Abstract
The effect of temperature on the community structure of ammonia-oxidizing bacteria was investigated in three different meadow soils. Two of the soils (OMS and GMS) were acidic (pH 5.0 to 5.8) and from sites in Germany with low annual mean temperature (about 10°C), while KMS soil was slightly alkaline (pH 7.9) and from a site in Israel with a high annual mean temperature (about 22°C). The soils were fertilized and incubated for up to 20 weeks in a moist state and as a buffered (pH 7) slurry amended with urea at different incubation temperatures (4 to 37°C). OMS soil was also incubated with less fertilizer than the other soils. The community structure of ammonia oxidizers was analyzed before and after incubation by denaturing gradient gel electrophoresis (DGGE) of the amoA gene, which codes for the α subunit of ammonia monooxygenase. All amoA gene sequences found belonged to the genus Nitrosospira. The analysis showed community change due to temperature both in moist soil and in the soil slurry. Two patterns of community change were observed. One pattern was a change between the different Nitrosospira clusters, which was observed in moist soil and slurry incubations of GMS and OMS. Nitrosospira AmoA cluster 1 was mainly detected below 30°C, while Nitrosospira cluster 4 was predominant at 25°C. Nitrosospira clusters 3a, 3b, and 9 dominated at 30°C. The second pattern, observed in KMS, showed a community shift predominantly within a single Nitrosospira cluster. The sequences of the individual DGGE bands that exhibited different trends with temperature belonged almost exclusively to Nitrosospira cluster 3a. We conclude that ammonia oxidizer populations are influenced by temperature. In addition, we confirmed previous observations that N fertilizer also influences the community structure of ammonia oxidizers. Thus, Nitrosospira cluster 1 was absent in OMS soil treated with less fertilizer, while Nitrosospira cluster 9 was only found in the sample given less fertilizer.
Temperature is one of the most important factors that influence nitrifier populations (6). Mahendrappa et al. (27) found that indigenous nitrifiers had temperature optima in correspondence to their climate region. This was also observed by Mahli and McGill (28), who compared soils from the tropics (Australia, 25°C annual mean temperature), temperate zone (Iowa, United States, 10°C annual mean temperature), and northern latitudes (Alberta, Canada, 2.5°C annual mean temperature) and showed that the optimum temperatures for communities from these soils were 35, 30, and 20°C, respectively. Stark and Firestone (42), on the other hand, found that the temperature optima of communities from different soil microsites appeared to be more related to differences in the temperature at particular times of the year than to the annual mean temperatures. Furthermore, the optimum temperature (31.8°C) for communities beneath the canopies of oak trees was lower than that in the open interspaces (35.9°C). However, in all these studies, the community structure of ammonia oxidizers was not analyzed. The question arising from these studies is whether these differences in optimal activities are due to physiological adaptation of the populations or to differences in the communities.
Changes in communities of ammonia-oxidizing bacteria can be analyzed by targeting the genes coding for the 16S rRNA and the α subunit of ammonia monooxygenase (amoA). The phylogeny of the amoA gene was found to largely correspond to the phylogeny of the 16S rRNA gene in ammonia oxidizers (1, 21, 36). The amoA gene has been used before for studying the community structure of ammonia oxidizers by denaturing gradient gel electrophoresis (DGGE) (31, 34) and in our previous studies (3, 4). These studies defined AmoA clusters 1, 2, 3a, 3b, 4, 9, 10, 11, and 12. Clusters 2, 3, and 4 can be related to corresponding 16S rRNA gene clusters as defined by Stephen et al. (45), although clusters 2 and 4 cannot be clearly distinguished. Cluster 1 can be related to the new 16S rRNA gene cluster 4 defined by Purkhold et al. (36). Two more clusters (cluster 10 and 11) have Nitrosospira sp. strain 24C and Nitrosospira sp. strain A16 as representative cultures. The last can be related to 16S rRNA gene sequences of Nitrosospira cluster 3. There is no representative pure culture for AmoA cluster 9 and thus it cannot be related to a 16S rRNA gene cluster. The definition of AmoA clusters is presently only tentative and will have to be redefined in future, when more pure cultures and clones are available.
Nitrosospira species of 16S rRNA gene clusters 2, 3, and 4 were frequently observed in soils (9, 14, 20, 22, 24, 29, 35, 44). These studies found that the community structure of ammonia-oxidizing bacteria in soil is influenced by different selective factors, such as pH, gravimetric water content, and fertilizer treatment (reviewed in reference 21). In our previous work, we found that temperature affects the community structure of ammonia oxidizers only after long incubation (>16 weeks) (4), not after short incubation (<4 weeks) (3). Community structure was in addition influenced by fertilizer treatment, indicating that ammonium was also a selective factor for different ammonia-oxidizing populations (4). It seemed desirable to study the effects of temperature and fertilizer in more soils with different communities of ammonia oxidizers. Therefore, we investigated the effect of temperature on the community structure of ammonia oxidizers in three meadow soils which originated from areas with different annual mean temperatures and partially differed in the community structure of ammonia oxidizers.
MATERIALS AND METHODS
Soil samples.
Soil samples were taken from the upper 10-cm layer of three different meadow fields. KMS was sampled from a field close to Moshav Kahal, Lake Kinneret (Israel), while GMS and OMS were sampled from fields near Giessen and Oppenrod (both in Germany), respectively. The main soil characteristics are given in Table 1. The soils were different in their pHs, ranging from acidic (pH 5.0) to slightly alkaline (pH 7.9). The annual mean temperature for the areas where the soil samples were obtained in Germany was lower (9.9 ± 1.9°C, measured in Giessen) than that for the Israeli soil (22.2 ± 1.8°C). All soil samples were air-dried to 15 to 21% gravimetric water content, sieved to <2-mm aggregate size, and stored at 4°C for a few days to 10 months. The soils were extracted with 1 M KCl, and ammonium concentrations were measured colorimetrically (18). The pH was determined after suspension of the soil in 0.01 M CaCl2 or in water. Soil gravimetric water content as well as maximal water-holding capacity were determined by standard protocols (39). At the beginning of each experiment, the soils were adjusted to 60% water-holding capacity.
TABLE 1.
Characteristics of three meadow soils
| Soil | Ammonium concn (μg of NH4+/g of soil) | pH with water/pH with CaCl2 | 60% water holding capacity (g of water/ 100 g of soil) | Mean annual temp (°C), yr | Soil texture | % Carbon | % Nitrogen | Location | Sampling date | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| GMS | 0.45 | 5.78/4.94 | 29.8 | 9.9 ± 1.9, 1999 | Sandy loam over clay | 3.66 | 0.31 | N50°32′, E8°47.3 | February 2000 | 17 |
| OMS | 0.39 | 5.0/4.7 | 27.36 | NDa | Clay loam | 2.7 | 0.21 | N50°35.022′, E8°47.101′ | April 2000 | 13 |
| KMS | 1.7 | 7.93/7.4 | 40.9 | 22.2 ± 1.8, 2001 | ND | 3.75 | 0.37 | Near Kinneret Lake | November 2000 |
ND, not determined.
Experimental set-up.
As in our previous work, long-term experiments were set up both as moist soil incubations and buffered (pH 7) soil slurries (4). All three soils in both set-ups were incubated at different temperatures for 20 weeks. Ammonium had been found to be a selective factor for ammonia oxidizer communities (4). Therefore, the soils were amended with an excess amount of a commercial fertilizer (Flatania, Terrasan) having a total N concentration of 13% (wt/wt). The fertilizer consisted mainly (70%) of horn and hoof material, allowing for gradual release of nitrogen. KMS soil was amended with 0.5 to 1.5% fertilizer at the beginning of the experiment and after 11 weeks of incubation. OMS soil was amended in the beginning of the experiments with two concentrations of fertilizer, high fertilizer (HF) treatment (1%, wt/wt) and low fertilizer (LF) treatment (0.3%, wt/wt). GMS soil received only HF treatment. HF treatments of GMS and OMS were set up in 120-ml bottles with 5 g of moist soil, while HF treatment of KMS and LF treatment of OMS were set up in 500-ml boxes with ca. 150 g of soil.
The fertilizer was mixed into the moist soil. Bottles were incubated at 4, 25, and 30°C. Boxes were incubated at 4, 10, 15, 20, 25, 30, and 37°C in KMS and at 10, 15, 20, 25, and 30°C in LF treatment of OMS. All incubations and samplings were done in duplicate. Both bottles and boxes were opened for aeration every 3 days for 10 min to maintain aerobic condition. Boxes were weighed before incubation and every 3 days during incubation. Since we used boxes that were not tightly closed, water was added occasionally to compensate for evaporation loss. After 6.5 weeks of incubation, the bottles were closed with parafilm instead of tight stoppers to avoid loss of water. After 20 weeks of incubation, the gravimetric water content of the soil samples was measured. On average, soil moisture had decreased from the initial 60% to 38% ± 5% water-holding capacity (GMS) and 42% ± 7% water-holding capacity (OMS). Ammonium was measured at time zero and after 6.5 weeks of incubation (bottles) or after 8, 16, and 20 weeks of incubation (boxes).
Potential nitrification activity was measured after 20 weeks of incubation (40, 46). Sterile Erlenmeyer flasks containing 18 ml of phosphate buffer (1 mM, pH 7.4), 0.04 ml of (NH4)2SO4 (0.25 M), and 2 g of soil were incubated at 25°C on a shaker for 6 h (bottles) or 38 h (boxes). Samples were taken at five time points, centrifuged for 5 min at 4°C, and filtered through regenerated cellulose membrane filters (0.2 μm; Schleicher & Schuell). Samples were stored at −20°C until analysis of nitrite and nitrate in a Sykam ion chromatograph system (5). No nitrite was detected. In both incubations (6 h and 38 h), nitrate increased linearly with time. Rates of potential nitrification activity were determined from the slope of a linear regression of nitrate production versus time. Samples for community analysis were taken after 6.5 and 20 weeks of incubation (bottles) or after 8, 16, and 20 weeks of incubation (boxes).
For comparison, all soils were also set up as slurries. Slurries with 5 g of soil and 15 ml of mineral medium (26) as modified (2) were set up in 250-ml Erlenmeyer flasks. The medium contained nitrogen as urea at a concentration of ca. 4 mM. The pH was adjusted to pH 7.0 to 7.5 with 1 M NaOH once a week. Urea solution (ca. 4 mM) was added occasionally (upon decrease of pH) during the incubation in total amounts of 40 to 220 μmol. The slurries were incubated without shaking in duplicate at 4, 10, 15, 20, 25, and 30°C. Samples for community analysis were taken after 5.5, 12.5, and 19.5 weeks of incubation.
DNA extraction.
Both moist soil and slurry set-ups were sampled for molecular analysis. Approximately 500 mg (wet weight) of soil was transferred into a 2-ml screw-cup tube. Slurry samples (2 ml) were centrifuged at 4°C, and the supernatant was removed. Samples were taken before and after incubation as well as after sampling in the field, all in duplicate. DNA was extracted from the soil samples with the Fast DNA Spin kit for soil (Bio 101, Carlsbad, Calif.), in accordance with the manufacturer's instructions. DNA was cleaned from humic acid, if necessary, with the Wizard DNA clean up kit (Promega, Madison, Wis.).
PCR amplification of amoA.
The primers used for PCR amplification were amoA-1F (38) and amoA-2R-GG (31). In a previous study, this primer (termed amoAR1) showed the best results (4). For DGGE analysis, a GC clamp (30) was added to the 5′ end of primer amoA-1F. Amplification was preformed with 0.5 μM each primer, 1 unit of AmpliTaq DNA polymerase (Perkin-Elmer Applied Biosystems, Weiterstadt, Germany), and 25 μl of MasterAmp 2×PCR premix E containing 100 mM Tris-HCl (pH 8.3), 100 mM KCl, 5 mM MgCl2, 400 μM each deoxynucleoside triphosphate, and the PCR enhancer betaine (Epicentre Technologies, Madison, Wis.). DNA and water (Sigma-Aldrich, Deisenhofen, Germany) were added to a final volume of 50 μl. If necessary, DNA was diluted. Amplifications always started by placing PCR tubes into the preheated (94°C) thermal block of a Mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany). The thermal profile used for amplification was the same as used before (4).
DGGE and cloning.
DGGE was preformed as described previously (30), with slight modifications. PCR products were separated on a polyacrylamide gel with a gradient of 45% (6% [wt/vol] acrylamide-bisacrylamide [37.5:1; Bio-Rad (Labratories, GmbH, Munich, Germany], 18% deionized formamide, 3.1 M urea) to 65% (6% [wt/vol] acrylamide-bisacrylamide [37.5:1; Bio-Rad], 26% deionized formamide, 4.5 M urea). If necessary, the gradient was changed to a narrower gradient, which gives a better resolution. Gels were electrophoresed with the D Gene system (Bio-Rad) with 0.5× Tris-acetate-EDTA at 60°C at 100 V for 17 h. Gels were stained with SYBR Green I (Biozym, Hessisch-Oldendorf, Germany) and scanned with a Storm 860 phosphorimager (Molecular Dynamics, Sunnyvale, Calif.).
Bands were excised from DGGE gels with a Dark Reader transilluminator (Clare Chemical Research, Ross on Wye, United Kingdom). The excised bands were suspended in 200 μl of PCR water, reamplified, and electrophoresed on DGGE again. Some bands were repeatedly electrophoresed and excised until only one band was detectable on DGGE. The purified bands were sequenced. However, in many cases there were still multiple bands after several cycles of excision and reamplification. Therefore, multiple bands were excised, reamplified, ligated to the pGEM T-easy vector, and transformed into Escherichia coli JM109 competent cells (Promega, Madison, Wis.). Alternatively, reamplified bands were cloned with the original TOPO cloning kit (pCR 2.1 vector for Escherichia coli; TOP 10F′; Invitrogen, Leek, The Netherlands) following the manufacturer's instructions. In some cases environmental or experimental samples were cloned. Clones containing a correct insert were reamplified with amoA primers and screened by DGGE, always compared with their environmental or experimental sample. Different clone types reamplified with amoA primers were sequenced as described previously (3).
Phylogenetic analysis.
Based on the sequence information, either deposited in public domain databases or generated in the course of this study, we established a database for amoA. This database was integrated into the ARB program package (http://www.arb-home.de). Derived AmoA sequence types with less than 99% amino acid identity were taken for phylogenetic analysis. For sequence types which exhibited at least 99% amino acid identity to each other, only one representative was considered for construction of trees. Phylogenetic analyses were performed based on 150 deduced amino acid positions with ARB and the Phylip software package, version 3.6a2.1 (12). Trees were reconstructed with the PAM matrix in combination with the neighbor-joining method (ARB and PHYLIP) or Fitch-Margoliash (PHYLIP) (38), parsimony (PHYLIP), or maximum likelihood (Institute Pasteur, Paris, France; http://bioweb.pasteur.fr/sequanal/interfaces/molphy.html).
Correspondence analysis.
DNA fingerprints from DGGE banding patterns on the gels were evaluated categorically, with undetected scored as 0, high intensity scored as 3, and intermediate intensities scored as either 1 or 2. Correspondence analysis was performed on the data with SYSTAT 9 (SPSS Inc., Chicago, Ill.). Correspondence analysis is similar to principal-component analysis but is preferable for analysis of species abundance data if many zero values are present (25). The analysis compared two sets of descriptors (DGGE bands versus samples) with chi-square distances and reduced the multidimensional relationships between them to two principal axes. In our data, the ordination of DGGE bands is used to predict the ordination of samples. That is, the DGGE bands are considered descriptors that create the two-dimensional space in which the samples are scattered.
Nucleotide sequence accession numbers.
The sequences of amoA genes have been deposited in the GenBank nucleotide sequence database under accession numbers AY249650 through AY249655, AY177937, AY177938, and AY254037 for clones retrieved from KMS soil after field sampling; AY249656 through AY249670 for clones and bands retrieved from moist soil incubations of KMS soil; AY249671 through AY249690 for clones and bands retrieved from GMS soil after field sampling; AY249691 through AY249718, AY177934, and AY251475 for clones and bands retrieved from moist soil and slurry treatment of GMS soil; AY249719 through AY249723 and AY177936 for clones and bands retrieved from OMS soil after field sampling; AY249724 through AY249737 and AY249750 for clones and bands retrieved from HF treatment and slurry treatment of OMS soil; and AY249738 through AY249749 for clones and bands retrieved from LF treatment of OMS soil.
RESULTS
Three meadow soils were studied in this work: KMS (Kahal soil) at HF treatment, GMS (Giessen soil) at HF treatment, and OMS (Oppenrod soil) at both HF treatment and LF treatment.
Ammonium measurements.
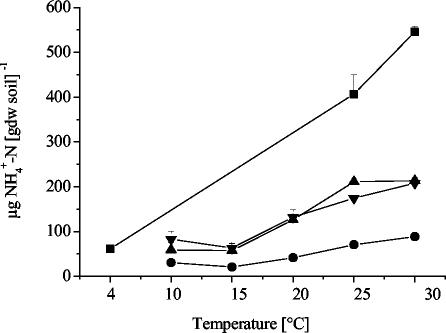
After incubation, ammonium concentrations in all soils were at least 130 times higher than in the field soil samples (Table 1; Fig. 1). The ammonium concentrations in OMS soil incubated at HF treatment increased with increasing temperature (Fig. 1). The concentrations were significantly lower in the LF treatment than in the HF treatment (Fig. 1). Since there was no overlap of the range of ammonium concentrations between HF and LF treatments, soil conditions could also be defined as relatively high and low ammonium concentrations, respectively. Nevertheless, the incubation conditions guaranteed an excess supply of ammonium to the nitrifier populations. The GMS samples at 4°C and 25°C were lost, but the concentration of the sample at 30°C was similar to that of the OMS soil at 30°C in the HF treatment, 590 ± 1 and 545 ± 13 μg of NH4+-N per g (dry weight) of soil, respectively. The ammonium concentrations of KMS soil after 16 and 20 weeks of incubation were high and ranged between 200 and 1,600 μg of NH4+-N per g (dry weight) of soil, with similar values (1,000 to 1,600 μg NH4+-N per g [dry weight] of soil) at 10 to 30°C.
FIG. 1.
Ammonium concentrations after incubation at different temperatures in high fertilizer (HF) treatments and low fertilizer (LF) treatments of OMS soil (Oppenrod, Germany). ▪, HF treatments after 6.5 weeks of incubation; •, LF treatments after 8 weeks of incubation; ▴, LF treatments after 16 weeks of incubation; and ▾, LF treatments after 20 weeks of incubation. Values are means ± standard error (n = 2).
Potential nitrification activity.
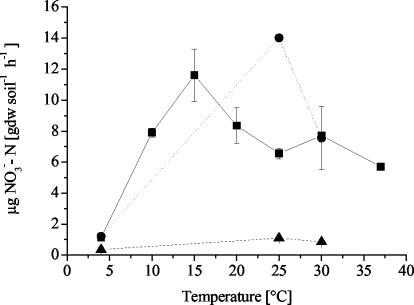
The potential nitrification activity of all soils was measured after incubation for 20 weeks. KMS and GMS soils were rather active (optima of 8.4 ± 1.15 and 6.6 ± 0.4 μg of NO3−-N per g of soil per h at 15°C and 25°C, respectively) (Fig. 2). The lowest activity was always found in the soils incubated at 4°C (between 0.35 and 1.15 μg of NO3−-N per g of soil per h). The potential activities of OMS soil in both HF and LF treatments were very low (<1.1 ± 0.2 μg of NO3−-N per g of soil per h), with an optimum around 15 to 25°C, and activities in the HF treatment were at least two times higher (<1.1 μg of NO3−-N per g of soil per h) than in the LF treatment (<0.6 μg of NO3−-N per g of soil per h).
FIG. 2.
Potential nitrification activity after 20 weeks of incubation at different temperatures. ▪, KMS soil; •, GMS soil; ▴, OMS soil high fertilizer (HF) treatment. Values are means ± standard error (n = 2).
Molecular analysis of field soil samples.
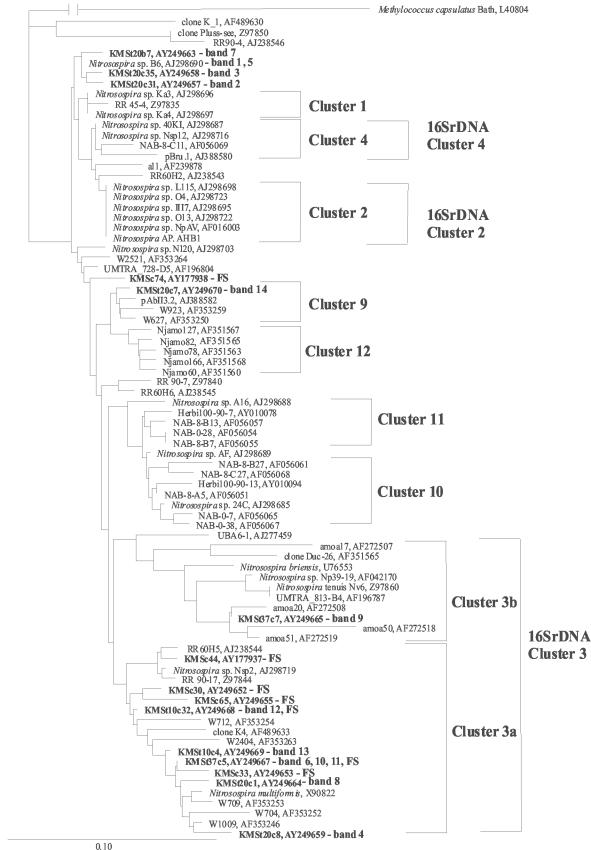
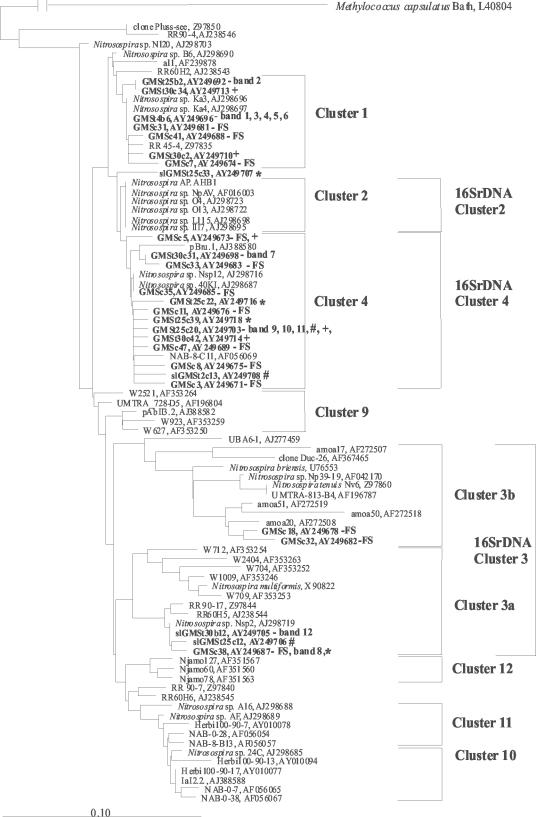
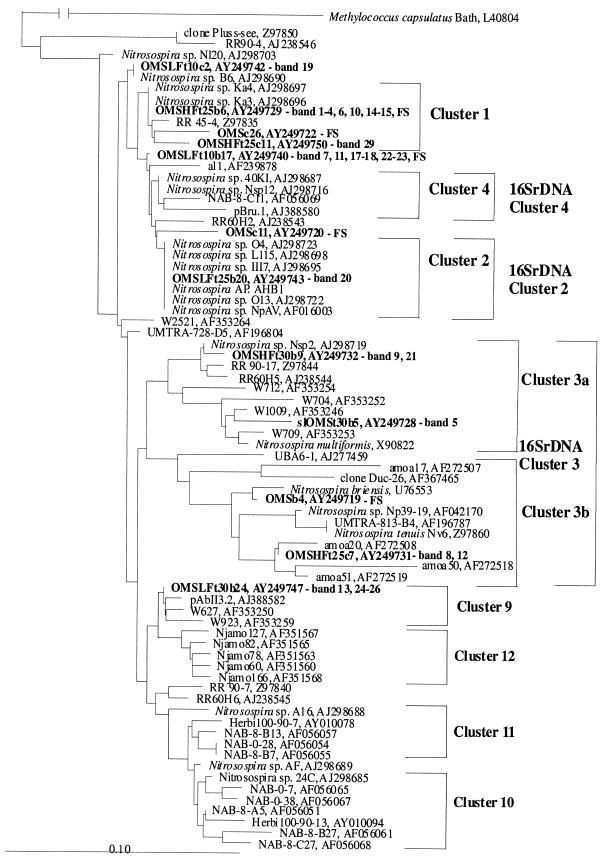
Samples from the three meadow soils were taken for molecular analysis after field sampling. Phylogenetic analysis of AmoA sequences with different algorithms showed the same tree topology and gave clusters 1, 2, 3a, 3b, 4, 9, 10, 11 and 12 (Fig. 3, 4, and 5). We used the same nomenclature for Nitrosospira amoA clusters as in our previous study (4). Interestingly, the majority of sequences from KMS soil grouped together in Nitrosospira cluster 3a, showing low diversity (Fig. 3). Sequences from GMS (Fig. 4) and OMS (Fig. 5) soils, on the other hand, were affiliated with several Nitrosospira AmoA clusters and thus showed a higher diversity than KMS soil. However, Nitrosomonas was not detected, even with a degenerate primer (amoA-2R) that allowed detection in a previous study (3).
FIG.3.
Fitch-Margoliash phylogenetic reconstruction (with global rearrangement and randomized input order) (three jumbles) based on partial AmoA sequences (150 amino acids) retrieved from KMS soil. Clones and bands obtained from this experiment are highlighted in bold. DNA was retrieved from field samples (FS) and the DGGE bands (band number) shown in Fig. 6. The designations of the clones and bands include the following information: t, incubation temperature; c, clone number; and b, band number. The scale bar indicates 10 changes per 100 nucleotide positions. The sequences of DGGE bands that are not mentioned in the tree due to >99% amino acid identity to another sequence: 1, KMSt20c6; 5, KMSt10c1; 6, KMSt37c18; and 10, KMSt37c1. Sequences from public databases are identified by their accession numbers. These sequences were published previously (1, 11, 15, 16, 19, 31, 32, 34, 36-38, 41, 43) or are unpublished sequences deposited in GenBank.
FIG. 4.
Fitch-Margoliash phylogenetic reconstruction (with global rearrangement and randomized input order) (three jumbles) based on partial AmoA sequences (150 amino acids) retrieved from GMS soil. Clones and bands obtained from this experiment are highlighted in bold. DNA was retrieved from field samples (FS) and the DGGE bands shown in Fig. 8. DGGE was conducted on DNA amplified from moist soil (GMS) and soil slurry (slGMS) incubations. Symbols: +, upper faint bands at 30°C of moist soil; *, upper faint bands at 25°C of moist soil; #, upperfaint bands at 25°C of slurry. The designations of the clones and bands include the following information: t, incubation temperature; c, clone number; and b, band number. The scale bar indicates 10 changes per 100 nucleotide positions. The sequences of DGGE bands that are not mentioned in the tree due to >99% amino acid identity to another sequence include: 1, GMSt25c41; 3, GMSt25c29; 4, GMSt25c10; 5, GMSt25c2; 8, GMSt30c4/slGMSt30b8; 9, slGMSt25c9; and 10, slGMSt25b10. Sequences from public databases are identified by their accession numbers. These sequences were published previously (1, 11, 15, 16, 19, 31, 32, 34, 36-38, 41, 43) or are unpublished sequences deposited in GenBank.
FIG.5.
Fitch-Margoliash phylogenetic reconstruction (with global rearrangement and randomized input order) (three jumbles) based on partial AmoA sequences (150 amino acids) retrieved from OMS soil. Clones and bands obtained from this experiment are highlighted in bold. DNA was retrieved from field sample (FS) and the DGGE bands shown in Fig. 9. DGGE was conducted on DNA amplified from moist soil (OMS) at both LF (OMSLF) and HF (OMSHF) treatments and from soil slurry (slOMS) incubations. The designations of the clones and bands include the following information: t, incubation temperature; c, clone number; and b, band number. The scale bar indicates 10 changes per 100 nucleotide positions. The sequences of DGGE bands that are not mentioned in the tree due to >99% amino acid identity to another sequence include: 1, slOMSt25b1; 2, OMSHFt25c16; 3, OMSHFt25b3; 4, OMSHFt30b4; 7, OMSHFt25b7; 8, OMSHFt25c7; 10, slOMSt20c35; 11, slOMSt25c1; 12, slOMSt15b12; 13, slOMSt25b13; 14, OMSLFt20c23; 15, OMSLFt15b15; 18, OMSLFt20b18; 21, OMSLFt30b21; 22, OMSLFt10c44; 23, OMSLFt20c14; 25, OMSLFt30b25; and 26, OMSLFt30b26. Sequences from public databases are identified by their accession numbers. These sequences were published previously (1, 11, 15, 16, 19, 31, 32, 34, 36-38, 41, 43) or are unpublished sequences deposited in GenBank.
Molecular analysis of moist soil and slurry incubations.
Samples were taken for molecular analysis after incubation at different temperatures and for different time periods. Moist samples of each soil were compared with slurry samples of the same soil. Occasional analysis of duplicate soil samples indicated good reproducibility of the DGGE banding patterns. Furthermore, sequences of bands which migrated identically but originated from different temperature treatments were compared and were always found to be identical by amino acid sequence. For example, the sequences of band 6 in GMS soil at 4 and 25°C (GMSt4b6 and GMSt25b6, respectively) showed differences at only 2 bp and no difference in the amino acid sequence.
KMS soil.
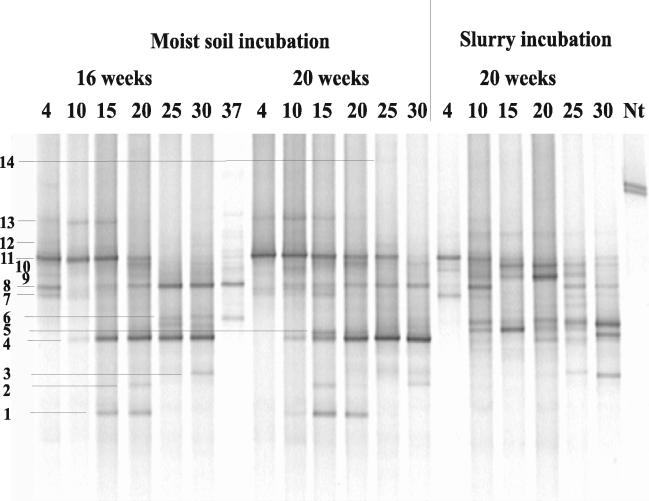
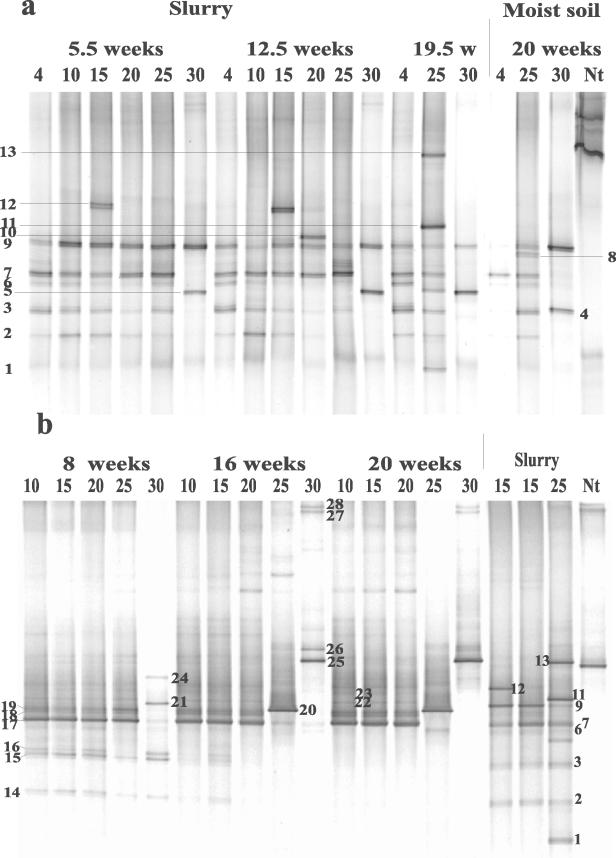
The community structure of the ammonia oxidizers in KMS soil analyzed by DGGE showed a clear change with temperature (4 to 37°C) (Fig. 6). While some DGGE bands (e.g., 4 and 8) could not be detected at low temperature, other bands (e.g., 11 and 13) could not be detected at high temperature. The soil slurries also showed differences at low and high temperatures, although the DGGE patterns were different from those in moist soil incubations (Fig. 6). For example, bands 4 and 8 were detected only at 30°C and only at 20 to 30°C, respectively, while band 11 was detected at 4°C but not at the other temperatures. The AmoA sequences of all these DGGE bands grouped within Nitrosospira cluster 3a, similar to the sequences retrieved after field sampling (Fig. 3). DGGE bands with AmoA sequences outside of AmoA clusters 3a and 3b showed up at low and intermediate temperatures in moist soil samples of KMS but not in the slurry. These were DGGE bands 1 to 2 (at 15 to 20°C), band 5 (at 15°C), and band 7 (at 4 to 20°C), which were all closely related to Nitrosospira sp. strain B6. Note that the pH of KMS soil decreased during incubation at all temperatures above 4°C from pH 7.9 in the field samples to pH 6.2 to 6.6 after incubation. At 4°C, it decreased only to pH 7.5.
FIG. 6.
DGGE analysis of amoA fragments retrieved from KMS soil after 16 and 20 weeks of incubation at different temperatures. Soil samples were from moist soil or slurry incubations. Band numbers are the same as in the corresponding AmoA tree in Fig. 3. Nt represents Nitrosospira tenuis as a reference bacterium.
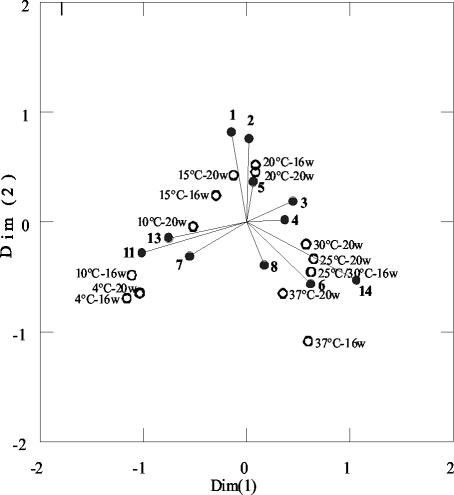
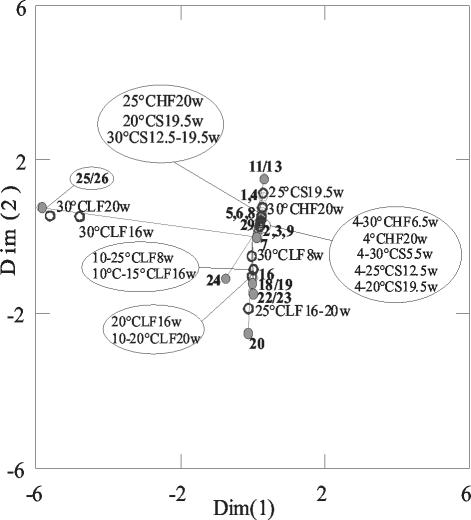
All DGGE patterns (including repeated experiments not shown in Fig. 6) were evaluated categorically (four band intensity categories) and used for correspondence analysis. Correspondence analysis of the DGGE patterns showed that there was a striking influence of temperature treatment on the community structure of ammonia oxidizers in KMS soil (Fig. 7). The results of the analysis were rather robust, since use of only two DGGE band categories (absent and present) allowed the same interpretation.
FIG. 7.
Correspondence analysis comparing the differences in DGGE banding patterns with the program SYSTAT 9. Open circles represent samples of KMS soil which were incubated at different temperatures (4 to 37°C) in the moist soil state. The names of samples indicate the incubation temperature and period of incubation in weeks (w). Solid circles with a line represent bands with numbers in bold.
GMS soil.
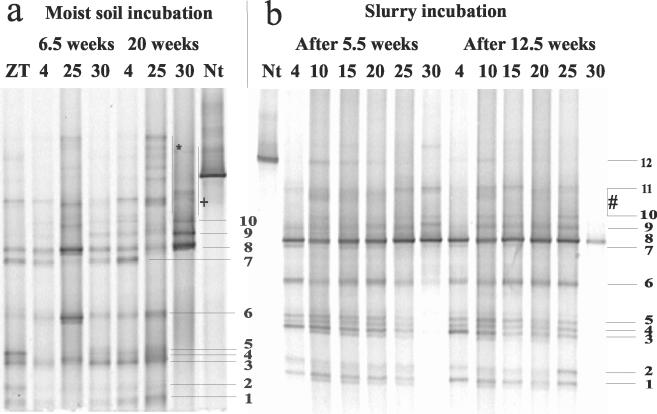
The DGGE patterns of the amoA gene after 6.5 weeks of incubation in moist soil at different temperatures were similar. However, after 20 weeks of incubation, marked differences developed (Fig. 8). The pattern at 30°C was strikingly different from that at the lower temperatures. The bands at the lower part of the gel (1 to 5 in AmoA cluster 1), which were only detected at 4°C and 25°C, could not be detected at 30°C. The dominant band at 30°C (no. 8, in AmoA cluster 3a) was also detected at lower temperatures but was much fainter. The band pattern at the upper part of the gel at 30°C was different from that at 4°C and 25°C. These bands were not always reproduced in repeated assays at 30°C, and the majority of sequences (marked + for 25°C and * for 30°C in Fig. 8) grouped in AmoA cluster 4. Similar to moist soil incubations, the DGGE patterns of slurry incubations showed differences mainly between 30°C and temperature treatments at ≤25°C (Fig. 8). After 5.5 weeks of incubation at 30°C, one dominant band was detectable (no. 8), while bands in the lower part of the gel (AmoA cluster 1) could not be detected. Correspondence analysis (data are not shown) of this soil supported the clear trend of individual bands with incubation temperature.
FIG. 8.
DGGE analysis of amoA fragments retrieved from GMS soil incubated for different times at different temperatures (4 to 30°C) as (a) moist soil and (b) soil slurry. Band numbers are the same as in the corresponding AmoA tree in Fig. 4. Zt, time zero of the experiment; Nt, Nitrosospira tenuis as a reference bacterium.
OMS soil.
Similar to GMS soil, the DGGE patterns of OMS soil in HF moist state treatments showed differences only after 20 weeks of incubation. Note that nitrification potential was low in this soil. The main differences were observed in incubations at 30°C, which were characterized by a small number of bands (only two). One of these bands could also be detected at both 4 and 25°C (band 9, in AmoA cluster 3a). The second band could not be detected at any other temperature than 30°C (band 4, in AmoA cluster 1). On the contrary, no marked differences were observed in DGGE patterns at 4°C and 25°C after 20 weeks of incubation (Fig. 9a) except band 8 (in AmoA cluster 3b), which could be detected only at 25°C (Fig. 9a; Fig. 5). Soil slurries showed a diversity and trends similar to those in the HF treatment of moist soil incubations, again with only two bands detected at 30°C. The sequences of the two bands at 30°C grouped in AmoA cluster 3a.
FIG. 9.
DGGE analysis of amoA fragments retrieved from OMS soil incubated for different times at different temperatures (4 to 30°C). Soil samples were from slurry and moist soil incubations at (a) high fertilizer (HF) treatment and (b) low fertilizer (LF) treatment. As a comparison, three samples from slurry incubations after 12.5 weeks (15°C) and 19.5 weeks (25°C) were loaded on the gel with LF-treated moist soil. Band numbers are the same as in the corresponding AmoA tree in Fig. 5. Nt, Nitrosospira tenuis as a reference bacterium.
The community structure of OMS soil in the moist LF treatment was different from that in the moist HF or the slurry treatment. Note that the nitrification potential was twofold lower in LF- than in HF-treated OMS soil. However, a temperature-related community change occurred (Fig. 9b). At 25°C, a community change occurred only after 16 weeks of incubation and was characterized by a new dominant band (band 20, identical to Nitrosospira sp. strain AHB1), which was not detected at any other temperature. However, at 30°C a first community change had occurred after just 8 weeks of incubation, with sequences of new bands grouping in AmoA cluster 1 (bands 14 and 15), in AmoA cluster 3a (band 21), and in AmoA cluster 9 (band 24). Only bands of cluster 9 could be detected after longer incubation (16 weeks). The DGGE banding pattern in samples at 10 to 20°C did not change during the incubation period of 20 weeks (with the exception of bands 14 to 15, which were not detected after 20 weeks of incubation in any of the temperatures). Most of the sequences at these temperatures were al1-like (accession no. AF239878) (bands 17, 18, 22, and 23).
Correspondence analysis showed that temperature was selecting different communities. However, the effect of ammonium was much stronger (Fig. 10). Temperature-related community changes after 16 to 20 weeks (especially between 10 to 20°C, 25°C, and 30°C) were observed in the LF treatments. In LF treatments, bands 16 to 19 and 22 to 23 (al1-like sequences and Nitrosospira sp. strain B6-like) had a large contribution at 10 to 20°C, band 20 (in AmoA cluster 2) had a large contribution at 25°C, and bands 25 to 26 (in AmoA cluster 9) had a large contribution at 30°C. Separate correspondence analyses for HF treatment and slurry also supported a trend of individual bands with temperature. The results of the analysis were rather robust, since use of only two DGGE band categories (absent and present) allowed the same interpretation.
FIG. 10.
Correspondence analysis comparing the differences in DGGE banding patterns with the program SYSTAT 9. Open circles represent samples of OMS soil which were incubated at different temperatures (4 to 30°C) and/or in different ammonium treatments. The names of samples indicate the temperature, slurry (S), low fertilizer treatment (LF), high fertilizer treatment (HF), and period of incubation in weeks (w). Solid circles with a line represent DGGE bands with numbers in bold. Bands 15, 17, and 21 are not shown because they are identical in amino acid sequence to bands 3, 7, and 9, respectively, but their contribution to the samples was considered in the calculations.
DISCUSSION
Phylogenetic analysis of AmoA sequences resulted in the same tree topology with different algorithms (the trees shown were calculated with the Fitch-Margoliash method), as was observed earlier (4). The only exception was Nitrosospira sp. strain B6, which in the present study did not group within any cluster. The same was the case with al1-like sequences. The phylogenetic affiliation of Nitrosospira sp. strain B6-like and al1-like sequences will become clearer in future when more sequences are available. Phylogenetic trees were calculated with only sequences with less than 99% amino acid, thus avoiding potential PCR bias or silenced mutations that did not contribute to evolution. Bias caused by multiple copies of amoA within one organism (8, 33) could be excluded because amplification of the amoA genes from 31 pure cultures resulted in unambiguous sequences with the amoA-2R primer set (1). Note that the definition of the AmoA clusters is still tentative and that some of them may turn out to be not warranted in future work (4).
The community structure of ammonia oxidizers was studied in three meadow soils. The sequences of ammonia oxidizers after field sampling of KMS soil (Israel) mainly grouped in Nitrosospira AmoA cluster 3a, indicating low diversity, while sequences of OMS and GMS soils (Germany) grouped in more clusters, indicating higher diversity. Sequences from the GMS soil mainly grouped in Nitrosospira AmoA clusters 1 and 4 but also in cluster 3a and 3b, while sequences from the OMS soil grouped in Nitrosospira AmoA clusters 1 and 3b and al1-like sequences.
The effect of temperature on the community structure of ammonia oxidizers was studied by incubation of the soils with excess ammonium, i.e., in moist soil treated with fertilizer and in buffered slurry containing urea. The latter treatment was chosen to keep variables other than temperature (e.g., ammonium concentration, pH, and moisture) at a relatively constant level. This allowed us to unmask the direct effects of temperature on community structure. Although community change usually occurred only after a long period of incubation (16 weeks), as observed before in an agricultural soil (EAS) (4), a community shift after LF treatment of OMS soil incubated at 30°C occurred after just 8 weeks of incubation. The community change among ammonia oxidizers was analyzed by DGGE with a nondegenerate primer set (amoA-2R GG/amoAR1) (31). The quality of this primer set was tested in our previous study (4). Nevertheless, we cannot exclude that we missed some amoA sequences that were actually present in the environmental samples.
None of the meadow soils showed any Nitrosomonas-like species independently of the incubation conditions. This was in agreement with previous observations that Nitrosospira species are frequently observed in soils (9, 14, 20, 22-24, 29, 35, 44). On the other hand, Phillips et al. (35) detected Nitrosomonas-like sequences in DNA extracted from most-probable-number cultures but not in the DNA extracted directly from the soil. The fact that we did not get any Nitrosomonas-like sequences in our slurry incubations could be explained by our use of a medium containing urea. Aarka et al. (2) observed that a medium containing urea supported the growth of Nitrosospira spp., while a medium containing ammonium sulfate supported the growth of Nitrosomonas spp. The failure to detect Nitrosomonas spp. in the field samples and moist soil incubations indicates that this ammonia oxidizer was not among the dominant populations.
Two patterns of community change due to temperature effect were observed in this study. One pattern was a community change within AmoA cluster 3a, and the second pattern was a community change between different clusters of Nitrosospira. The first pattern was observed in KMS soil and was supported by correspondence analysis of the DGGE patterns. Although different trends of temperature dependency were observed, most of the representative sequences were grouped within AmoA cluster 3a. This was in agreement with our previous study of EAS soil, where AmoA clusters 3a and 3b were found at most temperatures, although individual members of this cluster exhibited different trends with temperature (4). The variability within this cluster with respect to temperature was further supported in this study.
The second pattern of community change due to temperature was observed in GMS and OMS soils, and the change was between clusters of Nitrosospira. HF treatments resulted in a change from dominance of Nitrosospira AmoA clusters 1 and 4 or 1 and al1-like sequences at temperatures of ≤25°C to a dominance of AmoA cluster 3 at 30°C in the HF treatment but not in the LF treatment. In contrast to this study, in our previous study AmoA cluster 1 was found in EAS soil at 4 to 10°C (4) but not at the intermediate temperature range. These findings might be explained by the low ammonium concentrations in EAS soil at intermediate temperatures. The observed stimulation of AmoA cluster 3 in the HF treatment but not in the LF treatment of the present study is in agreement with the results of Kowalchuk et al. (23, 24), who showed that members of Nitrosospira 16S rRNA gene cluster 3 are dominant in early successional soils having relatively high ammonium concentrations, though the ammonium concentrations in our study were about 10-fold higher. Furthermore, it is known that cultured strains from Nitrosospira cluster 3 grow well in high-ammonium culture media (7). However, in our previous study of EAS soil, AmoA clusters 3a and 3b were not necessarily dominant at high ammonium concentrations (4). That might imply that individual members of AmoA clusters 3a and 3b show a different trend not only for temperature but also for ammonium. On the other hand, at 30°C in the LF treatment of OMS soil, Nitrosospira AmoA cluster 9 was the dominant group. Interestingly, AmoA cluster 9 was found only at high temperature and low fertilizer concentrations. This is in agreement with the results for the EAS soil, where AmoA cluster 9 could not be detected at high ammonium concentrations (4), and also with the results of Oved et al. (34), who found this cluster in irrigated agricultural soil which had been treated with low ammonium concentrations.
The OMS soil was more acidic (pH 5.0) than the other soils. However, bands representing Nitrosospira cluster 2 were hardly detected in this soil. Nitrosospira sp. strain AHB1, which was isolated in acidic medium, as well as environmental sequences and enrichment cultures from acidic soils (pH 4.2) all group in this cluster (23, 44, 45). On the other hand, two other native soils of pH 3.3 and 5.4 showed a high diversity represented by 16S rRNA gene environmental sequences grouping in Nitrosospira clusters 1, 3, 4, 6, and 7 (9, 47) but not in Nitrosospira cluster 2. Moreover, Carnol et al. (10) recently found a dominance of Nitrosomonas species in an acidic forest soil. Therefore, the detection of Nitrosospira cluster 2 probably depends on factors in addition to low pH.
In conclusion, a community change as a result of temperature was observed in the three soils examined as slurries and LF and HF treatments. Two patterns of community shift were observed, within Nitrosospira AmoA cluster 3a and between different clusters of Nitrosospira. Nitrosospira clusters 3a and 3b showed different trends with respect to temperature and ammonium, reflecting the high versatility of ammonia oxidizers within this cluster. Some AmoA clusters exhibited a clear trend with temperature even when other soil variables were kept at a relatively constant level (slurry experiments). These observations demonstrate that temperature had an effect on the community structure of ammonia oxidizers in soils. The effect of ammonium on the community structure of ammonia oxidizers which had been observed in an agricultural (EAS) soil (4) was further supported by fertilizer treatment of a meadow (OMS) soil. The results of our study are of ecological importance, showing that a community change among ammonia oxidizers can be expected in nature when the temperature changes for a long period (8 to 16 weeks), e.g., from winter to summer. In addition, it is possible that geographic areas with different mean annual temperatures may have different community structures of ammonia oxidizers. Our study also shows that the community structure of ammonia oxidizers is affected by treatment with ammonium-containing fertilizers. Temperature may be partially effective via changes in soil ammonium concentrations. Our results indicate, however, that temperature also has a direct effect on the community structure of ammonia oxidizers.
Acknowledgments
We thank Peter Dunfield for advice on correspondence analysis, Werner Eckert for sampling the Kahal soil, and Dror Minz for discussion.
This study was supported financially by the German Federal Ministry for Education and Research within the BIOLOG Biodiversity Program (01LC0021).
REFERENCES
- 1.Aakra, A., J. B. Utaker, and I. F. Nes. 2001. Comparative phylogeny of the ammonia monooxygenase subunit A and 16S rRNA genes of ammonia-oxidizing bacteria. FEMS Microbiol. Lett. 205:237-242. [DOI] [PubMed] [Google Scholar]
- 2.Aakra, A., J. B. Utaker, I. F. Nes, and L. R. Bakken. 1999. An evaluated improvement of the extinction dilution method for isolation of ammonia-oxidizing bacteria. J. Microbiol. Methods 39:23-31. [DOI] [PubMed] [Google Scholar]
- 3.Avrahami, S., R. Conrad, and G. Braker. 2002. Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl. Environ. Microbiol. 68:5685-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avrahami, S., W. Liesack, and R. Conrad. 2003. Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 5:691-705. [DOI] [PubMed]
- 5.Bak, F., G. Scheff, and K. H. Jansen. 1991. A rapid and sensitive ion chromatographic technique for the determination of sulfate and sulfate reduction rates in freshwater lake sediments. FEMS Microbiol. Ecol. 85:23-30. [Google Scholar]
- 6.Belser, L. W. 1979. Population ecology of nitrifying bacteria. Annu. Rev. Microbiol. 33:309-333. [DOI] [PubMed] [Google Scholar]
- 7.Belser, L. W., and E. L. Schmidt. 1978. Diversity of ammonia-oxidizing nitrifier populations in a soil. Appl. Environ. Microbiol. 36:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruns, M. A., M. R. Fries, J. M. Tiedje, and E. A. Paul. 1998. Functional gene hybridization patterns of terrestrial ammonia-oxidizing bacteria. Microb. Ecol. 36:293-302. [DOI] [PubMed] [Google Scholar]
- 9.Bruns, M. A., J. R. Stephen, G. A. Kowalchuk, J. I. Prosser, and E. A. Paul. 1999. Comparative diversity of ammonia oxidizer 16S rRNA gene sequences in native, tilled, and successional soils. Appl. Environ. Microbiol. 65:2994-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnol, M., G. A. Kowalchuk, and W. De Boer. 2002. Nitrosomonas europaea-like bacteria detected as the dominant beta-subclass Proteobacteria ammonia oxidisers in reference and limed acid forest soils. Soil Biol. Biochem. 34:1047-1050. [Google Scholar]
- 11.Chang, Y. J., A. K. S. J. R. Hussain, M. D. Mullen, D. C. White, and A. Peacock. 2001. Impact of herbicides on the abundance and structure of indigenous beta-subgroup ammonia-oxidizer communities in soil microcosms. Environ. Toxicol. Chem. 20:2462-2468. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1989. PHILIP — phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 13.Gödde, M., and R. Conrad. 1999. Immediate and adaptational temperature effects on nitric oxide production and nitrous oxide release from nitrification and denitrification in two soils. Biol. Fertil. Soils 30:33-40. [Google Scholar]
- 14.Hastings, R. C., C. Butler, I. Singleton, J. R. Saunders, and A. J. Mccarthy. 2000. Analysis of ammonia-oxidizing bacteria populations in acidic forest soil during conditions of moisture limitation. Lett. Appl. Microbiol. 30:14-18. [DOI] [PubMed] [Google Scholar]
- 15.Horz, H. P., J. H. Rotthauwe, T. Lukow, and W. Liesack. 2000. Identification of major subgroups of ammonia-oxidizing bacteria in environmental samples by T-RFLP analysis of amoA PCR products. J. Microbiol. Methods 39:197-204. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova, I. A., J. R. Stepeh, Y. J. Chang, J. Bruggemann, P. E. Long, J. P. McKinley, G. A. Kowlachuk, D. C. White, and S. J. Macnaughton. 2000. A survey of 16S rRNA and amoA genes related to autotrophic ammonia-oxidizing bacteria of the beta-subdivision of the class proteobacteria in contaminated groundwater. Can. J. Microbiol. 46:1012-1020. [DOI] [PubMed] [Google Scholar]
- 17.Kammann, C., L. Grünhage, and H. J. Jäger. 2001. A new sampling technique to monitor concentrations of CH4, N2O and CO2 in air at well-defined depths in soils with varied water potential. Eur. J. Soil Sci. 52:297-303. [Google Scholar]
- 18.Kandeler, E., and H. Gerber. 1988. Short-term assay of soil urease activity with colorimetric determination of ammonium. Biol. Fertil. Soils 6:68-72. [Google Scholar]
- 19.Klotz, M. G., and J. M. Norton. 1995. Sequence of an ammonia monooxygenase subunit A-encoding gene from Nitrosospira sp. NpAV. Gene 163:159-160. [DOI] [PubMed] [Google Scholar]
- 20.Kowalchuk, G. A., P. L. E. Bodelier, G. H. J. Heilig, J. R. Stephen, and H. J. Laanbroek. 1998. Community analysis of ammonia-oxidising bacteria, in relation to oxygen availability in soils and root-oxygenated sediments, with PCR, DGGE and oligonucleotide probe hybridisation. FEMS Microbiol. Ecol. 27:339-350. [Google Scholar]
- 21.Kowalchuk, G. A., and J. R. Stephen. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu. Rev. Microbiol. 55:485-529. [DOI] [PubMed] [Google Scholar]
- 22.Kowalchuk, G. A., J. R. Stephen, W. DeBoer, J. I. Prosser, T. M. Embley, and J. W. Woldendorp. 1997. Analysis of ammonia-oxidizing bacteria of the b subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol. 63:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowalchuk, G. A., A. W. Stienstra, G. H. J. Heilig, J. R. Stephen, and J. W. Woldendorp. 2000. Changes in the community structure of ammonia-oxidizing bacteria during secondary succession of calcareous grasslands. Environ. Microbiol. 2:99-110. [DOI] [PubMed] [Google Scholar]
- 24.Kowalchuk, G. A., A. W. Stienstra, G. H. J. Heilig, J. R. Stephen, and J. W. Woldendorp. 2000. Molecular analysis of ammonia-oxidising bacteria in soil of successional grasslands of the Drentsche A (The Netherlands). FEMS Microbiol. Ecol. 31:207-215. [DOI] [PubMed] [Google Scholar]
- 25.Legendre, P., and L. Legendre. 1998. Developments in environmental modelling, vol. 20. Elsevier, Amsterdam, The Netherlands.
- 26.MacDonald, R. M., and J. R. Spokes. 1980. A selective and diagnostic medium for ammonia oxidising bacteria. FEMS Microbiol. Lett. 8:143-145. [Google Scholar]
- 27.Mahendrappa, M. K., R. L. Smith, and A. T. Christiansen. 1966. Nitrifying organisms affected by climate region in western United States. Soil Sci. Soc. Am. J. 30:60-62. [Google Scholar]
- 28.Mahli, S. S., and W. B. McGill. 1982. Nitrification in three Alberta soils: effect of temperature, moisture and substrate concentrations. Soil Biol. Biochem. 14:393-399. [Google Scholar]
- 29.Mendum, T. A., R. E. Sockett, and P. R. Hirsch. 1999. Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the b subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer. Appl. Environ. Microbiol. 65:4155-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muyzer, G., T. Brinkhoff, U. Nuebel, C. Santegoeds, H. Schafer, and C. Wawer. 1997. Denaturing gradient gel electrophoresis (DGGE) in microbial ecology, p. 1-27. In A. D. L. Akkermans, J. D. VanElsas, and F. J. DeBruijn (ed.), Molecular microbial ecology manual. Kluwer, Dordrecht, The Netherlands.
- 31.Nicolaisen, M. H., and N. B. Ramsing. 2002. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J. Microbiol. Methods 50:189-203. [DOI] [PubMed] [Google Scholar]
- 32.Norton, J. M., J. J. Alzerreca, Y. Suwa, and M. G. Klotz. 2002. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch. Microbiol. 177:139-149. [DOI] [PubMed] [Google Scholar]
- 33.Norton, J. M., J. M. Low, and G. Martin. 1996. The gene encoding ammonia monooxygenase subunit A exists in three nearly identical copies in Nitrosospira sp NpAV. FEMS Microbiol. Lett. 139:181-188. [DOI] [PubMed] [Google Scholar]
- 34.Oved, T., A. Shaviv, T. Goldrath, R. T. Mandelbaum, and D. Minz. 2001. Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil. Appl. Environ. Microbiol. 67:3426-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips, C. J., D. Harris, S. L. Dollhopf, K. L. Gross, J. I. Prosser, and E. A. Paul. 2000. Effects of agronomic treatments on structure and function of ammonia-oxidizing communities. Appl. Environ. Microbiol. 66:5410-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purkhold, U., A. Pommerening-Röser, S. Juretschko, M. C. Schmid, H. P. Koops, and M. Wagner. 2000. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl. Environ. Microbiol. 66:5368-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotthauwe, J. H., W. DeBoer, and W. Liesack. 1995. Comparative analysis of gene sequences encoding ammonia monooxygenase of Nitrosospira sp AHB1 and Nitrosolobus multiformis C-71. FEMS Microbiol. Lett. 133:131-135. [DOI] [PubMed] [Google Scholar]
- 38.Rotthauwe, J. H., K. P. Witzel, and W. Liesack. 1997. The ammonia monooxygenase structural gene amoA as a functional marker — molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63:4704-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlichting, E., and H. P. Blume. 1966. Bodenkundliches Praktikum. Paul Parey, Hamburg, Germany.
- 40.Schmidt, E. L., and L. W. Belser. 1994. Autotrophic nitrifying bacteria, p. 160-177. In J. M. Mickelson, M. Weaver, S. Angle, P. Bottomley, D. Bezdicek, S. Smith, A. Tabatabai, and A. Wollum (ed.), Methods in soil analysis. American Society of Agronomy, Soil Science Society of America, Madison, Wis.
- 41.Semrau, J. D., A. Chistoserdov, J. Lebron, A. Costello, J. Davagnino, E. Kenna, A. J. Holmes, R. Finch, J. C. Murrell, and M. E. Lidstrom. 1995. Particulate methane monooxygenase genes in methanotrophs. J. Bacteriol. 177:3071-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark, J. M., and M. K. Firestone. 1996. Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol. Biochem. 28:1307-1317. [Google Scholar]
- 43.Stephen, J. R., Y. J. Chang, S. J. Macnaughton, G. A. Kowalchuk, K. T. Leung, C. A. Flemming, and D. C. White. 1999. Effect of toxic metals on indigenous soil beta-group proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl. Environ. Microbiol. 65:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephen, J. R., G. A. Kowalchuk, M. A. V. Bruns, A. E. McCaig, C. J. Phillips, T. M. Embley, and J. I. Prosser. 1998. Analysis of beta-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl. Environ. Microbiol. 64:2958-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephen, J. R., A. E. McCaig, Z. Smith, J. I. Prosser, and T. M. Embley. 1996. Molecular diversity of soil and marine 16S rRNA gene sequences related to beta-subgroup ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 62:4147-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stienstra, A. W., P. K. Gunnewiek, and H. J. Laanbroek. 1994. Repression of nitrification in soils under a climax grassland vegetation. FEMS Microbiol. Ecol. 14:45-52. [Google Scholar]
- 47.Webster, G., T. M. Embley, and J. I. Prosser. 2002. Grassland management regimens reduce small-scale heterogeneity and species diversity of β-proteobacterial ammonia oxidizer populations. Appl. Environ. Microbiol. 68:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]