Abstract
A region of 12 kb flanking the structural gene of the cyclic antibacterial peptide circularin A of Clostridium beijerinckii ATCC 25752 was sequenced, and the putative proteins involved in the production and secretion of circularin A were identified. The genes are tightly organized in overlapping open reading frames. Heterologous expression of circularin A in Enterococcus faecalis was achieved, and five genes were identified as minimally required for bacteriocin production and secretion. Two of the putative proteins, CirB and CirC, are predicted to contain membrane-spanning domains, while CirD contains a highly conserved ATP-binding domain. Together with CirB and CirC, this ATP-binding protein is involved in the production of circularin A. The fifth gene, cirE, confers immunity towards circularin A when expressed in either Lactococcus lactis or E. faecalis and is needed in order to allow the bacteria to produce bacteriocin. Additional resistance against circularin A is conferred by the activity of the putative transporter consisting of CirB and CirD.
Antimicrobial peptides or bacteriocins are produced by various gram-positive and gram-negative bacteria. Although many bacteriocins inhibit the growth of strains closely related to the bacteriocin producer, an ever-increasing number of bacteriocins have a broader activity range. Only a few antimicrobial peptides of clostridial origin have been characterized at the molecular level, despite their extensive use as a means of identifying and typing clostridia (24, 32). The three bacteriocins that have been partially characterized are BCN5, boticin B, and circularin A (9, 12, 25), produced by Clostridium perfringens, Clostridium botulinum, and Clostridium beijerinckii, respectively.
Circularin A, a circular bacteriocin produced by C. beijerinckii ATCC 25752, is active against a broad range of gram-positive bacteria (25). The circularization of the peptide involves a head-to-tail peptide bond formation between the fourth and last amino acid of the precursor peptide (25). Circularin A shares limited sequence homology with enterocin AS-48 (also known as Bac21), a cyclic bacteriocin from Enterococcus faecalis (34, 56), but its precursor lacks the long leader present in the enterocin AS-48 precursor. The circularin A gene cluster is chromosomally located, while the enterocin AS-48 operon is located on a plasmid.
Both circularin A and enterocin AS-48 belong to the recently defined class V bacteriocins of ribosomally synthesized, nonmodified, head-to-tail-ligated cyclic antibacterial peptides (25). Other class V bacteriocins are microcin J25 and gassericin A (4, 22). Microcin J25, peptide of 21 amino acid residues produced by Escherichia coli, is the only circular peptide known so far that is produced by a gram-negative bacterium (4). The genes involved in the production of microcin J25 are located in an operon immediately downstream of the structural gene (51). Gassericin A is produced by Lactobacillus gasseri as a 91-amino-acid precursor peptide that is circularized after removal of a leader peptide of 33 amino acids (22, 23). The coding regions of enterocin AS-48 of two strains have been sequenced and determined to be almost identical (35, 56). Enterocin AS-48 is a tightly packed peptide containing five α-helices and is structurally related to NK-lysin, a cytotoxic peptide from human natural killer or T cells (16).
Most bacteriocins require processing of a precursor peptide in order to become (fully) active. For many bacteriocins, the genes encoding processing, secretion, and immunity functions flank the structural gene. Processing can involve modification of amino acids, as is the case in lantibiotics, leader peptide removal, or, in the case of circular peptides, circularization. The mechanisms underlying these modifications are poorly understood, although the proteins involved are generally known. The secretion of most bacteriocins occurs via dedicated ABC transporters (26), while some can be secreted via the general secretion pathway (5, 21, 33).
Immunity systems for bacteriocins are poorly characterized, but it has been demonstrated that specialized immunity proteins confer immunity to bacteriocin action on cells by blocking access to a putative receptor, as is the case for the lactococcin A immunity protein LciA (58). In some cases ABC transporters have been shown to be involved, e.g., the NisFEG system in nisin resistance and McbFE in microcin B17 resistance (13, 43). Little homology exists among bacteriocin immunity proteins, even those that are involved in immunity against bacteriocins of the same class.
In this study we identified the genes required for functional heterologous expression of circularin A and showed that two independent mechanisms confer reduced circularin A sensitivity, one of which is based on the expression of cirE and the other on the combined expression of cirB and cirD. As such, this study will further the field of clostridial bacteriocins and that of class V (circular) bacteriocins in particular.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
The strains and plasmids used in this study are listed in Table 1. Clostridium beijerinckii ATCC 25752 was grown anaerobically at 30°C in AC broth (Difco, Detroit, Mich.). Anaerobicity was obtained by chemical absorption of the oxygen in closed bottles as previously described (25). Lactobacillus saké ATCC 15521 was grown in De Man Rogosa and Sharpe (MRS; Merck, Darmstadt, Germany) broth at 30°C. Twofold-diluted M17 broth (Difco) with a final concentration of 1.9% β-glycerophosphate (Merck) and 0.5% glucose (G[1/2]M17) was used for growth of Lactococcus lactis NZ9000 and Enterococcus faecalis JH2-2 at 30°C and 37°C, respectively. Escherichia coli DH5α was grown for 16 h in tryptone-yeast (TY) broth at 37°C with vigorous agitation (250 rpm). For growth on plates, medium containing 1.5% agar was used. Ampicillin (Sigma, Zwijndrecht, The Netherlands) and chloramphenicol (Sigma) were used at 100 and 10 μg/ml, respectively, for E. coli. Chloramphenicol and erythromycin (Sigma) were used at 5 μg/ml each for L. lactis NZ9000 and at 20 and 2 μg/ml, respectively, for E. faecalis JH2-2. When used together, chloramphenicol and erythromycin were employed at 2.5 μg/ml each for Lactobacillus saké ATCC 15521 or at 10 μg/ml (chloramphenicol) and 2 μg/ml (erythromycin) for E. faecalis JH2-2.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Reference or sourceb |
|---|---|---|
| C. beijerinckii ATCC 25752 | Circularin A producer | NIZO |
| E. faecalis JH2-2 | Plasmid-free derivative of E. faecalis JH-2 | 20 |
| L. lactis NZ9000 | Plasmid-free derivative of L. lactis MG1363, pepN::nisRK | 30 |
| Lactobacillus sake ATCC 15521 | Bacteriocin indicator | lab collection |
| E. coli Top10 | F′ mcrA (mrr-hsdRMS-mcrBC) φ80lacZM15 lacX74 recA1 deoR araD139 (ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| E. coli DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 17 |
| Plasmids | ||
| pMG36c | Cmr, pWV01-based cloning vector carrying the strong lactococcal promoter P32 | Laboratory collection |
| pMG36e | Emr, gene expression vector carrying P32 | 57 |
| pCR21 | Ampr Kmr | Invitrogen |
| pCRAE | Ampr Kmr | This study |
| pIL253 | Emr, theta replicating cloning vector | 49 |
| pIL-E | Emr, pIL253 derivative with cirE under control of P32 | This study |
| pIL-P32 | Emr, pIL253 derivative with P32 promoter | This study |
| pMGAE1 | Cmr, pMG36c derivative with cirABCDE under control of P32 | This study |
| pCir | Cmr, derivative of pMGAE1 overexpressing circularin A | This study |
| pCirΔA | Cmr, pCir ΔcirA | This study |
| pCirΔB | Cmr, pCir ΔcirB | This study |
| pCirΔC | Cmr, pCir ΔcirC | This study |
| pCirΔD | Cmr, pCir ΔcirD | This study |
| pCirΔE | Cmr, pCir ΔcirE | This study |
| pCirΔAE | Cmr, pCir ΔcirA ΔcirE | This study |
| pCirΔBE | Cmr, pCir ΔcirB ΔcirE | This study |
| pCirΔCE | Cmr, pCir ΔcirC ΔcirE | This study |
| pCirΔDE | Cmr, pCir ΔcirD ΔcirE | This study |
| pCirΔACE | Cmr, pCir ΔcirA ΔcirC ΔcirE | This study |
| pCirΔABCE | Cmr, pCir ΔcirA ΔcirB ΔcirC ΔcirE | This study |
| pCirΔACDE | Cmr, pCir ΔcirA ΔcirC ΔcirD ΔcirE | This study |
| pMG-E | Emr, pMG36e derivative with cirE under control of P32 | This study |
Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Emr, erythromycin resistance; Kmr, kanamycin resistance.
NIZO, NIZO Food Research Ede, The Netherlands; Invitrogen, Breda, The Netherlands.
Nucleotide sequencing.
Inverse PCR techniques with the nucleotide sequence of cirA (25) were employed to obtain the region surrounding the cirA gene. PCR products were sequenced either directly or after subcloning of restriction enzyme digestion fragments in pUC19. PCR products were purified with the High Pure PCR product purification kit of Roche (Roche Diagnostics GmbH, Mannheim, Germany). Sequencing was performed with indodicarbocyanine-labeled universal, reverse, or T7 primers (Amersham Pharmacia Biotech, Roosendaal, The Netherlands) and the ALFII system (Amersham Pharmacia Biotech) according to the protocols of the supplier with the following modifications: the power was set at 15 and 18 W for the long-read and high-resolution gels, respectively.
Bacteriocin assays.
Colony overlayer assays were performed as described previously (25). Bacteriocin activity in C. beijerinckii ATCC 25752 supernatant was quantified in triplicate by a critical dilution assay as described by Geis et al. (14), with the modification that assays were performed in microtiter plates. To 50 μl of serially diluted, bacteriocin-containing samples, 150 μl of medium containing the indicator strain Lactobacillus saké ATCC 15521(pMG36e; pMG36c), diluted 100-fold from a stationary-phase overnight culture, was added unless mentioned otherwise. Resistance to bacteriocin was determined by plating strains on plates containing 4 or 10% (vol/vol) filter-sterilized C. beijerinckii ATCC 25752 supernatant containing circularin A. Alternatively, a critical-dilution assay was performed with the strain of interest as an indicator strain. In these critical-dilution assays, 100 μl of bacteriocin-containing sample was mixed with 100 μl of freshly diluted (1,000-fold) indicator strains.
Cloning methods and materials.
Molecular cloning techniques were performed essentially as described by Sambrook et al. (45). Restriction enzymes, T4 DNA ligase, and Expand DNA polymerase were obtained from Roche (Roche Diagnostics GmbH) and used as specified by the supplier. L. lactis NZ9000 was transformed as described by Shepard and Gilmore (19) with 1% glycine (Merck). E. faecalis JH2-2 was transformed as described previously (47; http://w3.ouhsc.edu/enterococcus) with 8% glycine. After transformation, both strains were plated on G[1/2]M17 medium containing 0.5 M sucrose and the appropriate antibiotics. Plasmids from L. lactis NZ9000, Escherichia coli DH5α, and E. faecalis JH2-2 were isolated according to Birnboim (3), with the following modifications for E. faecalis: mutanolysin (1 U/ml; Sigma) was added to the suspension buffer to facilitate lysis, and plasmids isolated from 50 ml of culture were, after RNase (0.5 mg/ml; Sigma) treatment, further purified with the High Pure PCR product purification kit (Roche Diagnostics GmbH).
Cloning of the circularin A determinant.
The region encompassing cirA to cirE was amplified with primers located just upstream of cirA (B51426, 5′-ACGCGTCGACTCATGAGTTTTTCAAAAGGAGGTGATTAATT ATGTTTTTATTGCAGG-3′) and downstream of cirE (B51427, 5′-CGCGGATCCGTCGACCTCTCCCACTTTAACATTAGTTATTGCTC-3′). SalI-RcaI and BamHI-SalI sites, respectively, in the two primers are underlined. All enzymes (Roche) were used according to the manufacturer's instruction. The PCR product was cloned with the Zero-Blunt Topo PCR cloning kit (Invitrogen, Breda, The Netherlands), creating pCRAE. The plasmid pCRAE was digested with SpeI and XhoI. The fragment carrying cirA was ligated into pMG36c digested with XbaI and SalI, and the ligation mixture was used to transform E. coli DH5α. Transformants were identified by growth on TY agar with chloramphenicol. The correct plasmid, pMGAE1, was isolated as described above and introduced into E. faecalis JH2-2. Three consecutive selection steps of clean streaking and testing for a strain with a high and stable bacteriocin expression phenotype with the colony overlay assay yielded E. faecalis JH2-2 carrying a pMGAE1 derivative labeled pCir.
In-frame deletions of cirA through cirD were made by amplifying pCir by PCR with appropriate outward-facing primers, creating a PCR product of the entire plasmid but lacking the gene of interest. The primers used were 5′-AGTATGGCAAGAGCTATAGC-3′ and 5′-CACGCCTAGTGCTCCTGC-3′ for ΔcirA, 5′-TAATTATGCCTGTATCATACC-3′ and 5′-CCAAGAGTTATAGTTTGAGT CG-3′ for ΔcirB, 5′-GTGCACATAGGTAGGATTTTAAG-3′ and 5′-GAAACATTCC AACAATAATACC-3′ for ΔcirC, and 5′-GAACTTAATCTAGTTAACGGAAG-3′ and 5′-AGTTATCTCTAGCATAGGCTTC-3′ for ΔcirD.
Each PCR product was kinase treated with T4 polynucleotide kinase (Amersham Pharmacia Biotech) in T4 ligase buffer (Roche Diagnostics GmbH) and subsequently self-ligated with T4 ligase, creating the plasmids pCirΔA, pCirΔB, pCirΔC, and pCirΔD. Derivatives of pCir with a deletion of cirE and a deletion of one of the other genes cirABCD, pCirΔAE, pCirΔBE, pCirΔCE, or pCirΔDE, were made by the same method with the primers 5′-CATATATTCTACTACCTTTC-3′ and 5′-GTAATTAAAGGCTCTAATAAG-3′ for ΔcirE and the plasmids carrying the respective single deletions as templates in the PCR. A plasmid with a triple deletion, pCirΔACE, was constructed likewise by deleting cirC with pCirΔAE as a template and the primers used for the single deletion of cirC. Based on pCirΔACE, pCirΔABCE and pCirΔACDE were made with the primers employed for the single knockouts of cirB and cirD, respectively. All plasmids were isolated with E. faecalis JH2-2 as the cloning host.
The cirE gene was cloned behind the lactococcal chromosomal P32 promoter by digesting the PCR product obtained with primers B51426 and B51427 and HpaI and SalI and ligating the cirE-carrying fragment into SmaI- and SalI-digested pMG36e, leading to pMG-E. Colonies obtained after transformation of L. lactis NZ9000 were replica streaked onto G[1/2]M17 plates with 4% (vol/vol) C. beijerinckii ATCC 252752 supernatant to screen for circularin A immunity. The plasmid was isolated and used to transform E. faecalis JH2-2. In order to make pCirΔE, a fragment with the immunity gene cirE behind the P32 promoter was first cloned in pIL253 to avoid possible lethal effects of bacteriocin expression without immunity. This was done by digesting pMG-E with EcoRI and SalI and ligating the cirE-carrying fragment into pIL253 digested with the same enzymes. The resulting plasmid (pIL-E) was introduced into E. faecalis JH2-2. pCirΔE was subsequently made with pCir as a template and the appropriate primers. E. faecalis JH2-2(pIL-E) was used as the host for construction of pCirΔE.
Computational analyses.
Open reading frames were identified with the Glimmer 2.0 program (6). Predictions by the Glimmer 2.0 program were checked manually for validity. Homology comparisons were performed with the basic logical alignment tool (Blast) as described by Altschul et al. (1). Blast searches were performed against the NCBI nonredundant protein database and the NCBI microbial genomes database (http://www.ncbi.nlm.nih.gov/BLAST/). Homologies with conserved domains from the Pfam database (http://www.sanger.ac.uk/Software/Pfam/) (2) were also identified with Blast searches. Putative signal peptides were identified with signalP (http://www.cbs.dtu.dk/services/SignalP/) (38). Putative transmembrane helices were identified with the TMHMM2.0 program (http://www.cbs.dtu.dk/services/TMHMM-2.0/) (28). Dyad symmetries, isoelectric points, and molecular weights were determined with the program Clonemanager 4 (SEcentral; Scientific & Educational Software). Sequence alignments were performed with the ClustalW program available at http://www2.ebi.ac.uk/clustalw/ (54).
Nucleotide sequence accession number.
The cirA sequence is available under GenBank accession number AJ566621.
RESULTS
Sequence analysis of the region encompassing the structural gene of circularin A.
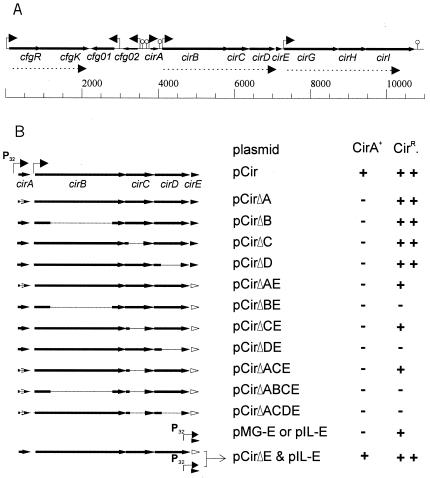
The structural gene of the circular bacteriocin circularin A of Clostridium beijerinckii ATCC 25752 (cirA) has previously been cloned and sequenced (25). A region of 11 kb surrounding cirA was sequenced and shown to contain 12 open reading frames (ORFs), including cirA (Fig. 1 and 2). Six putative promoters were identified, one upstream of each cfgR (cirA-flanking gene response regulator), cfg01 (cirA-flanking gene 01), cfg02, cirA, cirB, and cirG. The cirBCDE and cirGHI genes are putatively transcribed as polycistronic messengers, since no clear transcription initiation signals were detected other than the ones upstream of cirB and cirG, respectively. Translation of cirC and cirD can putatively start from alternative start codons within the same reading frame (Fig. 2). Overlap between the end of one gene (cfgR, cirC, cirD, cirH, and cirI) and the predicted start of the downstream gene, which is suggestive of regulation of expression by translational coupling, is a common feature in the entire region (Fig. 2). For cirH, translational coupling to cirG would be the only means of expression, as it lacks an obvious ribosome-binding site (Fig. 2).
FIG. 1.
(A) Physical map of the region surrounding the circularin A structural gene cirA of C. beijerinckii ATCC 25752. Solid arrows indicate genes; bent arrows show putative promoters; lollipops represent predicted regions of dyad symmetry (ΔG0 < −10 kcal/mol); dotted arrows show possible polycistronic messengers. Map units are base pairs. (B) Schematic representation of the cir DNA fragments in the indicated plasmids and locations of the deletions (indicated by the thin lines). Open arrowheads indicate a deletion in cirE. Promoters are shown by bent arrows. CirA+, circularin A production; CirR, circularin A resistance, denoted as ++, full protection against CirA [>24-fold increase relative to E. faecalis JH2-2(pMG36c)]; +, partial protection [2- to 16-fold increase relative to E. faecalis JH2-2(pMG36c)]; −, sensitive.
FIG. 2.
(A) Nucleotide sequences of promoter and translation initiation regions in the cir gene cluster. Putative −35, −10, and ribosome-binding site (RBS) sequences are underlined. Deduced amino acid sequences are indicated below the nucleotide sequences, and gene names are given below the amino acid sequences. Putative start codons are indicated in boldface and are numbered when more than one possibility exists. Termination codons are underlined and in italic.
Derived protein sequences and homologies are presented in Table 2. The gene products CirB through CirI all show some degree of homology to proteins involved in the production of enterocin AS-48, a bacteriocin produced by Enterococcus faecalis S-48 (34). Homologues of the putative proteins AS-48C1 and Bac21F are not encoded by the cir operon. CirD and CirH, like AS-48D and BacH, both contain an ATP-binding domain (Fig. 3). CirG belongs to the HlyD family of accessory proteins of ABC transporters, which includes proteins like EmrA, an accessory protein in the EmrAB multidrug transporter (31), and LcnD, an auxiliary protein involved in the secretion of the bacteriocin lactococcin A (11).
TABLE 2.
Characteristics of predicted proteins specified by the C. beijerinckii ATCC 25752 cir gene cluster
| Protein | Gene sizea (bp) | Protein
|
||||
|---|---|---|---|---|---|---|
| Size (kDa) | pl | TMb | Homologyc | Putative function | ||
| CfgR | 774 | 30.4 | 6 | 0 | Response regulators | |
| CfgK | 1,275 | 49.8 | 6.8 | 7 | Histidine kinases | |
| Cfg01 | 597 | 23.2 | 9.8 | 5 | AgrB regulatory protein | |
| Cfg02 | 390 | 14.4 | 10.17 | 4 | Unknown | |
| CirA | 216 | 7.2 | 10.9d | 2 | Enterocin AS-48 | CirA precursor |
| CirB | 1,743 | 68.8 | 9.4 | 11 | AS-48B (19%) | Secretion/immunity |
| CirC | 555 | 20.9 | 10.1 | 4 | AS-48C (21%) | Maturation |
| CirD | 663 | 25.7 | 6.4 | 0 | ATP-binding proteins; AS-48D (31.6%) | Secretion/immunity |
| CirE | 147 | 5.7 | 10.6 | 2 | AS-48D1 (30%) | Immunity |
| CirG | 1,425 | 51.7 | 4.6 | 1 | HlyD family of proteins/EmrA/BacG (17%) | |
| CirH | 744 | 27.6 | 6.1 | 0 | ATP-binding proteins/BacH (40%) | |
| CirI | 1,266 | 45.7 | 9.7 | 4 | Duf214 domain/permease of ABC transporter/Bacl (32%) | |
When multiple putative starts were possible, the longest product was used.
TM, number of putative transmembrane sequences.
Homologues were identified by using Blast searches against the NCBI nonredundant protein database or by direct comparison to the proteins involved in enterocin AS-48 production. The identity of the protein to its homologue involved in enterocin AS-48 production is shown in parentheses.
The pl of CirA is based on the linear unprocessed protein.
FIG. 3.
Alignment of the putative ATP binding proteins involved in circularin A (CirD, CirH) and enterocin AS-48 (AS-48D, BacH) production with LolD, a protein involved in lipoprotein secretion. Identical residues are indicated by an asterisk. Colons and periods indicate conserved and semiconserved amino acid substitutions, respectively, according to the ClustalW grouping of amino acids. Dashes indicate gaps introduced in the sequence to maximize alignment. Walker A, Walker, and ABC transporter B motifs are indicated.
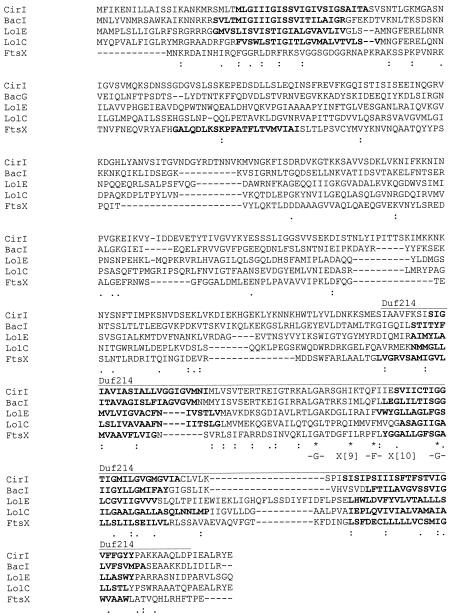
Based on the occurrence of the G-X9-F-X10-G motif, CirI can be classified in the ortholog group 3-1 of ABC transporters, as defined by Tomii and Kanehisa (55). Like the already characterized members of this group (FtsX [7] and LolC and LolE [37]), CirI contains four putative transmembrane domains, typical of group 3-1 ABC transporters (Fig. 4), and a Duf214 domain of predicted permeases, as defined in the Pfam database. CfgR and CfgK are homologous to response regulators and histidine kinases, respectively, of two-component regulatory systems. Cfg01 is homologous (24% identity) to the accessory gene regulator (AgrB) of Staphylococcus aureus, which is thought to be involved in processing and secretion of a signaling peptide (AgrD), regulating a large set of virulence factors (61). Cfg02 is homologous to two putative proteins with unknown function from C. acetobutylicum and C. perfringens (34% identity with each of these proteins) (39, 48).
FIG. 4.
Alignment of CirI with BacG (56), FtsX (6), and LolC and LolE (37). The consensus motif G-X9-F-X10-G for ortholog group 3-1 type ABC transporters is indicated. Predicted transmembrane domains are indicated in bold. The region constituting the predicted DUF214 domain is indicated by a line above the sequences. Identical residues are indicated by an asterisk, whereas colons and periods indicate conserved and semiconserved amino acid substitutions, respectively, according to the ClustalW grouping of amino acids. Gaps were introduced in the sequence to maximize alignment.
Circularin A production in a heterologous host.
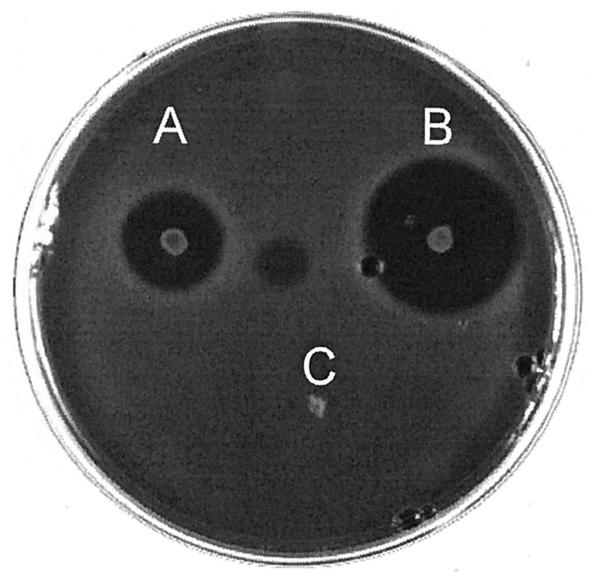
The region encompassing cirABCDE was cloned in the broad-host-range vector pMG36c downstream of the constitutive lactococcal promoter P32, creating pMGAE1. E. faecalis JH2-2(pMGAE1) produced a small amount of bacteriocin and was resistant to circularin A (Fig. 5), but bacteriocin production was not stable. Three consecutive cycles of selection for bacteriocin production by overlay assay and subsequent clean-streaking of producing colonies yielded a strain that stably expressed a high level of circularin A (Fig. 5). The plasmid in this strain, designated pCir, was identical to pMGAE1 by restriction enzyme analysis. The high bacteriocin expression level was maintained upon plasmid isolation and reintroduction into E. faecalis JH2-2. The copy numbers of pCir and pMGAE1 were clearly reduced compared to the copy number of the empty vector pMG36c (data not shown).
FIG. 5.
Heterologous production of circularin A by E. faecalis JH2-2, as visualized in a colony overlayer assay with Lactobacillus saké ATCC 15521 as the indicator strain. (A) E. faecalis JH2-2(pMGAE1); (B) E. faecalis JH2-2(pCir); (C) E. faecalis JH2-2(pMG36c).
Functional analysis of the circularin A gene cluster.
In order to determine which genes are involved in the production of circularin A, pCir was used as a template to create in-frame single deletions of cirA, cirB, cirC, or cirD. E. faecalis JH2-2 strains harboring the various plasmids all lost the CirA+ phenotype, as revealed by colony overlayer assays (Fig. 1). All strains remained resistant to circularin A, as they grew on plates containing filter-sterilized culture supernatant (10%, vol/vol) of C. beijerinckii ATCC 25752, while E. faecalis JH2-2(pMG36c) did not (Fig. 1). These results indicate that each of the four gene products is required for the production of active circularin A.
Removal of cirE from the cirABCDE cluster in pCir could not be achieved in several attempts with different cloning hosts [E. faecalis JH2-2 and E. faecalis JH2-2(pMGE)], while simultaneous deletion of cirA and cirE (pCirΔAE) was possible. This observation suggested that cirE is involved in bacteriocin immunity, an assumption that will be discussed further below. E. faecalis JH2-2(pCirΔAE) showed reduced sensitivity to circularin A present in filter-sterilized culture supernatant of C. beijerinckii ATCC 25752 but was clearly more resistant to circularin A than E. faecalis(pMG36c).
To determine which gene(s) is involved in this partial resistance towards circularin A, single deletions of cirB, cirC, and cirD were combined with a deletion in cirE. A mutation in either cirB or cirD in combination with deletion of cirE led to the loss of the circularin A-resistant phenotype, whereas cells carrying a pCir derivative with a deletion in cirA or cirC in combination with cirE remained partially resistant to circularin A (Fig. 1). These results indicate that both CirB and CirD are required for partial resistance in the absence of CirE. To confirm this hypothesis, three additional deletion constructs were made. E. faecalis JH2-2(pCirΔACE), specifying only CirB and CirD, still showed partial resistance to CirA. E. faecalis JH2-2 expressing only CirB (pCirΔACDE) or CirD (pCirΔABCE) was bacteriocin sensitive, confirming that both CirB and CirD are needed for the partial resistance phenotype in the absence of CirE.
Heterologous expression of circularin A in L. lactis NZ9000 was attempted, but pCir and any other vector containing cirB did not give transformants, while all control plasmids did. Apparently, an intact cirB gene is lethal to this host. Plasmids with a deletion in cirB could be stably maintained in L. lactis. L. lactis(pCirΔB) did not produce active CirA and was 2.5-fold more resistant to the bacteriocin than L. lactis(pNG8048e), a strain carrying an empty cloning vector.
cirE gene confers circularin A immunity.
To prove that cirE can confer bacteriocin resistance independent of the combination cirB and cirD, the gene was cloned downstream of the lactococcal promoter P32 in pMG36e (pMG-E). Unlike L. lactis(pMG36e), L. lactis NZ9000(pMG-E) was able to grow in a medium with culture supernatant (up to 50%, vol/vol) of C. beijerinckii ATCC 25752 (CirA+), as determined by serial dilution assay. L. lactis NZ9000(pMG-E) also formed normal colonies on plates containing filter-sterilized C. beijerinckii ATCC 25752 culture supernatant (4%, vol/vol), whereas the control strain did not grow at all. These results indicate that cirE alone gives rise to circularin A resistance. E. faecalis JH2-2(pMG-E) was also immune to the bacteriocin present in C. beijerinckii ATCC 25752 supernatant, as determined in a plate assay.
As mentioned above, initial attempts to remove cirE from cirABCDE with either E. faecalis JH2-2 or E. faecalis JH2-2(pMG-E) as the cloning host failed. This problem was circumvented by cloning cirE downstream of the P32 promoter in pIL253. The resulting strain, E. faecalis JH2-2(pIL-E), was immune to C. beijerinckii ATCC 25752 culture supernatant, as determined in a plate assay, and did not produce bacteriocin (Fig. 1), as determined by serial dilution and overlay assays. In E. faecalis JH2-2(pIL-E), we were able to introduce the cirE deletion plasmid pCirΔE (CirA+). E. faecalis JH2-2(pIL-E, pCirΔE) was immune to C. beijerinckii ATCC 25752 culture supernatant and produced bacteriocin (Fig. 1). Attempts to introduce pCirΔE in E. faecalis JH2-2 alone were unsuccessful, indicating that cirE is required for proper bacteriocin immunity.
The levels of resistance conferred by pMG-E and pIL-E in E. faecalis JH2-2, which is able to grow in medium containing filter-sterilized C. beijerinckii ATCC 25752 culture supernatant (at the most 3 to 6%, vol/vol), were only two- to fourfold increased compared to the control strain carrying only pMG36e, able to grow in medium containing at the most 1.5% (vol/vol) filter-sterilized C. beijerinckii ATCC 25752 culture supernatant. This level is lower than the resistance level of E. faecalis JH2-2(pCirΔB) or E. faecalis JH2-2(pCirΔD), both also expressing only cirE as a functional immunity system. Complementing pIL-E with pCirΔDE in E. faecalis JH2-2 did not restore the resistance level to that of E. faecalis JH2-2(pCirΔD), indicating that the lower resistance level is not due to the absence of auxiliary factors not present on pMG-E or pIL-E. Taken together, these results confirm that CirE is the dedicated circularin A immunity protein and that expression of cirE alone is sufficient for immunity.
DISCUSSION
The region surrounding the structural gene cirA of the circular bacteriocin circularin A of C. beijerinckii ATCC 25752 encompasses 11 genes. Upstream of cirA there are four genes, of which two could encode a two-component regulatory system. Together with the presence of an AgrB homologue (Cfg01), this presents the possibility of regulation of bacteriocin expression. Two-component systems are often involved in the regulation of bacteriocin expression, and their genes are normally located near the bacteriocin operon (27). The homology to the Agr system of Staphylococcus aureus, which consists of a two-component system, a processing protein (AgrB) and a signaling peptide (AgrD), involved in regulation of virulence factors (40, 61) suggests a similar regulatory mechanism, although we could not identify an ArgD homologue in our sequence and have not further addressed the possible involvement of CfgR and CfgK in CirA expression.
We show here that the region cirABCDEGHI is involved in bacteriocin production and secretion. The genes cirABCDE represent the minimal region required for bacteriocin processing and secretion in the heterologous host E. faecalis JH2-2, as deletion of only a single gene from this cluster causes loss of either bacteriocin production or cell viability. The genetic organization of the region cirABCDEGHI seems rather compressed, as several genes overlap. This set-up suggests that translational coupling, a gene-regulatory mechanism often used in operons in which the stoichiometry of gene expression is important (36), may occur.
The minimal requirements for extracellular circularin A activity are production, processing, circularization, and secretion of the bacteriocin, while the producer cell should be immune to the bacteriocin. All these features should in principle be encoded by cirABCDE. Here, we show that resistance to circularin A is acquired via at least two independent systems. First, expression of cirE confers a certain level of immunity to the expressing strain, which is essential for the bacteria to be able to produce and withstand CirA. CirE has a very high and contains two possible transmembrane helices, which make membrane localization of the protein very likely. Its small size, high isoelectric point, and two predicted transmembrane helices are characteristics that CirE has in common with AS-48D1, the immunity protein of the circular bacteriocin enterocin AS-48, and with the proteins PepI, EciI, LasJ, and DviA, which have all been shown or postulated to be involved in immunity to the unrelated bacteriocins Pep5, epicidin 280, lactocin S, and divergicin A, respectively (18, 41, 44, 50, 59). The immunity mechanism of these proteins is unknown, but PepI has been suggested to inhibit pore formation by Pep5 (46).
The second system conferring reduced sensitivity to CirA depends on the combined activity of CirB and CirD. Together, these proteins form a putative ABC transporter in which CirB is the transporter and CirD provides the nucleotide-binding domain. The putative transporter CirBD confers a basal level of CirA resistance, which is, however, insufficient to support bacteriocin production by the heterologous host that we used; a viable clone of E. faecalis JH2-2(pCirΔE) could not be obtained. CirBD (most likely) also function in CirA secretion, as ABC transporters are often implicated in bacteriocin secretion (10). The fact that proteins required for secretion of a bacteriocin can be involved in resistance has been shown for McbE and McbF, which are involved in microcin B17 production (13). The secretion proteins of the lantibiotic nisin were suggested to fulfill a similar role, but involvement of NisI and/or NisFEG, via a regulatory loop inducing expression of the respective genes, cannot be excluded (29, 43). Resistance is most probably obtained by the pumping out of the bacteriocin (42, 43, 46). In conclusion, CirBD confer low-level resistance by virtue of their ability to secrete CirA, while CirE shows structural homology to other bacteriocin immunity proteins, which identifies it as the dedicated CirA immunity protein.
Based on homology studies, CirGHI could constitute another transporter. CirG probably has an auxiliary function, as it is homologous to the HlyD family of proteins, many of which are accessory proteins in the export of drugs or toxic proteins, such as hemolysin (52), lactococcin A (53), and colicin V (15). CirH and CirI probably form an ABC transporter of the LolCDE type (60): CirH is homologous to LolD, while CirI is homologous to both LolC and LolE. The CirGHI homologues in the enterocin AS-48 system (BacGHI/AS-48FGH) enhance the expression of enterocin AS-48 (56) and the resistance to exogenous enterocin AS-48 (8), roles we have not investigated for CirGHI yet. The homology to the LolCDE system furthermore suggests that active transport, perhaps from the outer leaflet of the membrane, as shown for LolCDE (60), is involved in this enhancing effect by making more bacteriocin available. The NisFEG system fulfills such a function in enhancement of nisin secretion (43), but this system is not very homologous to CirGHI or LolCDE.
No experimental evidence has been obtained to identify the protein(s) involved in the processing and/or circularization of the CirA prepeptide. As CirBD together form a putative ABC transporter and cirE confers bacteriocin immunity, the essential protein CirC is a likely candidate to perform this function(s), either alone or together with CirB and/or CirD. This notion seems to be supported by the fact that the only other CirC homologue is AS-48C, encoded by the enterocin AS-48 gene cluster of E. faecalis.
In conclusion, we have identified five genes essential for circularin A production and have shown that three of these genes (cirBDE) are involved in bacteriocin resistance. Future studies will be performed to determine the mechanism of circularization and the possible role of CirC therein.
Acknowledgments
This work was supported by FCDF Corporate Research and the Dutch Ministry of Economic Affairs. Jan Kok was the recipient of a grant from the Royal Netherlands Academy of Arts and Sciences (KNAW).
We are grateful to G. Dunny (Institute of Food Research, Colney, United Kingdom) for kindly providing the E. faecalis JH2-2 strains used in this study.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blond, A., J. Peduzzi, C. Goulard, M. J. Chiuchiolo, M. Barthelemy, Y. Prigent, R. A. Salomon, R. N. Farias, F. Moreno, and S. Rebuffat. 1999. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur. J. Biochem. 259:747-755. [DOI] [PubMed] [Google Scholar]
- 5.Cintas, L. M., P. Casaus, L. S. Håvarstein, P. E. Hernandez, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Leeuw, E., B. Graham, G. J. Phillips, C. M. Hagen-Jongman, B. Oudega, and J. Luirink. 1999. Molecular characterization of Escherichia coli FtsE and FtsX. Mol. Microbiol. 31:983-993. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, M., E. Valdivia, M. Martinez-Bueno, M. Fernandez, A. S. Soler-Gonzalez, H. Ramirez-Rodrigo, and M. Maqueda. 2003. Characterization of a new operon, as-48EFGH, from the as-48 gene cluster involved in immunity to enterocin AS-48. Appl. Environ. Microbiol. 69:1229-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dineen, S. S., M. Bradshaw, and E. A. Johnson. 2000. Cloning, nucleotide sequence, and expression of the gene encoding the bacteriocin boticin B from Clostridium botulinum strain 213B. Appl. Environ. Microbiol. 66:5480-5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fath, M. J., and R. Kolter. 1993. ABC transporters: bacterial exporters. Microbiol. Rev. 57:995-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke, C. M., K. J. Leenhouts, A. J. Haandrikman, J. Kok, G. Venema, and K. Venema. 1996. Topology of LcnD, a protein implicated in the transport of bacteriocins from Lactococcus lactis. J. Bacteriol. 178:1766-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnier, T., and S. T. Cole. 1986. Characterization of a bacteriocinogenic plasmid from Clostridium perfringens and molecular genetic analysis of the bacteriocin-encoding gene. J. Bacteriol. 168:1189-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido, M. C., M. Herrero, R. Kolter, and F. Moreno. 1988. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 7:1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geis, A., J. Singh, and M. Teuber. 1983. Potential of Lactic Streptococci to produce bacteriocin. Appl. Environ. Microbiol. 45:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilson, L., H. K. Mahanty, and R. Kolter. 1990. Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 9:3875-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez, C., G. M. Langdon, M. Bruix, A. Galvez, E. Valdivia, M. Maqueda, and M. Rico. 2000. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc. Natl. Acad. Sci. USA 97:11221-11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 18.Heidrich, C., U. Pag, M. Josten, J. Metzger, R. W. Jack, G. Bierbaum, G. Jung, and H. G. Sahl. 1998. Isolation, characterization, and heterologous expression of the novel lantibiotic epicidin 280 and analysis of its biosynthetic gene cluster. Appl. Environ. Microbiol. 64:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalmokoff, M. L., S. K. Banerjee, T. Cyr, M. A. Hefford, and T. Gleeson. 2001. Identification of a new plasmid-encoded sec-dependent bacteriocin produced by Listeria innocua 743. Appl. Environ. Microbiol. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai, Y., T. Saito, H. Kitazawa, and T. Itoh. 1998. Gassericin A; an uncommon cyclic bacteriocin produced by Lactobacillus gasseri LA39 linked at N- and C-terminal ends. Biosci. Biotechnol. Biochem. 62:2438-2440. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, Y., T. Saito, M. Suzuki, and T. Itoh. 1998. Sequence analysis by cloning of the structural gene of gassericin A, a hydrophobic bacteriocin produced by Lactobacillus gasseri LA39. Biosci. Biotechnol. Biochem. 62:887-892. [DOI] [PubMed] [Google Scholar]
- 24.Keis, S., C. F. Bennett, V. K. Ward, and D. T. Jones. 1995. Taxonomy and phylogeny of industrial solvent-producing clostridia. Int. J. Syst. Bacteriol. 45:693-705. [DOI] [PubMed] [Google Scholar]
- 25.Kemperman, R. A., A. Kuipers, H. Karsens, A. Nauta, O. Kuipers, and J. Kok. 2003. Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Appl. Environ. Microbiol. 69:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 27.Kleerebezem, M., and L. E. Quadri. 2001. Peptide pheromone-dependent regulation of antimicrobial peptide production in gram-positive bacteria: a case of multicellular behavior. Peptides 22:1579-1596. [DOI] [PubMed] [Google Scholar]
- 28.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 29.Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. de Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281-291. [DOI] [PubMed] [Google Scholar]
- 30.Kuipers, O. P., P. G. G. A. De Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 31.Lomovskaya, O., and K. Lewis. 1992. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahony, D. E., R. Ahmed, and S. G. Jackson. 1992. Multiple typing techniques applied to a Clostridium perfringens food poisoning outbreak. J. Appl. Bacteriol. 72:309-314. [DOI] [PubMed] [Google Scholar]
- 33.Martinez, B., M. Fernandez, J. E. Suarez, and A. Rodriguez. 1999. Synthesis of lactococcin 972, a bacteriocin produced by Lactococcus lactis IPLA 972, depends on the expression of a plasmid-encoded bicistronic operon. Microbiology 145:3155-3161. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Bueno, M., M. Maqueda, A. Galvez, B. Samyn, J. Van Beeumen, J. Coyette, and E. Valdivia. 1994. Determination of the gene sequence and the molecular structure of the enterococcal peptide antibiotic AS-48. J. Bacteriol. 176:6334-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Bueno, M., E. Valdivia, A. Galvez, J. Coyette, and M. Maqueda. 1998. Analysis of the gene cluster involved in production and immunity of the peptide antibiotic AS-48 in Enterococcus faecalis. Mol. Microbiol. 27:347-358. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy, J. E., and C. Gualerzi. 1990. Translational control of prokaryotic gene expression. Trends Genet. 6:78-85. [DOI] [PubMed] [Google Scholar]
- 37.Narita, S., K. Tanaka, S. Matsuyama, and H. Tokuda. 2002. Disruption of lolCDE, encoding an ATP-binding cassette transporter, is lethal for Escherichia coli and prevents release of lipoproteins from the inner membrane. J. Bacteriol. 184:1417-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 39.Nolling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick, R. P., S. J. Projan, J. Kornblum, H. F. Ross, G. Ji, B. Kreiswirth, F. Vandenesch, and S. Moghazeh. 1995. The agrP2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol. Gen. Genet. 248:446-458. [DOI] [PubMed] [Google Scholar]
- 41.Pag, U., C. Heidrich, G. Bierbaum, and H. G. Sahl. 1999. Molecular analysis of expression of the lantibiotic Pep5 immunity phenotype. Appl. Environ. Microbiol. 65:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peschel, A., and F. Gotz. 1996. Analysis of the Staphylococcus epidermidis genes epiF, -E, and -G involved in epidermin immunity. J. Bacteriol. 178:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ra, R., M. M. Beerthuyzen, W. M. de Vos, P. E. Saris, and O. P. Kuipers. 1999. Effects of gene disruptions in the nisin gene cluster of Lactococcus lactis on nisin production and producer immunity. Microbiology 145:1227-1233. [DOI] [PubMed] [Google Scholar]
- 44.Reis, M., M. Eschbach-Bludau, M. I. Iglesias-Wind, T. Kupke, and H. G. Sahl. 1994. Producer immunity towards the lantibiotic Pep5: identification of the immunity gene pepI and localization and functional analysis of its gene product. Appl. Environ. Microbiol. 60:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Saris, P. E., T. Immonen, M. Reis, and H. G. Sahl. 1996. Immunity to lantibiotics. Antonie van Leeuwenhoek 69:151-159. [DOI] [PubMed] [Google Scholar]
- 47.Shepard, B. D., and M. S. Gilmore. 1995. Electroporation and efficient transformation of Enterococcus faecalis grown in high concentrations of glycine. Methods Mol. Biol. 47:217-226. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 50.Skaugen, M., E. L. Andersen, V. H. Christie, and I. F. Nes. 2002. Identification, characterization, and expression of a second, bicistronic, operon involved in the production of lactocin S in Lactobacillus sakei L45. Appl. Environ. Microbiol. 68:720-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solbiati, J. O., M. Ciaccio, R. N. Farias, J. E. Gonzalez-Pastor, F. Moreno, and R. A. Salomon. 1999. Sequence analysis of the four plasmid genes required to produce the circular peptide antibiotic microcin J25. J. Bacteriol. 181:2659-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanley, P., V. Koronakis, and C. Hughes. 1991. Mutational analysis supports a role for multiple structural features in the C-terminal secretion signal of Escherichia coli haemolysin. Mol. Microbiol. 5:2391-2403. [DOI] [PubMed] [Google Scholar]
- 53.Stoddard, G. W., J. P. Petzel, M. J. Van Belkum, J. Kok, and L. L. McKay. 1992. Molecular analyses of the lactococcin A gene cluster from Lactococcus lactis subsp. lactis biovar diacetylactis WM4. Appl. Environ. Microbiol. 58:1952-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomii, K., and M. Kanehisa. 1998. A comparative analysis of ABC transporters in complete microbial genomes. Genome Res. 8:1048-1059. [DOI] [PubMed] [Google Scholar]
- 56.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1997. Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 179:7843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van de Guchte, G. M., J. M. van der Vossen, J. Kok, and G. Venema. 1989. Construction of a lactococcal expression vector: expression of hen egg white lysozyme in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 55:224-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venema, K., R. E. Haverkort, T. Abee, A. J. Haandrikman, K. J. Leenhouts, L. de Leij, G. Venema, and J. Kok. 1994. Mode of action of LciA, the lactococcin A immunity protein. Mol. Microbiol. 14:521-532. [DOI] [PubMed] [Google Scholar]
- 59.Worobo, R. W., M. J. Van Belkum, M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1995. A signal peptide secretion-dependent bacteriocin from Carnobacterium divergens. J. Bacteriol. 177:3143-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yakushi, T., K. Masuda, S. Narita, S. Matsuyama, and H. Tokuda. 2000. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat. Cell Biol. 2:212-218. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, L., L. Gray, R. P. Novick, and G. Ji. 2002. Transmembrane topology of AgrB, the protein involved in the posttranslational modification of AgrD in Staphylococcus aureus. J. Biol. Chem.277:34736-34742. [DOI] [PubMed]