Abstract
A potential food-grade cloning vector, pND919, was constructed and transformed into S. thermophilus ST3-1, a plasmid-free strain. The vector contains DNAs from two different food-approved organisms, Streptococcus thermophilus and Lactococcus lactis. The 5.0-kb pND919 is a derivative of the cloning vector pND918 (9.3 kb) and was constructed by deletion of the 4.3-kb region of pND918 which contained DNA from non-food-approved organisms. pND919 carries a heterologous native cadmium resistance selectable marker from L. lactis M71 and expresses the Cdr phenotype in S. thermophilus transformants. With the S. thermophilus replicon derived from the shuttle vector pND913, pND919 is able to replicate in the two S. thermophilus industrial strains tested, ST3-1 and ST4-1. Its relatively high retention rate in S. thermophilus further indicates its usefulness as a potential food-grade cloning vector. To our knowledge, this is the first report of a replicative potential food-grade vector for the industrially important organism S. thermophilus.
Lactic acid bacteria are used as starter cultures for dairy fermentations. For decades, mesophilic Lactococcus strains have been the focus of research. More recently, Streptococcus thermophilus has gained increasing attention as an important industrial species and is being used in production of yogurt and some cheeses. Inevitably, with expanding dairy fermentation activities, the problem of bacteriophage infection of this particular starter species has intensified, with the majority of the phages isolated from S. thermophilus cultures classified as lytic phages rather than lysogenic phages (2). As a result, precautions have been implemented to avoid phage infection, including genetic manipulation to generate phage-resistant S. thermophilus starter cultures (16).
To date only a limited number of phage defense mechanisms have been reported for S. thermophilus (16). In contrast a large number have been characterized for Lactococcus lactis (3), and it is possible that the lactococcal phage defense systems could be used to provide phage resistance in S. thermophilus. To facilitate this, the construction of appropriate cloning vectors for S. thermophilus is necessary. While plasmids in S. thermophilus have been characterized (15), their development into food-grade vectors has not yet been realized. The lack of effective selectable markers in S. thermophilus has contributed to the lack of progress. With the increasing problems of phage infection in S. thermophilus, the development of suitable cloning vectors capable of delivering lactococcal phage resistance mechanisms to S. thermophilus is needed.
The approach adopted to construct a potential food-grade cloning vector for S. thermophilus was to use the S. thermophilus replicon in pND913 (15). A number of selectable markers have been characterized and used in Lactococcus. These include the nisin resistance gene (5), the lacF gene involved in lactose utilization (8), the pepN nonsense suppressor gene of the purine synthetic pathway (4), and the sucrose utilization genes (12). Recently, lactococcal heavy metal resistance determinants, such as cadmium resistance (Cdr) and copper resistance, have been used as food-grade markers in L. lactis (7). The usefulness of the Cdr system, isolated from L. lactis strain M71 and encoded by the cadA and cadC genes, has been demonstrated with L. lactis strains (7).
In this paper, we report the construction of a 5.0-kb potential food-grade cloning vector for S. thermophilus. The construct contains an S. thermophilus replicon and the Cdr selectable marker from Lactococcus; therefore, the construct contains DNAs from two different groups of food-approved organisms. The usefulness of this potential food-grade vector was indicated by its ability to replicate in two S. thermophilus industrial strains, ST3-1 and ST4-1.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli cultures used for plasmid isolation and cloning were propagated at 37°C in Luria-Bertani medium (14). L. lactis cultures were incubated at 30°C in M17 medium (17) supplemented with 0.5% (wt/vol) glucose (M17G). S. thermophilus strains were propagated at 37°C anaerobically in M17 medium supplemented with 0.5% (wt/vol) lactose (M17L). When appropriate, the following selective agents were added: for E. coli, 100 μg of carbenicillin per ml; for L. lactis, 5 μg of erythromycin per ml or 0.5 mM cadmium chloride (CdCl2); and for S. thermophilus, 3 μg of erythromycin per ml or 0.3 mM CdCl2.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| L. lactis subsp. lactis LM0230 | Lac− Prt− plasmid-free derivative of c2 | 9 |
| S. thermophilus | ||
| ST3-1 | Plasmid-free strain | 15 |
| ST4-1 | Industrial strain | DSM Food Specialties |
| E. coli JM109 | Transformation host | 14 |
| Plasmids | ||
| pGKV210 | 4.4 kb; Cmr Emr | 11 |
| pND312 | 7.0 kb; Cdr; 1.8-kb AccI fragment deleted from a native plasmid of L. lactis | 7 |
| pND913 | 6.4 kb; Ampr Emr Cdr; 2.9-kb fragment encoding Cdr from pND312 into pND913 | 15 |
| pND918 | 9.3 kb; Ampr Emr Cdr; 2.9-kb fragment from pND312 into pND913 | This study |
| pND919 | 5.0 kb; Cdr; 4.1-kb KpnI non-food-grade fragment deleted from pND918 | This study |
Ampr, ampicillin resistant (or Carbr); Cmr, chloramphenicol resistant; Lac, lactose fermenting; Prt, proteinase producing.
Plasmid isolation.
E. coli plasmid extraction was done as described by Sambrook et al. (14). For plasmid isolation from L. lactis transformants, the methods of Anderson and McKay (1) were used. Isolation of S. thermophilus plasmids was as described by O'Sullivan and Klaenhammer (10); however, rather than using overnight culture as recommended for plasmid isolation from the L. lactis hosts, a log-phase culture was used for plasmid extraction from the S. thermophilus strains.
Molecular cloning techniques and PCR.
General procedures for molecular cloning were performed as described by Sambrook et al. (14). PCR was used for the amplification of the 2.9-kb cadA-cadC region encoding the lactococcal Cdr marker, with pND312 (a modified version of native lactococcal plasmid pND302) (7) as a template. Primer cdR-L (7) was used as the forward primer, and its sequence is 5′-CGTTGGATCCATAACTTGAAAGCAC-3′. Primer cadB was designed for the backward primer, and its sequence is 5′-CGATCATTTTCGTACGGATGGCCATGGAGATCTGCG-3′. The amplification procedure was as described by Liu et al. (7).
Transformation.
E. coli cells were transformed as described by Sambrook et al. (14). For L. lactis cultures, LM0230 cells were transformed by electroporation with a Bio-Rad Pulse Controller and Gene-Pulser as described by Powell et al. (13). S. thermophilus was transformed by electroporation as described by Holo and Nes (6).
Characterization of pND919 in S. thermophilus.
The stability of pND919 was monitored for 30 generations. Thirty generations is more than the number of generations required to prepare an inoculum and then grow the culture in commercial fermentors. For segregational stability, transformants were incubated in M17L broth in the absence of any selective pressure. At the end of this growth period, transformed colonies were plated on nonselective medium and then patched onto selective medium, and the percent plasmid loss was calculated. The presence of a plasmid of the expected size in selected transformants was confirmed by plasmid isolation.
For structural stability of pND919, a similar procedure was used. However, the selective agent was added to the growth medium. After 30 generations of growth, plasmid extraction was performed on the transformants prior to examination by gel electrophoresis and comparison with plasmids extracted at generation 0.
Nucleotide sequencing and analysis.
Both DNA strands of the junction region of pND919 were sequenced. Sequencing reactions were set up with purified pND919 as the double-stranded template and two primers (primer 1, 5′-CAAGCAAGGGTTTCATCT-3′; primer 2, 5′-AAGCAGGCAAAAGCCTGC-3′). Each of the primers was designed according to the known sequences of the lactococcal Cdr determinant in pND312 and S. thermophilus DNA in pND913. Two dye terminator sequencing reactions were performed by the Sydney University and Prince Alfred Macromolecular Analysis Centre, using a Perkin-Elmer Catalyst 800 robotic work station. The sequence profile obtained was then compared with the databases from WebAngis as operated by the Australian Genomic Information Centre, University of Sydney. The BESTFIT program was used for the sequence comparison.
Nucleotide sequence accession numbers.
The GenBank accession number for the DNA sequence of the Cdr determinant is U78967. The GenBank accession number for S. thermophilus DNA (pND103) in pND913 and pND919 is AY250830.
RESULTS
Construction of pND919.
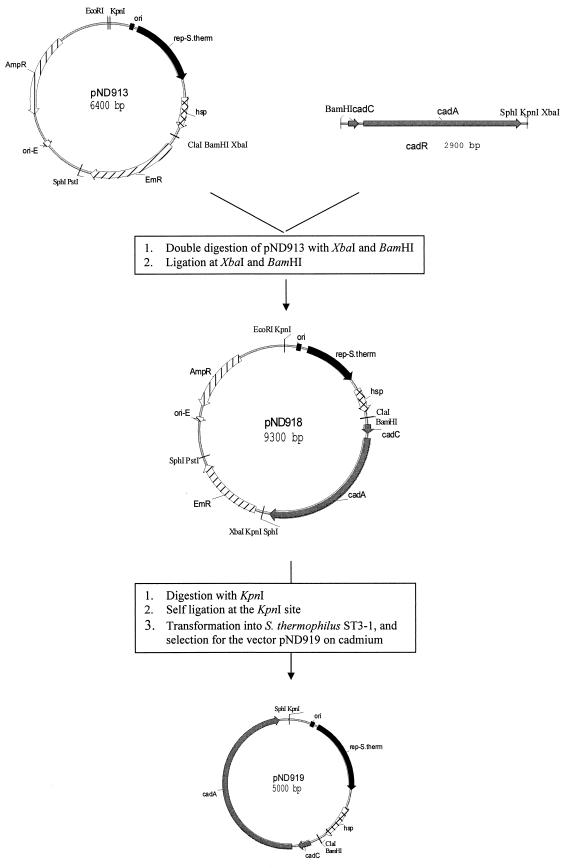
Construction of pND919 involved the generation of plasmid pND918. This plasmid was constructed by insertion of the 2.9-kb PCR-amplified fragment which carries the Cdr determinant of pND312 into the shuttle vector pND913. To enhance the cloning procedure, the fragment of the Cdr determinant was amplified so that it harbored a BamHI restriction site generated by the cdR-L forward primer and XbaI and KpnI restriction sites as designated by the cadB reverse primer. XbaI and BamHI restriction sites were created on the 2.9-kb insert to facilitate direct cloning into the vector pND913, while the addition of the KpnI site was made to facilitate generation of plasmid pND919 (Fig. 1).
FIG. 1.
Construction of the food-grade cloning vector pND919 for S. thermophilus.
Transformation of E. coli JM109 with the ligation mixture containing BamHI- and XbaI-digested and linearized pND913 ligated with the 2.9-kb PCR fragment produced carbenicillin-resistant (Carbr) transformants with a transformation efficiency of 2 × 103 transformants/μg of ligation mixture. Restriction analysis and gel electrophoresis revealed that the Carbr clones contained plasmid pND918, which was 9.2 kb in size. Restriction digestion showed that pND918 yielded the 2.9- and 6.4-kb fragments on double digestion with XbaI and BamHI and a 9.2-kb DNA fragment on digestion with XbaI. Based on these results, the cloning of the Cdr determinant into pND913 was confirmed (data not shown).
Transfer of pND918 to L. lactis and S. thermophilus.
To examine the expression of the Cdr determinant from pND918, electrotransformation of L. lactis LM0230 with pND918 was performed with the plasmid extracted from E. coli Carbr transformants. A transformation efficiency of 6 × 103 transformants/μg of DNA was achieved when selection was for erythromycin resistance (Emr). Patching of these Emr transformants onto M17G plates supplemented with glucose and Cd resulted in the identification of Emr Cdr clones. Plasmid profiles and restriction analysis confirmed that pND918 had remained unchanged during transfer to L. lactis LM0230 (data not shown).
pND918 prepared from L. lactis LM0230 was then used to electroporate S. thermophilus ST3-1. For comparison, another preparation of pND918 from E. coli was transformed into S. thermophilus ST3-1 under the same conditions. A transformation efficiency of approximately 104 transformants/μg of DNA was achieved with a positive control, pGKV210 (11). No transformation was observed when 1 μg of the E. coli plasmid preparation was used. Transformation with 10 ng of pND918 prepared from L. lactis produced 59 colonies on M17L-erythromycin plates and 16 colonies on M17L-Cd plates. Plasmid isolation from two Emr and three Cdr clones revealed the presence of plasmid pND918. Based on these results, the lactococcal Cdr system as encoded by pND918 was shown to be expressed in S. thermophilus. Furthermore, the inability of S. thermophilus to be transformed by pND918 prepared from E. coli is of interest.
The food-grade cloning vector pND919 was obtained by modification of pND918. To achieve this, the DNA components from non-food-approved organisms were removed from pND918 by KpnI digestion and self-ligation (Fig. 1). Electrotransformation into S. thermophilus ST3-1 was performed with ligation mixtures prepared from pND918 isolated from E. coli JM109, L. lactis LM0230, and S. thermophilus ST3-1. Similar to the results for pND918, S. thermophilus ST3-1 transformants were not obtained from the ligation mixture derived from E. coli, even though 2 μg of DNA was used to electroporate the host. By contrast, electrotransformation of S. thermophilus with 0.7 μg of ligation mixture derived from L. lactis or S. thermophilus generated 125 and 154 Cdr transformed colonies, respectively. Plasmid isolation and restriction analysis with KpnI indicated the presence of the 5.0-kb pND919.
The junction region of pND919 represents the point where the non-food-grade component was separated from the food-grade component of pND918; it spans the region of the cadA-cadC insert and pND913. Two sequencing reactions were set up with purified pND919 as the double-stranded template and two primers. The sequences obtained were analyzed and compared with the databases from WebAngis. Examination of the sequences with the aid of the BESTFIT program revealed 100% similarity with pND312 and pND913.
Stability of pND919 in S. thermophilus.
The segregational and structural stabilities of pND919 in S. thermophilus were examined. For comparison, pND913 was included, as well as pGKV210. pGKV210 was included because it has been reported to be functional and stable in various S. thermophilus strains (11). pND919 was found to be relatively stable. The plasmid loss was 19% after 30 generations of growth without selection. For plasmids pGKV210 and pND913, the plasmid losses were 82.5 and 35.9%, respectively.
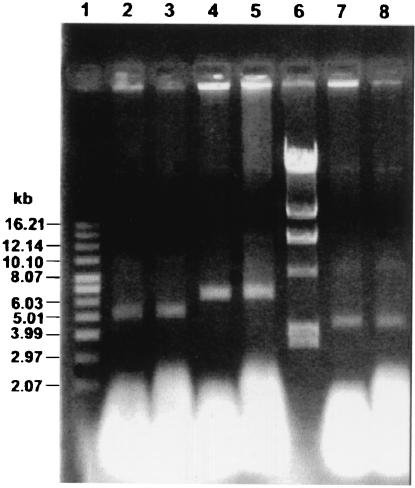
Transformants were incubated in M17L broth in the presence of cadmium (pND919) and erythromycin (pND913 and pGKV210) for approximately 30 generations. At the end of the growth period, single colonies were obtained by streaking the liquid cultures onto appropriate M17L selective plates. After plating, plasmid extraction was performed with selected colonies. The plasmid preparations were examined by gel electrophoresis and compared with those extracted from generation 0. As illustrated in Fig. 2, pND913, pND919, and pGKV210 were stably maintained for about 30 generations, as no obvious alteration in the size of any plasmids could be observed.
FIG. 2.
Structural stability of pND913, pND919, and pGKV210 in S. thermophilus ST3-1. Lane 1, supercoiled DNA ladder standard (GIBCO BRL Products); lanes 2 and 3, pND919; lanes 4 and 5, pND913; lane 6, lambda DNA marker digested with HindIII; lanes 7 and 8, pGKV210. Lanes 2, 4, and 7, 0 generations; lanes 3, 5, and 8, 30 generations.
Electrotransformation of pND919 into S. thermophilus ST4-1.
The industrial strain S. thermophilus ST4-1 was electrotransformed with 8 ng of plasmid extracted from S. thermophilus ST3-1 and plated on M17L plus erythromycin (3 μg per ml) and M17L plus Cd (0.3 mM). For comparison, pND913 and pND918 were included. Emr colonies were obtained with pND913 and pND918 and not with pND919, while Cdr colonies were obtained with pND918 and pND919. The transformation efficiencies of pND913, pND918, and pND919 were 2 × 104, 3 × 104, and 1 × 104 transformants/μg of DNA, respectively.
DISCUSSION
In this study, a potential food-grade vector, pND919, for S. thermophilus was constructed by using an S. thermophilus replicon together with the lactococcal cadA and cadC genes. The encoded Cdr determinant was shown to confer resistance to CdCl2 in S. thermophilus. The Cdr system has previously been shown to be a useful food-grade selectable marker in lactococcal strains (7). In S. thermophilus, sensitive strains are generally inhibited in media containing 0.3 to 0.5 mM CdCl2. Hence, this marker is considered to be a good selectable marker in S. thermophilus.
That pND919 is a potential food-grade cloning vector for S. thermophilus has been demonstrated in this study. The stability of vectors in any transformants is an important issue, especially if the vector needs to be stable enough to withstand the production process. To examine this requirement, the segregational and structural stabilities of pND919 in S. thermophilus ST3-1 were investigated. The results of the stability studies indicated that pND919 is both structurally stable and segregationally (without selection) relatively stable for approximately 30 generations. In industrial use, a fermentation takes approximately 21 generations. pND919 was also shown to replicate in a second S. thermophilus industrial strain (ST4-1).
In this study, plasmid DNA extracted from E. coli was unable to transform S. thermophilus. Successful S. thermophilus ST3-1 transformation was observed only when plasmid DNA was prepared from either L. lactis LM0230 or S. thermophilus ST3-1. It is possible that E. coli DNA was recognized as foreign while L. lactis and S. thermophilus DNA was recognized as self.
Plasmid pND919, which expresses Cdr in S. thermophilus, has the potential to be used in the food industry. The expression of Cdr in S. thermophilus transformants not only is an innovative approach but also should be effective enough to be useful as a food-grade selectable marker in S. thermophilus strains that have low natural cadmium resistance. The generation of such a potential food-grade vector, therefore, creates an opportunity to enhance bacteriophage defense systems in S. thermophilus by transferring the characterized lactococcal phage defense systems to S. thermophilus.
REFERENCES
- 1.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruttin, A., F. Desiere, S. Lucchini, S. Foley, and H. Brussow. 1997. Characterization of the lysogeny DNA module from the temperate Streptococcus thermophilus bacteriophage φSfi21. Virology 233:136-148. [DOI] [PubMed] [Google Scholar]
- 3.Coffey, A., and R. P. Ross. 2002. Bacteriophage-resistance systems in dairy starter strains: molecular analysis to application. Antonie Leeuwenhoek 82:303-321. [PubMed] [Google Scholar]
- 4.Dickely, F., D. Nilsson, E. B. Hansen, and E. Johansen. 1995. Isolation of Lactococcus lactis nonsense suppressors and construction of a food-grade cloning vector. Mol. Microbiol. 15:839-847. [DOI] [PubMed] [Google Scholar]
- 5.Froseth, B. R., and L. L. McKay. 1991. Development and application of pFM011 as a possible food-grade cloning vector. J. Dairy Sci. 74:1445-1453. [Google Scholar]
- 6.Holo, H., and I. F. Nes. 1995. Transformation of Lactococcus by electroporation. Methods Mol. Biol. 47:195-199. [DOI] [PubMed] [Google Scholar]
- 7.Liu, C. Q., N. Khunajakr, G. C. Lian, Y. M. Deng, P. Charoenchai, and N. W. Dunn. 1997. Genetic analysis of regions involved in replication and cadmium resistance of the plasmid pND302 from Lactococcus lactis. Plasmid 38:79-90. [DOI] [PubMed] [Google Scholar]
- 8.MacCormick, C. A., H. G. Griffin, and M. J. Gasson. 1995. Construction of a food-grade host/vector system of Lactococcus lactis based on the lactose operon. FEMS Microbiol. Lett. 127:105-109. [DOI] [PubMed] [Google Scholar]
- 9.McKay, L. L., K. A. Baldwin, and E. A. Zottola. 1972. Loss of lactose metabolism in lactic streptococci. Appl. Environ. Microbiol. 23:1090-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Sullivan, T. F., and G. F. Fitzgerald. 1999. Electrotransformation of industrial strains of Streptococcus thermophilus. J. Appl. Microbiol. 86:275-283. [DOI] [PubMed] [Google Scholar]
- 12.Platteeuw, C., I. van Alen Boerrigter, S. van Schalkwijk, and W. de Vos. 1996. Food-grade cloning and expression system for Lactococcus lactis, Appl. Environ. Microbiol. 62:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell, B. I., G. M. Achen, J. A. Hillier, and E. B. Davidson. 1988. A simple and rapid method for genetic transformation of lactic streptococci by electroporation, Appl. Environ. Microbiol. 54:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 15.Su, P., K. Jury, G. E. Allison, W. Y. Wong, W. S. Kim, C.-Q. Liu, T. Vancov, and N. W. Dunn. 2002. Cloning vectors for Streptococcus thermophilus derived from a native plasmid. FEMS Microbiol. Lett. 216:43-47. [DOI] [PubMed] [Google Scholar]
- 16.Tanrney, M., and G. F. Fitzgerald. 2002. AbiA, a lactococcal abortive infection mechanism functioning in Streptococcus thermophilus. Appl. Environ. Microbiol. 68:6388-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]