Abstract
In this study we provide further evidence associating activated cells of the monocyte lineage with the lesions of multiple sclerosis (MS). Using a combination of immunohistochemistry and reverse transcriptase-dependent in situ polymerase chain reaction analysis, we have identified monocytes expressing inducible nitric oxide synthase (iNOS) to be prevalent in the plaque areas of post mortem brain tissue from patients with MS. In addition, we have obtained evidence of the nitration of tyrosine residues in brain areas local to accumulations of iNOS-positive cells. In parallel studies we have assessed the effects of inhibitors of iNOS induction, as well as scavengers of nitric oxide and peroxynitrite in the experimental allergic encephalomyelitis model. Significant therapeutic effects were seen with the inhibitor of iNOS induction, tricyclodecan-9-xyl-xanthogenate, a nitric oxide scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, and a peroxynitrite scavenger, uric acid. In particular, treatment with high doses of uric acid virtually prevented clinical symptoms of the disease. Together with our demonstration of the presence of activated macrophages expressing high levels of iNOS and evidence of peroxynitrite formation in brain tissue from patients with MS, these findings are of importance in the development of approaches to treat this disease.
Evidence for the activation of the enzyme (iNOS) responsible for the production of the free radical nitric oxide (NO) in an immune response and the release of NO in the central nervous systems (CNS) of animals with experimental allergic encephalomyelitis (EAE) or viral encephalomyelitis (1–6) has led to further study of the role of NO and its products in CNS pathology. Convincing evidence for the involvement of NO overproduction in the pathogenesis of EAE comes from studies that have correlated spinal cord and brain NO levels with clinical symptoms in animals with EAE (1, 5). The relevance of these animal studies to multiple sclerosis (MS) is emphasized by the fact that high levels of mRNA specific for iNOS can be observed in brain lesions from MS patients (7, 8). In this case, there has as yet been no consensus as to the nature of the iNOS-positive cells with either cells of the monocyte lineage or astrocytes being implicated by different studies (7, 8). To determine more specifically the nature and origin of the activated cells expressing iNOS in the MS brain we have expanded our analysis of MS brain tissue specimens using a refined combination of immunohistochemistry and reverse transcriptase in situ PCR (RT-IS-PCR).
Several possibilities exist regarding the nature of NO involvement in the pathogenesis of CNS lesions. A variety of destructive effects have been postulated, both through direct interaction with cell surface and internal molecules (9) and through chemical interactions with oxygen species, such as superoxide (O2−), to form other potentially more toxic intermediates (10, 11). One of these, peroxynitrite (ONOO−), the reaction product of NO and superoxide, is a potent oxidant (12), which can be formed in an inflammatory response and can cause a variety of toxic effects, including lipid peroxidation (12) and tyrosine nitration (13). It has therefore been suggested that peroxynitrite may be responsible for a significant proportion of the damage attributed to NO. Thus, peroxynitrite may be an important factor in the generation of CNS lesions. Indeed, in a previous study we found evidence of tyrosine nitration in the brain tissue of MS patients (7). To further clarify the direct or indirect role of NO and peroxynitrite in the pathogenesis of CNS lesions, we analyzed the effect of substances thought to inhibit iNOS activation or to selectively scavenge NO or peroxynitrite on the development of the clinical symptoms of EAE in an acute model.
MATERIALS AND METHODS
Brain Tissue Specimens.
Brain tissue was obtained post mortem from patients who had suffered with MS for at least 10 years. Brain tissue was removed within 12 hr of death during autopsy at Thomas Jefferson University and areas with and without plaques were immediately frozen on dry ice and stored at −80°C until use. The diagnoses of MS, based on clinical presentation, were confirmed by the post mortem histopathological examination of brain tissues. All MS brains were found to contain pathognomonic MS plaques. In most cases autopsy samples were of frontal lobes, which provided mixed white and gray matter.
Immunohistochemistry and RT-IS-PCR.
To define cell types positive for iNOS mRNA expression in frozen brain sections, immunohistochemistry was performed in conjunction with RT-IS-PCR as described (7, 14). Staining was carried out with a variety of antibodies and lectins: (i) cells of the macrophage/microglia cell lineage were identified with rhodamine-conjugated Ricinus communis agglutinin (RCA-1) lectin (Sigma), which specifically binds these cells (15); (ii) microvascular endothelial cells were distinguished with polyclonal antibodies specific for von Willebrand factor (vWF; factor VIII-related antigen), which is expressed in the cytoplasm of human endothelial cells (Sigma) (16); (iii) astrocytes were identified using polyclonal antibodies against glial fibrillary acidic protein (GFAP) (17); (iv) oligodendrocytes were stained with monoclonal antibodies to 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase; Sigma), which is specifically expressed in oligodendrocytes and Schwann cells (18); and (v) neurons were identified with a cocktail of three monoclonal antibodies (NF5139, NF160, and NF389), which bind three different neurofilament epitopes (19). Antibodies to nitrotyrosine were purchased from Upstate Biotechnology (Lake Placid, NY). The GFAP- and neurofilament-specific antibodies employed were kind gifts from John Trojanowski (University of Pennsylvania). All of the primary antibodies used in these studies were unconjugated and developed using appropriate rhodamine-labeled secondary antibodies (Sigma). The product of RT-IS-PCR for iNOS mRNA was identified by hybridization with a fluorescein isothiocyanate-conjugated oligonucleotide probe specific for the iNOS-amplified sequence (25-mer oligonucleotide: 5′-AACATTGCTGTGATCCATAGTFTrC-3′). Using this approach, examination with a fluorescent microscope usually reveals cytoplasmic staining for mRNA versus nuclear staining for DNA (20). At least 10,000 cells per stain combination were examined for the immunohistochemistry and mRNA signals.
Induction of Acute EAE.
Six- to 8-week-old SWXJ-14 female mice were purchased from The Jackson Laboratory. For most experiments, 3–7 mice were included per treatment group or time point for analysis. EAE was elicited by immunizing mice subcutaneously at two sites on days 0 and 7 with complete Freund’s adjuvant and 100 μg of the peptide PLP139–151 (PLP; HSLGKWLGHPDKF) derived from the sequence of proteolipid protein, a major component of myelin. PLP was synthesized by the peptide synthesis facility of the Kimmel Cancer Center, Thomas Jefferson University. This immunization protocol results in a progressive, often fatal, form of acute EAE in female SWXJ-14 mice (21, 22). Clinical symptoms in these mice manifest first as an ascending paralysis approximately 13 days after immunization, with rapid disease progression until death at days 16–20. The clinical signs of EAE were scored as: (i) piloerection, tail weakness; (ii) tail paralysis; (iii) tail paralysis plus hind limb weakness/paralysis; (iv) tail, hind and fore limb paralysis; (v) moribund.
NO Measurement.
Semiquantitative measurement of NO levels in the brain and spinal cord of control and experimental animals was performed as described (5). Briefly, NO was spin-trapped in vivo using diethyldithiocarbamate (DETC) and iron administered subcutaneously at two separate sites. Mice were euthanized 30 min later, and spinal cord and brain tissue were snap-frozen for analysis by electron paramagnetic resonance (EPR) spectroscopy. NO concentrations were estimated from EPR spectra using a calibration curve obtained as described (5).
Treatment of Acute EAE.
To test their action in the treatment of the development of acute EAE, the compounds of interest were administered i.p. in PBS beginning on day 4 or 5 after PLP immunization. Administration was continued daily until at least 2 days after all untreated PLP-immunized mice became severely paralyzed. Tricyclodecan-9-yl-xanthogenate (D609) inhibits iNOS induction by blocking the activity of phosphatidylcholine-specific phospholipase C, a key enzyme in the signal transduction pathway leading to iNOS activation (23). Mice were given 1 mg of D609 (Biomol, Plymouth Meeting, PA), a dose which was selected as one-half the amount suspected to have toxicity based on in vitro experiments. A water soluble, carboxylated derivative of 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) was used as a NO scavenger in a dose range proven to be effective in the treatment of endotoxic shock in mice, apparently through the reduction of NO levels (24, 25). C-PTIO (Dojindo Chemical, Bethesda) was administered at the doses indicated in the figure legends. Uric acid (2,6,8-trihydroxypurine) was used to target peroxynitrite, based on evidence for its peroxynitrite scavenging activity (26) and its low level of toxicity as a natural product of purine metabolism in vivo. Uric acid, which is poorly soluble in water, was administered as a suspension in an arbitrarily determined dose range as detailed in the figure legends.
RESULTS
Identification of Cells Expressing iNOS mRNA in MS Brain Tissue.
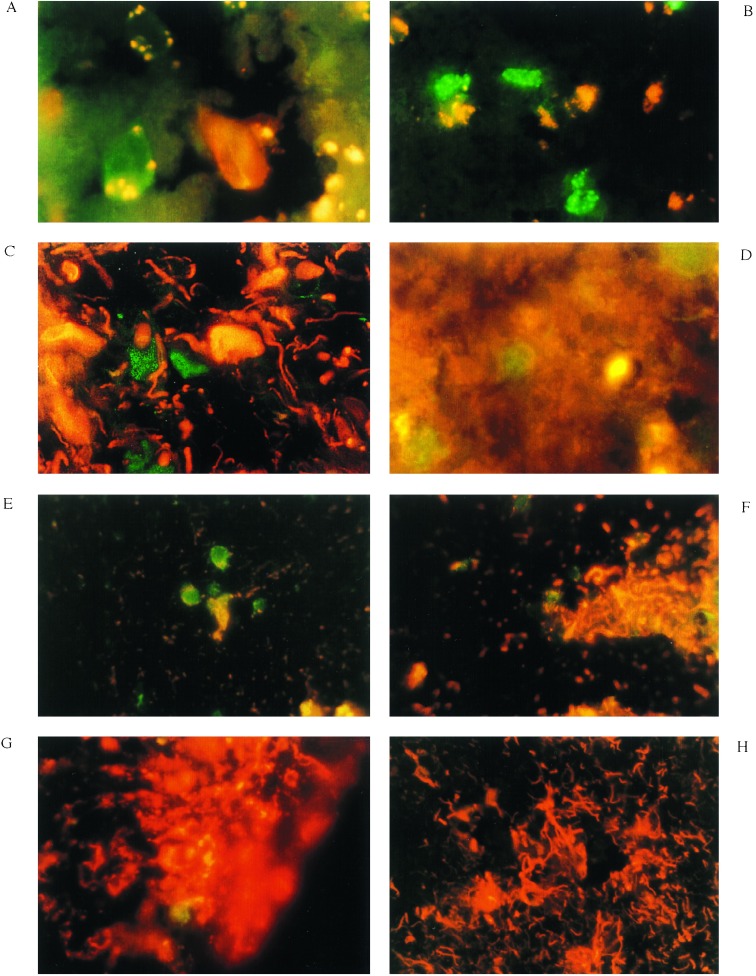
In Figs. 1 and 2, cells in sections of brain tissue from MS patients have been double-labeled with RT-IS-PCR for iNOS mRNA (green fluorescein isothiocyanate probe) and antibodies specific for selective cell surface markers (orange stains). In Fig. 1A, the cells staining positively for iNOS-specific mRNA (green) are also stained with RCA-1 (red), a lectin specific for cells of the monocyte lineage, the double stain appearing as patchy yellow. Fig. 1B shows that cells stained green for iNOS-specific mRNA are clearly distinguishable from those that stain with antibodies to vWF (yellow/orange) specific for endothelial cells. Fig. 1C, reveals that neurons and neuronal processes, identified by staining with a cocktail of three monoclonal anti-neurofilament antibodies (NF) (orange), are negative for iNOS mRNA (green), which is evident in other cells in the field. In Fig. 1D, oligodendrocytes stained with anti-CNPase antibody (orange) are distinguished from cells identified as iNOS mRNA-positive cells (green).
Figure 1.
Identification of cells expressing iNOS mRNA in MS brain tissue. As described, sections from MS brain were concurrently stained with RT-IS-PCR for iNOS mRNA (green) and a panel of cell-specific reagents (red/orange). The sections were photographed through a fluorescent microscope at a magnification of ×1000 using dual filters for orange and green fluorescence. (A) This section, from the region of a plaque, was stained for iNOS mRNA (green) and RCA-1 (orange). (B) A similar section was stained for iNOS mRNA (green) and vWF (yellow/orange). (C) A similar section was stained for iNOS mRNA (green) and neurofilament antigens using a cocktail of three distinct monoclonal antibodies (orange). (D) This section, again from the vicinity of a plaque, was stained for iNOS mRNA (green) and CNPase (orange). (E–H) Different views of a single section stained for iNOS mRNA (green), as well as GFAP (orange): E, the center of the plaque; F, the edge of the plaque; G, 2 mm from the plaque; H, 7 mm from the plaque.
Figure 2.
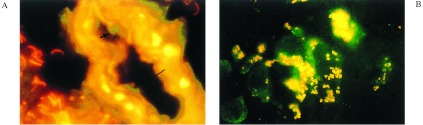
Identification of iNOS mRNA-positive mononuclear cells in the lumen of a blood vessel in the vicinity of an MS plaque (A) and the association of iNOS mRNA-positive cells with accumulations of nitrotyrosine in an MS plaque (B). (A) A brain section containing an MS plaque was stained with antibody to vWF (yellow/orange) and with RT-IS-PCR using a fluorescein isothiocyanate-labeled probe specific for iNOS mRNA (green). (B) A section of an MS plaque was stained for iNOS with RT-IS-PCR (green) and antibodies to nitrotyrosine. The sections were prepared, stained, and visualized as described in Materials and Methods and the legend to Fig. 1. The image in A was photographically enlarged approximately ×10.
Expression of iNOS mRNA in Plaque Versus Nonplaque Areas of MS Brain.
If local production of NO is central to the pathogenesis of MS as appears to be the case in EAE (5), it may be expected that areas containing plaques in MS brain sections should exhibit higher accumulations of iNOS mRNA-positive cells than areas without pathology. As shown in Fig. 1E–H, this appears to be the case. Fig. 1E shows an MS plaque area with large numbers of cells resembling monocytes stained green after RT-IS-PCR for iNOS. Massive destruction of astrocytic processes (stained orange with antibodies for the astrocyte-specific marker GFAP) is apparent. Notably, none of the cells positive for iNOS mRNA reacted with the GFAP-specific reagent. Fig. 1F is a photograph of the same MS-positive brain section taken at the edge of the MS plaque, showing partial tissue destruction. The astrocytic processes are positive for GFAP (orange) and clearly distinct from the cells staining for iNOS-specific mRNA (green). In Fig. 1G, the same MS brain section is shown 2 mm away from a plaque. Only a single iNOS mRNA-positive cell (green) can be seen in this area, among a number of intact astrocytic processes stained with anti-GFAP (orange). In Fig. 1H, a view of the same section from the same MS brain, taken 7 mm away from a plaque, is shown. At this distance from the plaque, astrocytes stained with anti-GFAP antibodies (orange) show no signs of damage and no iNOS mRNA-positive cells are present.
Fig. 2A shows a blood vessel from a MS plaque containing iNOS mRNA-positive mononuclear cells (green), evidently adherent to the luminal surface of the vessel (stained yellow/orange with antibody to vWF). Careful microscopic examination of the slide showed evidence of possible infiltration by diapedesis of the iNOS-positive cell through the vascular endothelium into the brain tissue (arrows). In Fig. 2B, a section of an MS plaque area exhibiting monocytes positive for iNOS mRNA (green) and accumulations of cell debris positive for nitrotyrosine residues (yellow) is shown.
Effect of an Inhibitor of iNOS Induction, D609, on EAE.
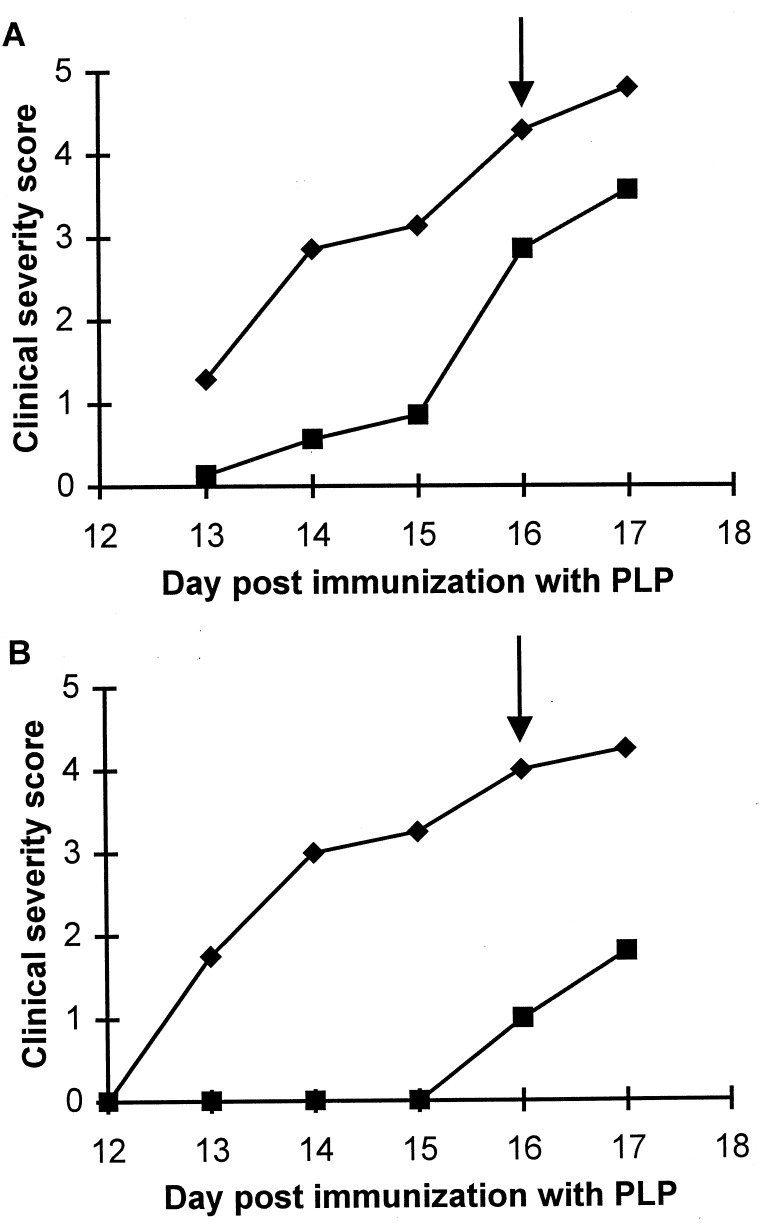
Clinical symptoms of EAE in SWXJ-14 mice immunized with PLP failed to develop in parallel with untreated controls when the mice were treated daily with 1 mg of D609 beginning 5 days after PLP immunization and terminating on day 14 after immunization (Fig. 3). Twenty-four hours after concluding treatment, the first clinical signs of EAE developed, progressing into significant disease over the next few days (Fig. 3).
Figure 3.
Effect of D609 on the development of EAE in SWXJ-14 mice. SWXJ-14 mice were immunized with PLP as described. Mice were either left untreated (♦) or treated with 1 mg of D609 (▪) daily from days 5 through 14 after immunization (arrow). Severity scores were graded and are presented as a mean.
Effect of the NO Scavenger PTIO on EAE.
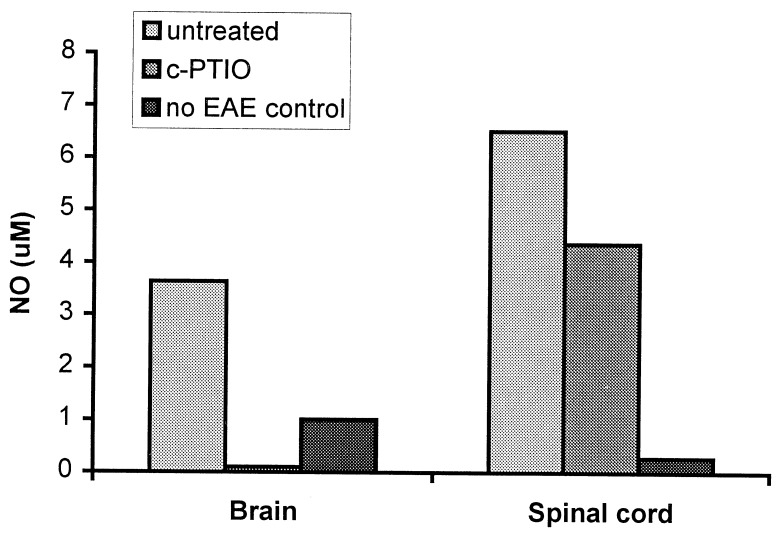
Daily treatment with a single dose of c-PTIO (100 mg/kg) from days 5–16 after PLP immunization caused a delay in the onset and inhibited the progression of the clinical signs of EAE (Fig. 4). However, at this level of treatment, disease eventually developed (Fig. 4A). Administration of the same amount of c-PTIO twice daily had a more profound therapeutic effect on the PLP-immunized mice, delaying the onset of clinical EAE by at least 3 days (Fig. 4B). When c-PTIO treatment was terminated 16 days after immunization the control mice were extensively paralyzed, whereas the treated animals exhibited only tail weakness and piloerection (Fig. 4B). Assessment of NO levels in the brains and spinal cords of the mice 1 day afterwards, revealed that spinal cord NO was only marginally lower in c-PTIO-treated versus untreated mice (Fig. 5). In contrast, the level of NO in brain tissue of c-PTIO-treated mice was considerably lower than those of the untreated mice (Fig. 5).
Figure 4.
Effect of administration of c-PTIO on EAE in SWXJ-14 mice. Four days after immunization with PLP, SWXJ-14 mice were either left untreated (♦) or treated (▪) either once (A) or twice (B) daily with 2 mg c-PTIO. In both cases, treatment was continued until day 16 after immunization (arrows). Severity scores were graded and are expressed as a mean.
Figure 5.
Effect of daily administration of c-PTIO on NO levels in brain and spinal cord of SWXJ-14 mice immunized with PLP. NO levels in brain and spinal cords of the SWXJ-14 mice described in Fig. 4A were semiquantitated using spin-trapping with DETC and EPR spectroscopy at day 17 after immunization with PLP and are presented as a mean.
Effect of the Peroxynitrite Scavenger Uric Acid on EAE.
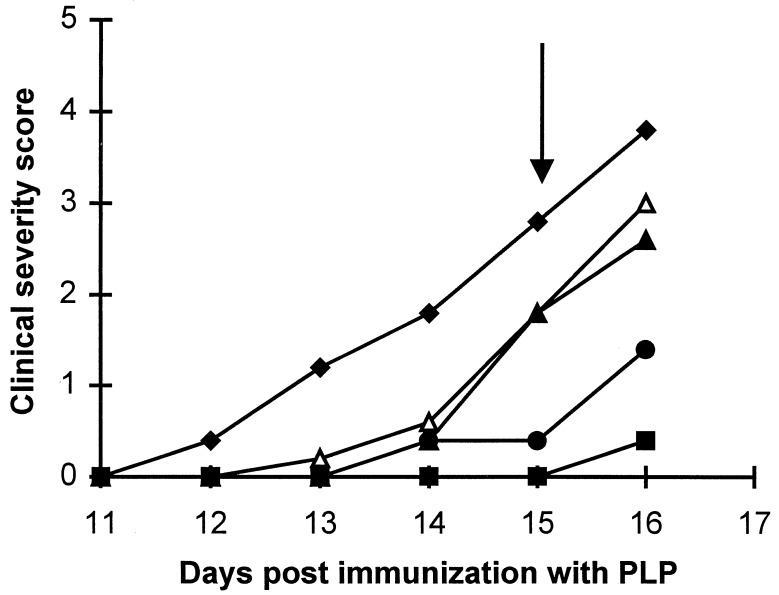
Analysis of SWXJ-14 mice treated with different doses of uric acid beginning 5 days after PLP immunization and continuing through day 15 showed that as little as one dose of 2 mg per day of uric acid can delay the onset of clinical signs of EAE (Fig. 6). Moreover, a daily dose of 20 mg of uric acid completely protected the mice, whereas all the controls became severely paralyzed. As detailed in Table 1, on the 16th day after PLP immunization, NO levels in the brains and spinal cords of mice treated with uric acid were significantly higher, like their untreated PLP-immunized counterparts, than those of nonimmunized controls. Nevertheless, three out of four of the uric acid-treated mice showed no signs of disease.
Figure 6.
Effect of the administration of various doses of uric acid on the development of EAE in SWXJ-14 mice. Beginning on day 5 after immunization with PLP, SWXJ-14 mice were either left untreated (♦) or treated once daily with either 2 mg (Δ), 4 mg (▴), 8 mg (•), or 20 mg (▪) of uric acid given i.p. Treatment was terminated on day 15 after immunization. Severity scores were graded and are expressed as a mean.
Table 1.
Effect of administration of uric acid on brain and spinal cord NO levels in EAE
| No. | PLP* | Uric acid treatment | Clinical severity | NO brain, μM | NO spinal cord, μM |
|---|---|---|---|---|---|
| 1 | No | None | 0 | ∼0 | ∼0 |
| 2 | No | None | 0 | 1.3 | 0.6 |
| 3 | Yes | None | 4 | 0.9 | 2.0 |
| 4 | Yes | None | 5 | 3.5 | 25.0 |
| 5 | Yes | 8 mg/day | 4 | 1.8 | 1.8 |
| 6 | Yes | 8 mg/day | 0 | 1.9 | 1.5 |
| 7 | Yes | 20 mg/day | 0 | 3.0 | 1.4 |
| 8 | Yes | 20 mg/day | 0 | 5.2 | 4.4 |
*SWXJ-14 mice, immunized with PLP as detailed in Materials and Methods, were treated once daily with 8 or 20 mg of uric acid i.p. At day 16 after immunization, clinical severity was scored and NO levels in brain and spinal cord were assessed by EPR as described.
DISCUSSION
Our concurrent analysis of tissue sections from the brains of MS patients with RT-IS-PCR for iNOS mRNA and a battery of stains for subsets of CNS resident or infiltrating cells demonstrated that cells expressing iNOS message also stained with RCA-1 lectin, which selectively binds to cells of the monocyte lineage. Stains for astrocytes, neurons, oligodendrocytes, Schwann cells, and endothelial cells were not found to react with the iNOS mRNA-positive cells. Cells positive for iNOS mRNA were concentrated in regions immediately surrounding the MS plaque areas of extensive damage to white matter and a diminishing gradient of positives and evidence of damage to astrocytes could be seen at increasing distances from the plaques. Regions further removed (7 mm) from plaques contained few if any iNOS mRNA-positive cells and little evidence of the destruction of astrocytes. Furthermore, nitration of tyrosine was evident in the vicinity of plaques implicating peroxynitrite in the pathogenesis of the lesions.
While our reagents were unable to distinguish between monocyte/macrophages and glial cells, it is important to note that we observed cells positive for iNOS mRNA either in the lumen of blood vessels or apparently adherent to the vascular endothelium in sections from MS brain. The apparent emergence of iNOS-positive cells from the blood vessel in the MS-plaque area indicates the likelihood that these are monocytes that have been activated in peripheral lymphoid tissues prior to infiltrating into the brain.
In EAE animal models, inhibition of the induction of iNOS has been successfully used for treatment of clinical disease (3). D609 indirectly suppresses several biological processes, including iNOS activation and tumor necrosis factor-α-induced apoptosis, by inhibiting the activation of phosphatidylcholine-specific phospholipase C, an enzyme involved in the regulation of the promoter NF-κB (27). In our hands, PLP-immunized SWXJ-14 did not develop EAE while under treatment with 1 mg of D609 (Fig. 1). Clinical signs of EAE appeared 24 hr after discontinuing D609. Because the dosage of D609 was chosen more or less arbitrarily, it is possible that a higher dose or prolonged treatment may have a more lasting effect. Alternatively, D609-insensitive pathways leading to the formation of active iNOS may exist (e.g., ref. 28). In the latter case, a scavenger of NO, like c-PTIO, would be expected to be more effective.
Indeed, c-PTIO had significant effects on the development and severity of the clinical signs of EAE in our experiments. The therapeutic action of c-PTIO was improved by more frequent administration (Fig. 4B) as expected based on the short biological half-life of c-PTIO in the blood of experimental animals. In fact, it has been reported that 82% of the c-PTIO administered to mice is excreted within 4.5 hr (25), raising the possibility that the therapeutic effects of c-PTIO seen in this study might not entirely depend on its continued presence in vivo. The finding that NO levels in the brains of PLP-immunized mice treated with c-PTIO remain much lower than those in spinal cord evidently suggest that the clinical signs of EAE, in this model, may be associated with high levels of NO in brain rather than spinal cord (Fig. 5). Even though c-PTIO may have an effect on the other biological roles of NO in the nervous and circulatory systems (29, 30), its strong therapeutic effect in EAE was without notable side effects.
We have found that treatment with uric acid, a known scavenger of peroxynitrite, can virtually block clinical disease in SWXJ-14 mice. The fact that mice remain healthy when treated with uric acid despite having elevated NO levels in their brains and spinal cords provides evidence for the assumption that NO derivatives may be more toxic in EAE than NO itself. Because uric acid is highly insoluble in aqueous solutions (31), a significant proportion of the uric acid administered i.p. as a suspension in PBS accumulated in the peritoneum. It was therefore difficult for us to accurately determine the effective therapeutic dose. In the future, analysis of serum uric acid levels will be used to shed more light on this problem. Since, in contrast to man, mice metabolize uric acid in an additional step to allantoin (32), it is conceivable that this compound, although not known to be a free radical scavenger, may be the protective molecule rather than uric acid. However, based on its known peroxynitrite binding activity, we speculate that uric acid is the active protective molecule in the mouse EAE system.
In this investigation we have shown that treatment with an inhibitor of iNOS activation and scavengers of NO and peroxynitrite are effective in preventing the development of EAE in an acute model. If these substances all function mechanistically as believed, they should also be effective in the treatment of chronic recurrent EAE. We are now investigating the mode of action of D609, c-PTIO, and uric acid in mice with chronic recurrent EAE. If treatment is successful under these circumstances, one may consider the use of these substances in the therapy of chronic degenerative CNS diseases of humans such as MS.
The factors involved in the formation of plaques in the brains of MS patients have been hitherto unknown. Our data strongly suggest that activated macrophages are causing these lesions through the production of NO and, subsequently, peroxynitrite. Inhibitors of these substances provide a strong therapeutic effect in EAE and, because the inhibitors act at the postinduction effector stage of the disease, should be useful in blocking the development of CNS lesions in MS regardless of its etiology.
The properties of c-PTIO are not as well known as those of the natural compound uric acid, initially isolated in the late 18th century (33). In the case of c-PTIO, toxicity studies must be performed before further experimentation, which may lead to applications in humans. In the case of uric acid, further studies are necessary to clarify our hypothesis that there is a negative association between uric acid on the one hand and peroxynitrite and its pathogenic role in MS on the other. These studies may include a determination of whether abnormalities in the metabolism of uric acid may have some effect on the incidence or course of MS. For instance, a statistical survey of patients with gout for incidence of MS may indicate that hyperuricemia may preclude the development of MS and provide a basis for the extension of our experimental studies to the treatment of MS with uric acid.
Acknowledgments
We thank Inglis House (Philadelphia) for assisting in the procurement of tissue samples, Dr. Mark Curtis (Department of Neurology, Thomas Jefferson University) for preparation of tissue specimens, and Dr. Robert Korngold (Department of Microbiology and Immunology, Thomas Jefferson University) for valuable assistance in helping us establish the SWXJ-14 EAE model. This work was supported by the Biotechnology Foundation at Thomas Jefferson University.
ABBREVIATIONS
- MS
multiple sclerosis
- NO
nitric oxide
- EAE
experimental allergic encephalomyelitis
- RT-IS-PCR
reverse transcriptase in situ PCR
- iNOS
inducible nitric oxide synthase
- CNS
central nervous system
- c-PTIO
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- D609
tricyclodecan-9-xyl-xanthogenate
- GFAP
glial fibrillary acidic protein
- vWF
von Willebrand Factor
References
- 1.Lin R F, Lin T-S, Tilton R G, Cross A H. J Exp Med. 1993;178:643–648. doi: 10.1084/jem.178.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koprowski H, Zheng Y M, Heber-Katz E, Fraser N, Rorke L, Fu Z F, Hanlon C, Dietzschold B. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross A H, Misko T P, Lin R F, Hickey W F, Trotter J L, Tilton R G. J Clin Invest. 1994;93:2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akaike T, Weihe E, Schaefer M K-H, Fu Z F, Zheng Y M, Vogel W H, Schmidt H H H W, Koprowski H, Dietzschold B. J Neurovirol. 1995;1:118–125. doi: 10.3109/13550289509111016. [DOI] [PubMed] [Google Scholar]
- 5.Hooper D C, Ohnishi T S, Kean R, Numagami Y, Dietzschold B, Koprowski H. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin R, McFarland H F. Crit Rev Clin Lab Sci. 1995;321:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 7.Bagasra O, Michaels F H, Zheng Y M, Bobroski L E, Spitsin S V, Fu Z F, Tawadros R, Koprowski H. Proc Natl Acad Sci USA. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bo L, Dawson T M, Wesslingh S, Mork S, Choi S, Kong P A, Hanley D, Trapp B D. Ann Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- 9.Beckman J S, Ye Y Z, Anderson P G, Chen J, Accavitti M A, Tarpey M M, White C R. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 10.Farias-Eisner R, Chaudhuri G, Aeberhard E, Fukuto J M. J Biol Chem. 1996;271:6144–6151. doi: 10.1074/jbc.271.11.6144. [DOI] [PubMed] [Google Scholar]
- 11.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin J C, Smith C D, Beckman J S. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 12.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 13.Beckman J S. J Dev Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- 14.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Tawadros R, Pomerantz R. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Dollard S C, James H J, Sharer L R, Epstein L G, Gelbard H A, Dewhurst S. Neuropathol Appl Neurobiol. 1995;21:518–528. doi: 10.1111/j.1365-2990.1995.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 16.McComb R D, Jones T R, Pizzo S V, Bigner D D. J Neuropathol Exp Neurol. 1982;41:479–489. [PubMed] [Google Scholar]
- 17.Janss A J, Yachnis A T, Silber J H, Trojanowski J Q, Lee V M, Sutton L N, Perilongo G, Rorke L B, Phillips P C. Ann Neurol. 1996;39:481–489. doi: 10.1002/ana.410390410. [DOI] [PubMed] [Google Scholar]
- 18.Sprinkle T J. Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- 19.Tlhyama T, Lee V M, Trojanowski J Q. Am J Pathol. 1993;142:883–892. [PMC free article] [PubMed] [Google Scholar]
- 20.Bagasra O, Seshamma T, Pomerantz R J, Hansen J. In: Current Protocols in Molecular Biology. Ausubel F, Brent R, Kingston R E, Moore D D, Smith D A, Seidman J G, Struhl K, editors. New York: Wiley–Interscience; 1994. pp. 14.8.1–14.8.24. [Google Scholar]
- 21.Knobler R L, Lublin F D, Linthicum D S, Cohn M, Melvold R D, Lipton H L, Taylor B A, Beamer W G. Ann NY Acad Sci. 1988;540:735–737. doi: 10.1111/j.1749-6632.1988.tb27230.x. [DOI] [PubMed] [Google Scholar]
- 22.Korngold R, Feldman A, Rorke L B, Lublin F D, Doherty P C. Immunogenetics. 1986;24:309–315. doi: 10.1007/BF00395536. [DOI] [PubMed] [Google Scholar]
- 23.Tschaikowsky K, Meisner M, Schonhuber F, Rugheimer E. Br J Pharmacol. 1994;113:664–668. doi: 10.1111/j.1476-5381.1994.tb17043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazadi K, Ueda S, Maeda H. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Akaike T, Wada Y, Sato K, Ikeda K, Ueda S, Maeda H. Biochem Biophys Res Commun. 1994;202:923–930. doi: 10.1006/bbrc.1994.2018. [DOI] [PubMed] [Google Scholar]
- 26.Szabo C, Zingarelli B, Salzman A L. Circ Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- 27.Pastorino J G, Simbula G, Yamamoto K, Glascott P A, Jr, Rothman R J, Farber J L. J Biol Chem. 1996;271:29792–29798. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- 28.Simmons M L, Murphy S. Glia. 1994;11:227–234. doi: 10.1002/glia.440110303. [DOI] [PubMed] [Google Scholar]
- 29.Snyder S H, Bredt D S. Sci Am. 1992;5:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- 30.Moncada S, Higgs A. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 31.Seidell A. Solubilities of Organic Compounds. 3rd. Ed. Vol. 2. New York: Van Nostrand; 1941. [Google Scholar]
- 32.Schulman M P. In: Metabolic Pathways. 3rd Ed. Greenberg D M, editor. Vol. 2. New York: Academic; 1961. pp. 389–457. [Google Scholar]
- 33.Lehninger A L. Biochemistry. New York: Worth; 1970. Appendix A, p. 795. [Google Scholar]