Abstract
Characterizing denitrification rates in aquatic ecosystems is essential to understanding how systems may respond to increased nutrient loading. Thus, it is important to ensure the precision and accuracy of the methods employed for measuring denitrification rates. The acetylene (C2H2) inhibition method is a simple technique for estimating denitrification. However, potential problems, such as inhibition of nitrification and incomplete inhibition of nitrous oxide reduction, may influence rate estimates. Recently, membrane inlet mass spectrometry (MIMS) has been used to measure denitrification in aquatic systems. Comparable results were obtained with MIMS and C2H2 inhibition methods when chloramphenicol was added to C2H2 inhibition assay mixtures to inhibit new synthesis of denitrifying enzymes. Dissolved-oxygen profiles indicated that surface layers of sediment cores subjected to the MIMS flowthrough incubation remained oxic whereas cores incubated using the C2H2 inhibition methods did not. Analysis of the microbial assemblages before and after incubations indicated significant changes in the sediment surface populations during the long flowthrough incubation for MIMS analysis but not during the shorter incubation used for the C2H2 inhibition method. However, bacterial community changes were also small in MIMS cores at the oxygen transition zone where denitrification occurs. The C2H2 inhibition method with chloramphenicol addition, conducted over short incubation intervals, provides a cost-effective method for estimating denitrification, and rate estimates are comparable to those obtained by the MIMS method.
Nitrogen (N) is an essential nutrient in aquatic ecosystems. Denitrification, a microbially mediated process whereby N is removed via nitrate (NO3−) reduction to dinitrogen gas (N2), is a key flux in the N cycle and ultimately determines N availability (3). Anthropogenic changes in the N cycle, including fossil fuel burning and fertilizer production, have increased N loading into freshwater systems 6 to 50 times from historical levels, with much of the N exported to rivers, estuaries, and oceans (e.g., see references 8 and 33). Increases in N loading may lead to eutrophication, alteration of food webs, hypoxia, and methylhemoglobinemia (e.g., see reference 11). Such problems have resulted in more attention on the potential for aquatic sediments to remove nitrogenous wastes, particularly via denitrification. Characterizing denitrification is essential to understanding how ecosystems handle increased N loads. It is important to ensure the precision and accuracy of the methods employed for measuring denitrification rates.
The acetylene (C2H2) inhibition method is a simple and widely used technique for measuring denitrification rates in aquatic (20) and terrestrial sediments (e.g., for reviews see references 9 and 32). Since C2H2 inhibits nitrous oxide (N2O) reduction by several denitrifying bacteria (4, 23, 34), it has been used to assess denitrification rates in many ecosystems and sediment types (e.g., see references 7, 10, 13, and 19). Acetylene is soluble in water (ca. 1.06 [vol/vol]) and can be distributed in aquatic systems to reach metabolic sites where denitrification occurs. However, problems associated with this method may constrain its usefulness. For instance, the inhibition of N2O reductase by C2H2 may not be complete, particularly at low NO3− concentrations, and C2H2 added to sediment cores may not penetrate the total depth of sediment available, resulting in an underestimation of denitrification rates (20, 27). The concurrent inhibition of nitrification by acetylene (29) may lead to nitrification-denitrification decoupling and underestimation of denitrification rates (15, 18). Furthermore, some C2H2 inhibition methods, especially laboratory assays, cause disturbance to substrata via mixing, which may stimulate denitrification by providing carbon (C) or NO3− or inhibit denitrification by sediment aeration.
An additional complication associated with the C2H2 inhibition method is that the techniques employed by researchers are not consistent. Many researchers estimate potential (as opposed to actual) denitrification rates by using C2H2 inhibition in conjunction with NO3− and/or dissolved organic C additions and anoxic conditions induced during incubations through flushing techniques. Although such treatments provide ideal conditions for denitrification, they do not represent in situ conditions.
A variant of the C2H2 inhibition assay includes the use of chloramphenicol, an antibiotic that prohibits de novo protein synthesis but does not inhibit existing enzymes, except at extremely high concentrations (6). In the presence of chloramphenicol, denitrifying bacteria cannot produce additional enzymes in response to ideal conditions (anoxic conditions and adequate NO3− and C) created during the assay or as a result of sampling (e.g., lysis of cells during core collection). Without chloramphenicol, rates may be influenced by synthesis of new denitrifying enzymes and microbial growth due to reduced O2 tension and a newly available substrate (30). The results then represent only enzymatic potential of the sediments for denitrification, not actual in situ rates of denitrification. Thus, chloramphenicol may prove useful in quantifying realistic rates of denitrification via laboratory assays.
A relatively new method for measuring denitrification rates involves measuring N2 concentrations in water samples by membrane inlet mass spectrometry (MIMS) (12, 16). This method has several advantages over other methods, including rapid throughput (∼20 to 30 samples h−1), lack of sample water preparation (e.g., no degassing step), small sample size (∼15 ml), and precise measurement of concentration and gas isotope ratio data (16). A benefit of MIMS is that it also allows the simultaneous measurement of N fixation after isotope addition (1).
A limitation of this method is inaccurate measurement of gases in supersaturated water (16). MIMS uses a quadrupole mass spectrometer to detect dissolved gases in water samples, but the ion source within the quadrupole mass spectrometer ionizes gases prior to detection and produces O+, which reacts with N2 to form NO (12). This error increases as the dissolved-oxygen concentration decreases. However, the error is instrument dependant and must be evaluated case by case (12). MIMS may underestimate denitrification if reduction stops at an intermediate step (i.e., nitrous oxide) rather than continuing to completion. Nitrous oxide production via denitrification may be greater than N2 production in some cases (5, 34). Another potential limitation of MIMS is total incubation time, which can vary from several hours to several days, potentially influencing the microbial community. Finally, the acquisition and assembly of MIMS equipment is costly (∼$30,000) and time-consuming.
The objectives of this study were to (i) compare estimates of denitrification measured by C2H2 inhibition and MIMS methods, (ii) compare estimates of potential and actual denitrification rates measured using C2H2 inhibition techniques, (iii) compare denitrification estimates by using sediment slurries and cores, (iv) gauge the effects of sample treatment on microbial assemblage structure, and (v) identify mechanisms responsible for observed differences in denitrification estimates by examining dissolved-O2 sediment profiles before and after incubations. We hypothesized that the C2H2 inhibition method would underestimate denitrification rates, relative to MIMS, due to the concurrent inhibition of nitrification. We also hypothesized that differences between results of C2H2 inhibition and MIMS would be smallest in comparisons of potential denitrification rates. We propose this hypothesis because potential rates are estimated by adding NO3− into the cores and incubating the cores under anoxic conditions, which may alleviate the decoupling effect of C2H2 on nitrification-denitrification. If high rates of denitrification require high rates of nitrification, adding an abundant substrate will have only a slight effect when nitrification is inhibited.
MATERIALS AND METHODS
Sediment collection and preparation.
Sediments were collected within the Corpus Christi Bay estuary near Corpus Christi, Texas, in July 2002. Two stations, in Nueces Bay and Corpus Christi Bay, were selected for measurement of water column characteristics (temperature, salinity, chlorophyll a concentration, and dissolved-O2 concentration [determined by using a Hydrolab]) and collection of sediment cores and slurries for incubation experiments. Water column and sediment characteristics at each site are presented in Table 1.
TABLE 1.
Characteristics of water and sediment at Nueces River and Corpus Christi Bay sites
| Site | Sediment O2 demand (μmol of O2 m−2 h−1) ± SD | Temp (°C) | Salinity (ppt) | Concn of:
|
Mean sediment C-to-N ratio (by mass) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dissolved O2 (mg liter−1) | Chlorophyll (μg liter−1) | NO3−-N (μM) | NH4+-N (μM) | PO43−-P (μM) | |||||
| Nueces River | 736 ± −2 | 30 | 0.29 | 5.3 | 15.5 | 66.7 | 1.4 | 4.4 | 29 |
| Corpus Christi Bay | 1,077 ± −70 | 32.3 | 1.94 | 11.2 | 38.1 | 1.95 | 0.78 | 4.1 | 46 |
Intact sediment cores (7.6 cm in diameter and 10 to 20 cm in length) were collected using a coring device equipped with a polyvinyl chloride handle and a one-way rubber valve to maintain core integrity and retain overlying water during core retrieval. Cores were collected with care to preserve sediment structure and avoid stimulating denitrification by providing a new supply of substrate. After collection, cores were capped immediately and placed into a cooler for transport. Additional cores were retrieved for composite sediment slurry samples at each site. Slurries were composed of the uppermost 10 to 15 cm of sediment from three separate cores. Two 20-liter carboys of bottom water were collected from each site for the MIMS flowthrough system and C2H2 inhibition assays. Cores were transported to the laboratory within 5 h of collection and divided randomly into treatment groups. Initial O2 concentrations were measured using microelectrodes (see below).
Potential rates in both cores and slurries were assessed under conditions of enriched NO3− and C concentrations and reduced O2 conditions. Nitrate (NO3−-N in the form of KNO3; final concentration, >9 mg liter−1) and carbon (as C6H12O6; final concentration, >30 mg liter−1) were added to cores for measurement of potential rates, and headspace was purged with helium (He) for 5 min, with periodic shaking, prior to the introduction of pure C2H2 gas. This process lowered dissolved-O2 concentrations in the slurries to <1.0 mg liter−1 as measured with an O2 microelectrode at the beginning of the experiment. The cores and slurries used for measurement of potential rates will hereafter be referred to as “amendment” cores and slurries. Cores selected for measurement of actual denitrification rates had no nutrient additions prior to pure C2H2 gas introduction. These cores and slurries will hereafter be referred to as “no-amendment” cores and slurries.
To estimate denitrification rates by using sediment slurries, 25 cm3 of sediment slurry was placed into 150-ml medium bottles capped with butyl septa. Unfiltered bottom water collected at the site was added to bring the total sediment-water volume to 75 ml. Chloramphenicol was added to achieve a final concentration of 5 mM (0.121 g/bottle); NO3− and C additions were made to the amendment sediment slurries as described above.
Dissolved-oxygen profiles.
Sediment O2 concentrations within the cores and slurries were measured using cathode-type dissolved-O2 microelectrodes (18). Electrodes were glass coated and had a gold-plated platinum wire tip of <10 μm in diameter. They were not sensitive to water velocity and could be used without stirring. The sensing tip of the electrode was placed inside a 16-mm-gauge hypodermic needle and held adjacent to the bevel of the needle with epoxy resin to avoid breakage during sediment penetration (18). Probes were recalibrated in every core by using water column O2 concentration, determined with a YSI model 58 macroprobe with gentle stirring, and anoxic sediment. Measurements of dissolved O2 were made (1) immediately upon return to the laboratory, before any experimental manipulations or addition of C2H2, and (2) within 1 h after the final gas or water sample was extracted.
Acetylene inhibition.
Acetylene inhibition procedures were conducted according to standard methods (e.g., see references 7, 19, and 32). Approximately 20% of the core length was reserved for headspace gas sampling. Pure C2H2 gas was injected into each core and slurry to 10% saturation (10 kPa) (32). To promote distribution of C2H2, headspace was reduced and increased alternately by pumping with a large syringe immediately after the C2H2 had been added. Sediments were incubated in environmental chambers for 6 h at ambient temperatures. Gas samples were taken with disposable plastic syringes and transferred to evacuated gas vials at time point 0 (before C2H2 addition), approximately 10 min after C2H2 introduction (to ensure distribution at the initial point), and every hour for 6 h following. Septa on gas vials were covered with rubber silicone beads to eliminate any gas seepage prior to gas analysis. Samples were analyzed on a Shimadzu 5890 gas chromatograph equipped with a Porapak Q column and a 63Ni electron capture detector for measurement of N2O concentration after creation of a standard curve from known concentrations of N2O (see references 21 and 25 for specifications). Rate calculations were corrected using the Bunsen coefficient (31). Control samples without addition of C2H2 had no accumulation of N2O over the incubation period.
MIMS.
Upon return to the laboratory, cores to be used for MIMS analyses were placed in a water bath maintained at ambient temperatures and equipped with a flowthrough plunger with Teflon inlet and outlet tubes installed over each sediment core. Bottom water from the site was passed over the core surface at 1.2 ml min−1 (0.072 liter h−1). Water column depth over the sediment was maintained at ∼5 cm to give a water volume of ca. 570 ml in each core. Triplicate samples of inflow and outlet water were retrieved at discreet time intervals (daily) for dissolved-gas analysis, after an initial incubation period of 1 day to allow steady-state conditions to develop. For 2 days after the preincubation, net N2 flux and sediment O2 demand were estimated by multiplying the difference between the inflow and outflow concentrations by a constant based on flow rate and core surface area. For measurement of N2 fixation in conjunction with denitrification, inflow water was enriched with 15NO3− (98% pure; ∼100 μM final concentration) and three masses of N2 gas were measured for two more days (28N2 from 14NO3−, 30N2 from 15NO3−, and 29N2 from 14NO3− and 15NO3−) (22). The total incubation time for the MIMS flowthrough method was 5 days.
Molecular analysis.
Samples for molecular analysis of the microbial assemblages were taken from the sediment cores immediately after field collection and following the flowthrough and C2H2 inhibition experiments. Subcores were collected with a 25-mm-diameter corer, flash frozen in liquid N2, and stored at −20°C until further processing. Frozen subcores were cut at 1-cm intervals, and sections were thawed and homogenized. DNA was extracted from ca 0.5 g of each homogenized slice by using a Mo Bio Soil DNA extraction kit and purified with a Stratagene GeneClean spin kit according to the manufacturers' instructions. DNA yield was determined with gel electrophoresis, and DNA extracts were stored frozen at −20°C.
DNA was amplified in a PCR mixture containing 2.25 mM Mg2+, 1 mM deoxynucleoside triphosphates (equimolar mixture of dATP, dTTP, dGTP, and dCTP), and 0.05 U of Taq DNA polymerase/μl with universal primers P2 and P3, which contain a GC clamp and are designed to amplify a ca. 200-bp fragment of the 16S rRNA gene. Amplification was conducted in an Idaho Technologies Indy thermocycler. Cycling was set to 2 min at 96°C; five cycles of 15 s at 92°C, 15 s at 55°C, and 45 s at 72°C; five cycles of 15 s at 94°C, 15 s at 50°C, and 45 s at 72°C; and 25 cycles of 15 s at 96°C, 15 s at 45°C, and 45 s at 72°C. The sizes of the fragments were determined by agarose gel electrophoresis (at 10 V/cm with 2% agarose in TAE buffer for 60 min) against a 100-bp DNA ladder (New England Biolabs) of known concentration. PCR products were concentrated to ca. 40 ng/μl via isopropanol precipitation, resuspended in 1× TAE buffer, and loaded onto a 20-to-60% gradient denaturing gradient gel electrophoresis (DGGE) gel at 10 μl/lane. Electrophoresis was conducted at 65°C and 10 V/cm for 5 h. The resulting gel was stained with ethidium bromide, destained in deionized water, and recorded digitally on a UV transilluminator.
RESULTS
Site physiochemical parameters.
The Corpus Christi Bay site had salinity approximately seven times higher than that of the Nueces River mouth, although salinities for both sites were uncharacteristically low due to recent flooding (Table 1). Water column O2 and chlorophyll concentrations were higher in the Corpus Christi Bay site. Sediment O2 demand and C-to-N content were higher in Corpus Christi Bay sediments. Nitrate was present in the water of both sites, but concentrations in Corpus Christi Bay (2 μM) were 30 times lower than those in the Nueces River (67 μM), suggesting potential NO3− limitation. Both sites had relatively low phosphate concentrations.
Dissolved-oxygen profiles.
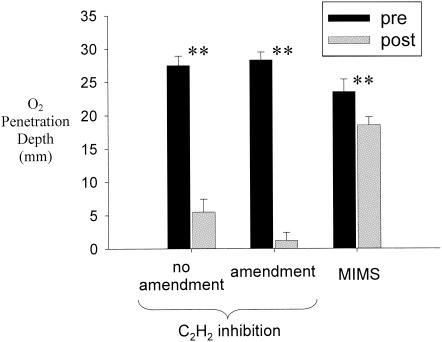
Significant differences in O2 penetration into the sediment cores were observed in connection with different sites, measurement times, and treatments applied (Fig. 1 and 2). All treatment groups demonstrated a decline in O2 penetration into the sediment, with depth to anoxia decreasing significantly over the incubation period (α = 0.05; P < 0.001 [analysis of variance {ANOVA}]) (Fig. 2). This decline in O2 penetration into the sediment was least pronounced in the cores subjected to the flowthrough system and most pronounced in the cores subjected to C2H2 addition in the amendment treatment (those purged with He and receiving C and N additions). Sediment slurries also demonstrated a significant decline in O2 concentrations, with average sediment O2 concentrations of ∼6 mg of O2 liter−1 prior to incubation and <1 mg of O2 liter−1 postincubation.
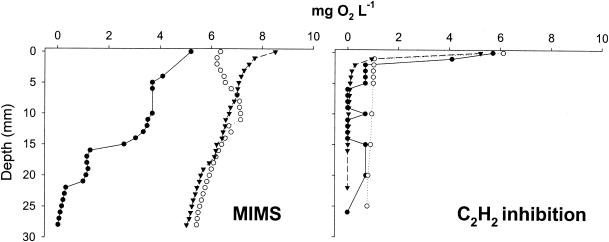
FIG. 1.
Selected profiles of dissolved-oxygen (O2) concentration with depth in sediment cores subjected to MIMS flowthrough incubation and C2H2 inhibition incubation. Concentrations were measured post-incubation periods. Different lines and symbols represent different replicate cores, with both sites represented.
FIG. 2.
Comparison of depths of O2 penetration into the sediment cores pre- and postincubation under different incubation techniques for all samples collected at both sites. Amendment cores were incubated with He headspace and carbon and nitrogen additions; no-amendment cores were incubated without additions; MIMS cores were incubated with a flowthrough system. **, significant difference within treatment group (α = 0.05; P < 0.001 [ANOVA]). Bars indicate standard deviations (SD).
Denitrification rates.
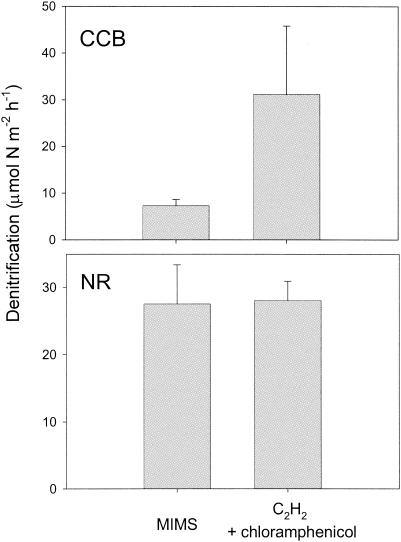
Significant differences in denitrification rates among sediments collected from the Nueces River and Corpus Christi Bay sites were detected for all methods and treatments (P > 0.002; α = 0.05 [ANOVA]; n = 13) (data not shown). Despite large differences among sites, differences among methods were also detected (Fig. 2 and 3). No significant differences in denitrification estimates were found at either site when results of the MIMS techniques were compared to those of C2H2 inhibition with chloramphenicol (Fig. 3) (P > 0.10). However, denitrification estimates made by using C2H2 in conjunction with chloramphenicol were higher than MIMS estimates for the Corpus Christi Bay site.
FIG. 3.
Average denitrification estimates obtained by MIMS flowthrough treatment and acetylene inhibition with the addition of chloramphenicol for Corpus Christi Bay (CCB) and the Nueces River (NR). Bars indicate SD.
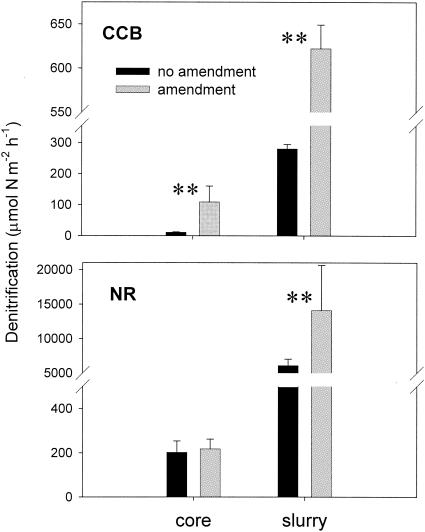
Denitrification rates estimated using the C2H2 inhibition method varied with the treatments applied (Fig. 4). Rate estimates were highest for amendment sediments without chloramphenicol addition. However, adding substrate and creating an anoxic habitat by purging with He proved ineffective for measuring potential denitrification rates in cores, as opposed to slurries, from the Nueces River, resulting in lower mean rates (Fig. 4) similar to estimates for cores without amendments (Fig. 4). Core sediments at this site were more silty and impermeable (Table 1), likely resulting in less diffusion of nutrients and C2H2 into the sediment.
FIG. 4.
Average denitrification rates estimated using the C2H2 inhibition method with sediment cores and slurries from the Corpus Christi Bay (CCB) and the Nueces River (NR). Amendment cores and slurries were incubated with a helium headspace, and additions of nitrogen and carbon were made prior to incubation. **, significant difference within treatment group (α = 0.05; P < 0.001 [ANOVA]). Bars indicate SD.
Molecular analyses.
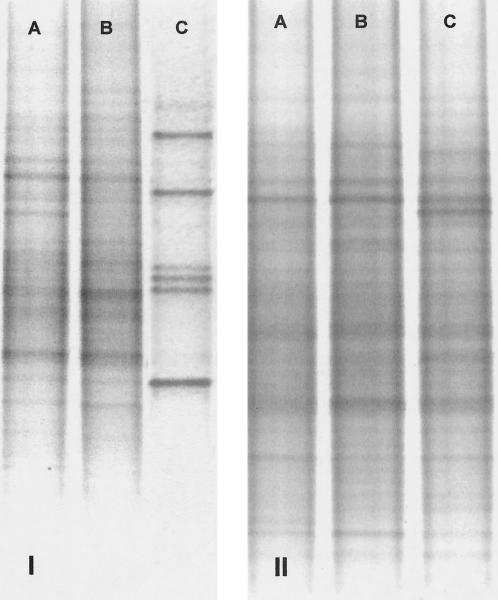
A degree of heterogeneity between fresh sediment cores with respect to 16S rRNA gene diversity was detected by DGGE analysis (Fig. 5, lines A). The changes in the bacterial community structures were most pronounced in the top centimeter of the cores, while the compositions of the communities deeper in the sediment cores, where denitrification is expected to occur, remained relatively unchanged. The flowthrough treatment altered the surface community composition over the 5-day incubation period to a greater extent than did the C2H2 inhibition over the 5-h incubation.
FIG. 5.
DGGE profiles of 200-bp 16S rRNA gene fragments. Panel I represents the 0- to 1-cm-depth horizon, and panel II represents the 3- to 4-cm-depth horizon. A, B, and C indicate, respectively, before treatment, after C2H2 inhibition, and after flowthrough incubation.
DISCUSSION
Contrary to our preliminary hypotheses, denitrification rates measured using the MIMS technique yielded significantly lower estimates of denitrification than did those measured using traditional C2H2 inhibition methods, except when the C2H2 inhibition method included chloramphenicol addition (Fig. 2 and 3). No significant differences were found between results of MIMS and those of the C2H2 inhibition method with chloramphenicol use. Not surprisingly, the highest denitrification rates were associated with amendment slurries and cores (representing potential denitrification) in which rates were measured using C2H2 inhibition without the addition of chloramphenicol (Fig. 4). These sediments had lower O2 tension after the incubation period than did cores subjected to the MIMS flowthrough technique (Fig. 1), a factor that may have contributed to increased rates due to more-abundant anoxic zones. Alternatively, the MIMS technique involved preincubation for 1 day, during which overlying water was recirculated through intact cores. Thus, the nitrate in the water may have been depleted during preincubation, resulting in lower rates. Lower rates obtained with MIMS for Corpus Christi Bay may also be an artifact of limited data, as these estimates are the average of only two measurements.
When ambient NO3− concentrations in the water column are maintained, populations of denitrifying bacteria are most dense at or just below the surface (24). In contrast, populations in cores supplemented with NO3− can exhibit the capacity for denitrification down to a depth of >9 cm (24). The combination of increased capacity of the denitrifying bacteria (because of high NO3− concentrations and decreased O2 penetration into the sediment core) and the synthesis of additional denitrification enzymes in the absence of chloramphenicol may have resulted in the increased denitrification rates observed in sediments subjected to an abundant substrate (amendment cores and slurries). Increases in amounts of NO3− likely did not influence denitrification in the Nueces River site due to the already high ambient NO3− concentrations (Table 1), but the addition of C and the creation of an anoxic environment yielded an increase in rates within amendment sediments at this site.
The C2H2 inhibition method lacks the ability to detect coupled nitrification-denitrification and can result in >50% decreases in denitrification rates in some systems (26, 27). If high denitrification rates require high nitrification rates, adding abundant substrate should alleviate this effect, resulting in minimal changes due to concurrent inhibition when C2H2 is added. In this study, adding NO3− to sediment subjected to the C2H2 inhibition method had a pronounced effect (twofold increase in denitrification rates) (Fig. 4), indicating that these two processes may be tightly coupled within these sediments, at least within the 5-h incubation time. In contrast, the finding that denitrification rates estimated for Corpus Christi Bay sediment by using C2H2 plus chloramphenicol were slightly higher than those estimated using MIMS (Fig. 3) may indicate the lack of coupling. These results suggest that nitrification-denitrification coupling does not significantly alter estimates within the time frame necessary for each technique when chloramphenicol addition is used. Higher denitrification estimates determined for Corpus Christi Bay by using C2H2 and chloramphenicol may be due to the ability of this technique to measure total denitrification (i.e., including that yielding either N2 or N2O as an end product). The MIMS method may underestimate denitrification rates by not incorporating the denitrification that yields N2O as an end product, although in this experiment we found no accumulation of N2O in control samples with no C2H2 additions.
Results (Fig. 4) were not consistent with results of previous research demonstrating that similar denitrification rates are estimated by C2H2 inhibition in intact sediment cores and sediment slurries (25). In this study, sediment slurries collected from the top 2 cm of a sediment core had denitrification rates similar to those of the entire sediment core only in the presence of added chloramphenicol (Fig. 2 and 3). Sediment slurries used without the addition of chloramphenicol may have had higher rates due to the production of new enzymes in response to abundant substrate and anoxic environments and the release of existing enzymes via lysis of cells during collection of composite slurry samples. Sampling sediment from the top 2 cm provides accurate estimates of denitrification rates within the entire sediment core when chloramphenicol is used because denitrification rates are typically highest in the upper layers of sediment and soils (Fig. 5) (25).
Overall, the flowthrough system used for the MIMS measurements maintained the natural physical structure of the sediment cores (as monitored through O2 concentrations) (Fig. 1 and 2) but not the microbial community structures in the surface sediments (Fig. 5), likely a result of the longer incubation times. More O2 penetrated into the sediment core over the incubation period when the MIMS flowthrough technique was used because of the gas-saturated continuous flow of water running across the surface of the sediment core. Without such flowthrough, cores quickly became anoxic (Fig. 1 and 2), changing the physiochemical properties of the sediment cores. Although some decreases in O2 concentration within the sediment occurred with all the methods during the incubation period, the changes were much lower with the MIMS flowthrough system, and thus, the in situ O2 concentrations were probably more closely represented in these sediments.
The MIMS flowthrough treatment had the most pronounced effect upon the 16S rRNA gene diversity at the core surface and appeared to favor a small subpopulation of bacteria (seven principal bands were significantly enhanced compared to the starting condition) (Fig. 5, lines C). However, gene diversity was not affected in the transition zone where denitrification is expected to occur. The bacterial assemblages in samples undergoing C2H2 treatment retained most of the original diversity, even those in surface sediments (Fig. 5, lines B). This observation may seem counterintuitive, as the C2H2 inhibition method involves introduction of a metabolic disrupter, but the long incubation times associated with the MIMS method may have a stronger effect on community composition. The C2H2 inhibition lasted a few hours, whereas the flowthrough incubation used in conjunction with MIMS measurements lasted 5 days.
The ability to denitrify is widely distributed across taxonomic lines of bacteria (5). Molecular analysis was limited to the general 16S rRNA gene population, and even though major changes were observed, those changes do not necessarily indicate changes in the denitrifying community. On the other hand, changes in the denitrifying community may pass unnoticed by the 16S analysis. However, our results indicate that the flowthrough incubation appears to be a more intrusive treatment, eliciting a long-term community response at the sediment surface and establishing a new equilibrium bacterial community appropriate for the given chemical conditions. The short incubation time required for C2H2 inhibition estimates makes the method more appropriate for studying responses of existing bacterial populations. However, the constant supply of oxygenated water causes redox conditions to be affected less in the flowthrough system than in the C2H2 inhibition system even though incubations are longer.
Even though the flowthrough treatment resulted in pronounced changes in bacteria at the sediment surface, changes were limited to the upper 20 mm of the sediment column (Fig. 5, lines C), and little change was observed at depths of over 20 mm with either treatment. Given that nitrification and denitrification generally occur in or near the oxygen transition zone and that the O2 penetration depth in the sediment cores was generally 2 to 3 cm, it seems unlikely that the major bacterial assemblage change in the top centimeter of the flowthrough system would have affected the structures of populations of anaerobic denitrifying bacteria.
Various methods have been used to quantify denitrification, including NO3− disappearance (4), 15N tracer studies (31), C2H2 inhibition (4, 14, 19, 35), and MIMS (1, 12, 16, 17), and the methodology for these techniques has been discussed (e.g., see references 20, 27, 28, and 32). However, differences among methods have not been predictable (9, 32). Rates measured using different methods are within the same order of magnitude, and the results of our experiments indicate that C2H2 inhibition with the addition of chloramphenicol and MIMS techniques yielded comparable results. These data suggest that the longer incubation times associated with the flowthrough system yield a change in community structure and potentially lower estimates of actual denitrification by not incorporating denitrification yielding N2O as an end product. The C2H2 inhibition method with chloramphenicol addition provides a simple, cost-effective method for estimating denitrification, and this method can be used if the ambient conditions are measured and taken into account. Conducting incubations over short intervals should minimize potential errors caused by simultaneous nitrification inhibition.
Acknowledgments
We thank Randy Bernot for field assistance, Andrew Laursen and Todd Royer for helpful discussion and technical assistance, Sarah Eichler and Lareina Wall for comments on an early version of the manuscript, and two anonymous reviewers for helpful comments.
Travel funds were provided by a Francis M. Kobayashi research grant to M.J.K. and J.L.T. and support from the National Science Foundation to W.K.D.
Authors are listed in alphabetical order after the first author.
REFERENCES
- 1.An, S., W. S. Gardner, and T. Kana. 2001. Simultaneous measurement of denitrification and nitrogen fixation using isotope pairing with membrane inlet mass spectrometry analysis. Appl. Environ. Microbiol. 67:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, J. M. 1977. Rates of denitrification of undisturbed sediment from six lakes as a function of nitrate concentration, oxygen and temperature. Arch. Hydrobiol. 80:147-159. [Google Scholar]
- 3.Atlas, R. M., and R. Bartha. 1998. Microbial ecology: fundamentals and applications. Benjamin Cummings, Menlo Park, Calif.
- 4.Balderston, W. L., B. Sherr, and W. J. Payne. 1976. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl. Environ. Microbiol. 31:504-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsma, T. T., N. E. Ostrom, M. Emmons, and G. P. Robertson. 2001. Measuring simultaneous fluxes from soil of N2O and N2 in the field using the 15N-gas “nonequilibrium” technique. Environ. Sci. Technol. 35:4307-4312. [DOI] [PubMed] [Google Scholar]
- 6.Brock, T. D. 1961. Chloramphenicol. Bacteriol. Rev. 25:32-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, Y. K., and R. Knowles. 1979. Measurement of denitrification in two freshwater sediments by an in situ acetylene inhibition method. Appl. Environ. Microbiol. 37:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conley, D. J. 2000. Biogeochemical nutrient cycles and nutrient management strategies. Hydrobiologia 410:87-96. [Google Scholar]
- 9.Cornwell, J. C., W. M. Kemp, and T. M. Kana. 1999. Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls, a review. Aquat. Ecol. 33:41-54. [Google Scholar]
- 10.DeLaune, R. D., L. M. Salina, R. S. Knox, M. N. Sarafyan, and C. J. Smith. 1991. Water quality of a coastal river receiving nutrient inputs: ammonium nitrogen transformations. J. Environ. Sci. Health 26:1287-1302. [Google Scholar]
- 11.Dodds, W. K., and E. Welch. 2000. Establishing nutrient criteria in streams. J. North Am. Benthol. Soc. 19:186-196. [Google Scholar]
- 12.Eyre, B. D., S. Rysgaard, T. Dalsgaard, and P. B. Christensen. 2002. Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification—assumptions, modifications, and implications. Estuaries 25:1077-1087. [Google Scholar]
- 13.Henriksen, K., T. H. Blackburn, B. A. Lomstein, and C. P. McRoy. 1993. Rates of nitrification, distribution of nitrifying bacteria and inorganic N fluxes in northern Bering-Chukchi shelf sediments. Continental Shelf Res. 13:629-651. [Google Scholar]
- 14.Holmes, R. M., J. B. Jones, Jr., S. G. Fisher, and N. B. Grimm. 1996. Denitrification in a nitrogen-limited stream ecosystem. Biogeochemistry 33:125-146. [Google Scholar]
- 15.Joye, S. B., S. V. Smith, J. T. Hollibaugh, and H. W. Paerl. 1996. Estimating denitrification rates in estuarine sediments: a comparison of stoichiometric and acetylene based methods. Biogeochemistry 33:197-215. [Google Scholar]
- 16.Kana, T. M., C. Darkangelo, M. D. Hunt, J. B. Oldham, G. E. Bennett, and J. C. Cornwell. 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2 and Ar in environmental water samples. Anal. Chem. 66:4166-4170. [Google Scholar]
- 17.Kana, T. M., M. B. Sullivan, J. C. Cornwell, and K. M. Groszkowski. 1998. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol. Oceanogr. 43:334-339. [Google Scholar]
- 18.Kemp, M. J., and W. K. Dodds. 2001. Centimeter-scale patterns in dissolved oxygen and nitrification rates in a prairie stream. J. North Am. Benthol. Soc. 20:347-357. [Google Scholar]
- 19.Kemp, M. J., and W. K. Dodds. 2002. Comparison of nitrification and denitrification in prairie and agriculturally influenced streams. Ecol. Appl. 12:998-1009. [Google Scholar]
- 20.Knowles, R. 1982. Denitrification. Microbiol. Rev. 46:43-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosier, A. R., and L. Mack. 1980. Gas chromatographic system for precise, rapid analysis of nitrous oxide. Soil Sci. Soc. Am. J. 44:1121-1123. [Google Scholar]
- 22.Nielsen, L. P. 1992. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol. Ecol. 80:357-362. [Google Scholar]
- 23.Payne, W. J. 1973. Reduction of nitrogenous oxides by microorganisms. Bacteriol. Rev. 37:409-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne, W. J. 1991. A review of methods for field measurements of denitrification. For. Ecol. Manag. 44:5-14. [Google Scholar]
- 25.Raymond, N., P. Bonin, and J. C. Bertrand. 1992. Comparison of methods for measuring denitrifying activity in marine sediments from the Western Mediterranean coast. Oceanol. Acta 15:137-143. [Google Scholar]
- 26.Rudolph, J., P. Frenzel, and N. Pfennig. 1991. Acetylene inhibition technique underestimates in situ denitrification rates in intact cores of freshwater sediments. FEMS Microbiol. Ecol. 85:101-106. [Google Scholar]
- 27.Seitzinger, S. P. 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol. Oceanogr. 33:702-724. [Google Scholar]
- 28.Siegel, R. S., R. D. Hanck, and L. T. Kurtz. 1982. Determination 30N2 and application to measurement of N2 evolution during denitrification. Soil Sci. Soc. Am. J. 46:68-74. [Google Scholar]
- 29.Sloth, N. P., L. P. Nielsen, and T. H. Blackburn. 1992. Nitrification in sediment cores measured with acetylene inhibition. Limnol. Oceanogr. 37:1108-1112. [Google Scholar]
- 30.Smith, M. S., and J. M. Tiedje. 1979. Phases of oxygen depletion in soil. Soil Biol. Biochem. 11:261-267. [Google Scholar]
- 31.Tiedje, J. M. 1982. Denitrification, p. 1011-1026. In A. L. Page (ed.), Agronomy monographs, 2nd ed., vol. 9. Methods of soil analysis. American Society of Agronomy, Madison, Wis.
- 32.Tiedje, J. M., S. Simkins, and P. M. Groffman. 1989. Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. Plant Soil 115:261-284. [Google Scholar]
- 33.Vitousek, P. M. 1994. Beyond global warming: ecology and global change. Ecology 75:1861-1876. [Google Scholar]
- 34.Weier, K. L., J. W. Doran, J. F. Power, and D. T. Walters. 1993. Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci. Soc. Am. J. 57:66-72. [Google Scholar]
- 35.Yoshinari, T., R. Hynes, and R. Knowles. 1977. Acetylene inhibition of nitrous oxide reduction and measurement of denitrification and nitrogen fixation in soil. Soil Biol. Biochem. 9:177-183. [Google Scholar]