2.1b |
Results are the mean ± SD for the number of mice given in parenthesis for 8- to 10-month-old mice. Letters indicate significant differences (P = 0.05) among strains.
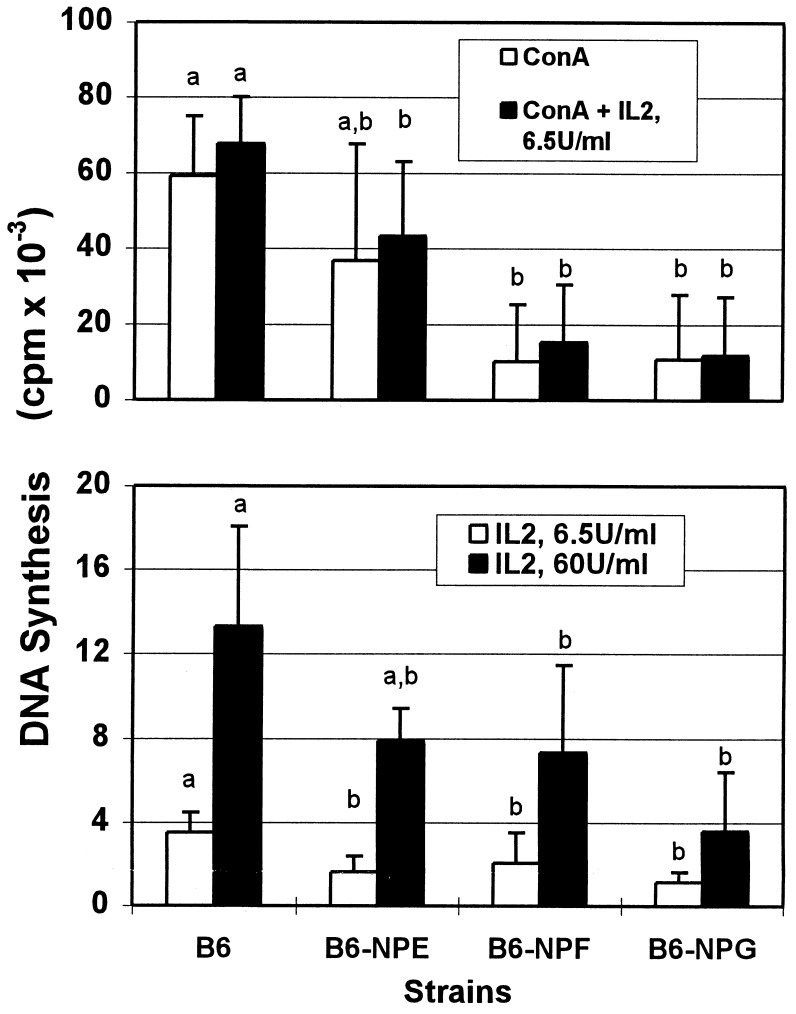
Spleen leukocyte response to con A and IL-2 was significantly impaired, being only 18–20% of normal for B6-NPG and 17–28% of normal for B6-NPF (Fig. 5). The less severe mutation, B6-NPE, showed a partial reduction in response at 60–69% of that for control mice. Even with adjustment of these responses for the reduction in Thy-1+ cells, the mutants have a decreased overall response to con A and IL-2, 35–57% for B6-NPF and 34–38% for B6-NPG spleen leukocytes as compared with levels for the controls. The severity of depression in peripheral T cell numbers (Table 3) and response (Fig. 5) shows a positive correspondence with the degree of decrease in PNP enzyme activity (Table 1).
Figure 5.
Response of spleen leukocytes from PNP-deficient mice to con A and IL-2. Spleen leukocytes were stimulated alone or in combination with con A (0.5 μg/ml) and/or IL-2 (6.5 or 60 units/ml). Results are the mean ± SD for the following numbers of animals: C57BL/6J, 7; B6-NPE, 3; B6-NPF, 6; B6-NPG, 4. Letters indicate significant differences (P = 0.05) among strains for each set of conditions.
dGTP in Thymocytes of PNP-Deficient Mice.
Attempts to examine changes in cellular dGTP levels in the PNP-deficient mice were initially carried out by liquid chromatography (27). Although no gross increase in dGTP was apparent, the methodology was not sufficiently sensitive to measure the background levels in control mice. We have examined dGTP levels using a sensitive nucleotide-specific oligonucleotide template–DNA polymerase method (28). These results reveal no change in thymocyte dGTP for the B6-NPE mutation; however, dGTP was increased 5- and 2.5-fold for the B6-NPF and B6-NPG mutations, respectively, as compared with C57BL/6J (Table 4). Examination of dCTP levels in thymocytes showed no significant change among the mutants as compared with the parental background strain.
Table 4.
Thymocyte dGTP and dCTP concentrations in PNP-deficient mice
| Strain | dGTP, pmol/106 cells | dCTP, pmol/106 cells | Ratio to C57BL/6J
|
|
|---|---|---|---|---|
| dGTP | dCTP | |||
| C57BL/6J | 1.63 ± 0.82a | 4.52 ± 1.13 | ||
| B6-NPE | 1.84 ± 0.86a | 4.54 ± 0.65 | 1.13 | 1.00 |
| B6-NPF | 8.72 ± 4.10b | 5.32 ± 0.70 | 5.35 | 1.18 |
| B6-NPG | 4.15 ± 0.23b | 3.60 ± 0.95 | 2.55 | 0.80 |
Three mice were examined for each strain except B6-NPF (n = 4). Male mice were 2 months of age (C57BL/6J, B6-NPE, and B6-NPF) or 4 months of age (B6-NPG). Statistical comparison was male by the Student–Neumann–Keuls (P = 0.05) test; letters represent significant differences. There were no significant differences among strains for dCTP.
DISCUSSION
Three independent point mutations at the PNP locus in the mouse have been recovered following mutagenesis and, by extensive backcross, have been isolated from other potentially extraneous mutational events. None of the three deduced amino acid substitutions (Table 1) were active site determinants when compared with the assignments for human PNP, as determined by x-ray structure analysis (32). The B6-NPE mutation, Met-87 → Lys, is next to His-86, a reversible proton donor involved in the catalytic site, and Tyr-88 may be involved in binding ribose (32). The B6-NPF and B6-NPG substitutions Ala-228 → Thr and Trp-16 → Arg are located in α helices and may destabilize these structures as each are poorer helix-forming residues than the native residue. Congruent with this information, the Km values for nucleoside and phosphate were not substantially altered for the B6-NPE and B6-NPF mutations, and these proteins were previously shown to be markedly more labile than the normal enzyme (25). The B6-NPF and B6-NPG mutations are substantially more severe, < 1% residual PNP activity, than the B6-NPE mutation, 5% (Table 1 and refs. 25 and 27).
T cell development in the thymus proceeds from CD4−CD8− precursors through double-positive CD4+CD8+ intermediates into single-positive CD4+CD8− and CD4−CD8+ mature T cells (33, 34). The majority of thymocytes (80–85%) are normally the immature or pre-T cells that coexpress both the CD4 and the CD8 accessory molecules and reside in the cortex. The phenotypic consequence of PNP deficiency in the B6-NPF and B6-NPG mutants is an increase in the steady-state numbers of CD4−CD8− double-negative precursors to 170–240% of the numbers for the control and a marked deficit of CD4+CD8+ double-positive thymocytes to 14–50% of that for the control (Table 2 and Fig. 4). Histopathology of the thymus in PNP-deficient mice reveals a narrowing of the cortex and a reduction in size in proportion to the medullary compartment (A. Pinto and F.F.S., unpublished observations).
In comparison, a complete deficiency of adenosine deaminase in the mouse results in death around birth due to severe liver cell degeneration (35, 36). A less severe phenotype is produced by inhibition of adenosine deaminase activity by deoxycoformycin, which causes a reduction in CD4+CD8+ double-positive thymocytes (37), as in the PNP-deficient mice; however, only in PNP-deficient mice were the double-negative precursor cells increased. Mature single-positive thymocytes were nonetheless essentially normal for both PNP deficiency (Fig. 4) and the pharmacological model of adenosine deaminase deficiency (37). In the PNP-deficient mice, excessive loss of double-positive CD4+CD8+ thymocytes during productive T cell receptor rearrangement and transition to mature T cells may be sufficient to account for the observed changes either alone or in combination with constraints on the transition of precursor double-negative cells to double-positive thymocytes.
The mouse model shares several similarities with human PNP deficiency where affected individuals have a small thymus, decreased numbers of T cells, and poor response to mitogens with normal levels of B cells (5, 12). There is also evidence of progressive loss of T cell function that may be normal at birth but decreases with age (3–5). The cellular features of the PNP-deficient mice include a progressive age-dependent decline in total thymocytes, with the marked decrease occurring during the 2- to 3-month period for the more severely deficient mutants (Fig. 2). This time period is not characterized by significant developmental changes in thymocyte or spleen leukocyte PNP or deoxyguanosine kinase activities (38). Spleen leukocyte response to mitogen and IL-2 was also shown to be decreased for the B6-NPF and B6-NPG mutants (Fig. 5). Thus the age-dependent decrease in thymocyte numbers and significant decrease in Thy-1+ spleen leukocytes (Table 3) for the two severe mutations are parallel features to the human disorder and reflect a reduced rate of production of mature thymocytes.
There is ample evidence that deoxyguanosine does induce thymocyte death and perturb T cell development in the mouse. In embryonic thymus organ culture models, deoxyguanosine pretreatment is used to deplete the organ of thymocytes and bone marrow-derived precursor cells (39). Suppressor T cell development in the mouse is also impaired by deoxyguanosine (40, 41). Among the PNP substrates, only deoxyguanosine has been shown to induce DNA strand breaks in mouse thymocytes, whereas inosine and guanosine do not (42). As yet, the mechanism whereby these effects are achieved has not been established; however, somatic cell mutants defective in deoxycytidine kinase do show reduced sensitivity to deoxyguanosine, thereby implicating dGTP in the mechanism of toxicity (16, 43).
We previously showed that PNP-deficient mice excrete PNP substrates in proportion to the severity of the enzyme deficiency, with B6-NPE mutants excreting the combination of inosine and guanosine at 10-fold the normal level and B6-NPF excreting these substrates plus deoxyguanosine and deoxyinosine at a total of 100-fold greater than the level of control mice (25). Cellular GTP was not changed in the PNP-deficient mutants (27), but the methodology was not sufficiently sensitive to evaluate the nearly 100-fold lower dGTP concentration. Using a sensitive DNA polymerase assay, an increase in thymocyte dGTP pools was observed for the B6-NPF and B6-NPG mutations; however, the magnitude of the change is not great (Table 4). Minimal changes are also apparent in a pharmacological model in which the combined administration of both a PNP inhibitor and deoxyguanosine in the rat produced a less than 2-fold increase in dGTP, and this only in thymocytes (44). The minimal change in dGTP is not surprising in view of the secondary deficiency of deoxyguanosine kinase observed in homozygous PNP-deficient mice (27). The reduction in deoxyguanosine kinase appears to be a compensatory phenomenon that prevents the detrimental accumulation of dGTP, rather than exacerbating the metabolic consequence of the primary mutation. The recent cloning of human deoxyguanosine kinase (45, 46) will facilitate clarification of the role of this enzyme and that of deoxycytidine kinase in phosphorylating deoxyguanosine. dGTP is believed to regulate the levels of dCTP through ribonucleotide reductase (12); however, there was no distortion in dCTP pools (Table 4). The possibility remains, however, of there being markedly greater increases in dGTP and reduced dCTP in a differentiating subset that is masked by the total thymocyte population. A link among PNP deficiency, the minimal increase in thymocyte dGTP, and the observed perturbations in T cell maturation remains to be established. Increases in the dGTP pools could be deleterious to the template-independent N region component of T cell receptor gene rearrangement (47, 48), which involves terminal deoxynucleotidyltransferase (49) and is presumably responsive to endogenous dNTP pool sizes.
The association of autoimmune disease with PNP deficiency has been thought to reflect a B cell hyperactivity phenomenon, possibly resulting from a loss of suppressor (CD8) T cell function (12). Of relevance, there appears to be a preferential deficit of CD8 cells in the periphery for both B6-NPF and B6-NPG mice, 57% and 41% of control levels, respectively, whereas in general terms, the CD4 fraction is not so substantially altered (Table 3). High titer antibodies to 120- and >200-kDa nuclear proteins have been detected by immunoblotting in the B6-NPF mutant, providing preliminary evidence for autoreactivity in the PNP-deficient mouse (M. J. Fritzler, personal communication).
Analysis of three mutations at the PNP locus in the mouse revealed impaired T cell differentiation in proportion to the severity of the enzyme deficiency. Significant metabolic and physiological phenotypic criteria have now been defined for the PNP-deficient mouse against which metabolite, enzyme, and gene therapy approaches may be tested.
Acknowledgments
We gratefully acknowledge M. J. Fritzler for autoantibody analyses, F. G. Biddle for providing inbred mouse stocks, L. Bryan for gross pathology studies, L. Robertson for assistance with fluorescence-activated cell sorter analyses, and F. Yang for manuscript preparation. This work was supported by the Medical Research Council of Canada (Grant MT6376) and by a studentship (J.P.J.) and scholarship (F.F.S.) from the Alberta Heritage Foundation for Medical Research.
ABBREVIATIONS
- PNP
purine nucleoside phosphorylase
- con A
concanavalin A
- IL-2
interleukin 2
Footnotes
References
- 1.Giblett E R, Ammann A J, Wara D W, Sandman R, Diamond L K. Lancet. 1975;i:1010. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- 2.Cohen A, Doyle D, Martin D W, Jr, Ammann A J. N Engl J Med. 1976;295:1449–1454. doi: 10.1056/NEJM197612232952603. [DOI] [PubMed] [Google Scholar]
- 3.Stoop J W, Zegers B J M, Hendrickx G F M, Siegenbeek van Heukelom L H, Staal G E J, De Bree P K, Wadman S K, Ballieux R E. N Engl J Med. 1977;296:651–655. doi: 10.1056/NEJM197703242961203. [DOI] [PubMed] [Google Scholar]
- 4.Rijksen G, Kuis W, Wadman S K, Spaapen L, Duran M, Voorbrood B S, Staal G, Stoop J W, Zegers B. Pediatr Res. 1987;21:137–141. doi: 10.1203/00006450-198702000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Markert M L. Immunodefic Rev. 1991;3:45–81. [PubMed] [Google Scholar]
- 6.Ammann A J, Wara D A, Allen T. Clin Immunol Immunopathol. 1978;10:262–269. doi: 10.1016/0090-1229(78)90180-0. [DOI] [PubMed] [Google Scholar]
- 7.Rich K C, Arnold W J, Palella T, Fox I H. Am J Med. 1979;67:172–176. doi: 10.1016/0002-9343(79)90100-1. [DOI] [PubMed] [Google Scholar]
- 8.Carapella-De Luca E, Aiuti F, Lacarelli P, Bruni L, Baroni C D, Imperato C, Roos D, Astaldi A. J Pediatr (Berlin) 1978;93:1000–1003. doi: 10.1016/s0022-3476(78)81237-2. [DOI] [PubMed] [Google Scholar]
- 9.Watson A R, Evans D K, Marsden H B, Miller V, Rogers P A. Arch Dis Child. 1981;56:563–565. doi: 10.1136/adc.56.7.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmonds H A, Fairbanks L D, Morris G S, Morgan G, Watson A R, Timms P, Singh B. Arch Dis Child. 1987;62:385–391. doi: 10.1136/adc.62.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soutar R L, Day R E. Arch Dis Child. 1991;66:982–983. doi: 10.1136/adc.66.8.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershfield M S, Mitchell B S. In: The Metabolic and Molecular Bases of Inherited Disease. 7th Ed. Scriver C R, Beaudet A L, Sly S W, Valle D, editors. Vol. 2. New York: McGraw–Hill; 1995. pp. 1725–1768. [Google Scholar]
- 13.Lukey T, Snyder F F. Can J Biochem. 1980;58:677–682. doi: 10.1139/o80-095. [DOI] [PubMed] [Google Scholar]
- 14.Osborne W R A. Proc Natl Acad Sci USA. 1986;83:4030–4034. doi: 10.1073/pnas.83.11.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chottiner E G, Shewach D S, Datta N S, Ashcraft E, Gribbin D, Ginsburg D, Fox I H, Mitchell B S. Proc Natl Acad Sci USA. 1991;88:1531–1535. doi: 10.1073/pnas.88.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnér E S J, Eriksson S. Pharmacol Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 17.Cohen A, Gudas L J, Ammann A J, Staal G E J, Martin D W., Jr J Clin Invest. 1978;61:1405–1409. doi: 10.1172/JCI109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmonds H A, Fairbanks L D, Morris G S, Webster D R, Harley E H. Clin Chim Acta. 1988;171:197–210. doi: 10.1016/0009-8981(88)90145-3. [DOI] [PubMed] [Google Scholar]
- 19.Simmonds H A, Watson A R, Webster D R, Sahota A, Perret D. Biochem Pharmacol. 1982;31:941–946. doi: 10.1016/0006-2952(82)90324-0. [DOI] [PubMed] [Google Scholar]
- 20.Carson D A, Kaye J, Seegmiller J E. Proc Natl Acad Sci USA. 1977;74:5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne W R A, Scott C R. Biochem J. 1983;214:711–718. doi: 10.1042/bj2140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carson D A, Kaye J, Seegmiller J E. Proc Natl Acad Sci USA. 1979;76:2430–2434. doi: 10.1073/pnas.76.5.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairbanks L D, Taddeo A, Duley J A, Simmonds H A. J Immunol. 1990;144:485–491. [PubMed] [Google Scholar]
- 24.Gelfand E W, Lee J J, Dosch H-M. Proc Natl Acad Sci USA. 1979;76:1998–2002. doi: 10.1073/pnas.76.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mably E R, Fung E, Snyder F F. Genome. 1989;32:1026–1032. doi: 10.1139/g89-547. [DOI] [PubMed] [Google Scholar]
- 26.Chapman V M, Miller D R, Armstrong D, Caskey C T. Proc Natl Acad Sci USA. 1989;86:1292–1296. doi: 10.1073/pnas.86.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder F F, Jenuth J P, Dilay J E, Fung E, Lightfoot T, Mably E R. Biochim Biophys Acta. 1994;1227:33–40. doi: 10.1016/0925-4439(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 28.Sherman P A, Fyfe J A. Anal Biochem. 1989;180:222–226. doi: 10.1016/0003-2697(89)90420-x. [DOI] [PubMed] [Google Scholar]
- 29.Gao W-Y, Johns D G, Mitsuya H. Anal Biochem. 1994;222:116–122. doi: 10.1006/abio.1994.1462. [DOI] [PubMed] [Google Scholar]
- 30.Jenuth J P, Snyder F F. Nucleic Acids Res. 1991;19:1708. doi: 10.1093/nar/19.7.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenuth J P, Mangat R K, Snyder F F. Mamm Genome. 1993;4:598–603. doi: 10.1007/BF00361392. [DOI] [PubMed] [Google Scholar]
- 32.Ealick S E, Rule S A, Carter D C, Greenhough T J, Babu Y S, Cook W J, Habash J, Helliwell J R, Stoeckler J D, Parks R E, Jr, Chen S, Bugg C E. J Biol Chem. 1990;265:1812–1820. doi: 10.2210/pdb2pnp/pdb. [DOI] [PubMed] [Google Scholar]
- 33.Pearse M, Wu L, Egerton M, Wilson A, Shortman K, Scollay R. Proc Natl Acad Sci USA. 1989;86:1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kisielow P, von Boehmer H. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 35.Migchielsen A A J, Breuer M L, van Roon M, te Riele H, Zurcher C, Ossendorp F, Toutain S, Hershfield M S, Berns A, Valerio D. Nat Genet. 1995;10:279–287. doi: 10.1038/ng0795-279. [DOI] [PubMed] [Google Scholar]
- 36.Wakamiya M, Blackburn M R, Jurecic R, McArthur M J, Geske R S, Cartwright J, Jr, Mitani K, Vaishnav S, Belmont J M, Kellems R E, Finegold M J, Montgomery A C, Jr, Bradley A, Caskey C T. Proc Natl Acad Sci USA. 1995;92:3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beneviste P, Cohen A. Proc Natl Acad Sci USA. 1995;92:8373–8377. doi: 10.1073/pnas.92.18.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukey T, Snyder F F. Biochem Pharmacol. 1983;32:1399–1406. doi: 10.1016/0006-2952(83)90453-7. [DOI] [PubMed] [Google Scholar]
- 39.Jenkinson E J, Franchi L L, Kingston R, Owen J T O. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 40.Dosch H-M, Mansour A, Cohen A, Shore A, Gelfand E W. Nature (London) 1979;285:494–496. doi: 10.1038/285494a0. [DOI] [PubMed] [Google Scholar]
- 41.Bril H, van den Akker T W, Molendijk-Lok B D, Bioanchi A T J, Benner R. J Immunol. 1984;132:599–604. [PubMed] [Google Scholar]
- 42.Kizaki H, Ahimada H, Ahsaka F, Sakurada T. J Immunol. 1990;141:1652–1657. [PubMed] [Google Scholar]
- 43.Hershfield M S, Felter J E, Small W C, Bagnara A S, Williams S R, Ullman B, Martin D W, Jr, Wasson D B, Carson D A. J Biol Chem. 1982;257:6380–6386. [PubMed] [Google Scholar]
- 44.Osborne W R A, Barton R W. Immunology. 1986;59:63–67. [PMC free article] [PubMed] [Google Scholar]
- 45.Johansson M, Karlsson A. Proc Natl Acad Sci USA. 1996;93:7258–7262. doi: 10.1073/pnas.93.14.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Hellman U, Eriksson S. FEBS Lett. 1996;390:39–43. doi: 10.1016/0014-5793(96)00623-0. [DOI] [PubMed] [Google Scholar]
- 47.Ma D D F, Sylwestrowicz T A, Janossy G, Hoffbrand A V. Immunol Today. 1983;4:65–69. doi: 10.1016/0167-5699(83)90120-2. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Valdez H, Cohen A. Proc Natl Acad Sci USA. 1988;85:6900–6903. doi: 10.1073/pnas.85.18.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komori T, Okada A, Stewart V, Alt F W. Science. 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]