Abstract
Bacterial chemotaxis has the potential to increase the rate of degradation of chemoattractants, but its influence on degradation of hydrophobic attractants initially dissolved in a non-aqueous-phase liquid (NAPL) has not been examined. We studied the effect of chemotaxis by Pseudomonas putida G7 on naphthalene mass transfer and degradation in a system in which the naphthalene was dissolved in a model NAPL. Chemotaxis by wild-type P. putida G7 increased the rates of naphthalene desorption and degradation relative to rates observed with nonchemotactic and nonmotile mutant strains. While biodegradation alone influenced the rate of substrate desorption by increasing the concentration gradient against which desorption occurred, chemotaxis created an even steeper gradient as the cells accumulated near the NAPL source. The extent to which chemotaxis affected naphthalene desorption and degradation depended on the initial bacterial and naphthalene concentrations, reflecting the influences of these variables on concentration gradients and on the relative rates of mass transfer and biodegradation. The results of this study suggest that chemotaxis can substantially increase the rates of mass transfer and degradation of NAPL-associated hydrophobic pollutants.
Nonaqueous-phase liquids (NAPLs) pose great challenges in the remediation of contaminated soil and sediment (21, 23). Residual NAPLs are often trapped in pores, leading to small-scale heterogeneity in contaminant distribution and slow rates of contaminant transfer into the surrounding aqueous phase (5, 12, 13). In situ biodegradation by indigenous microorganisms may be a low-cost means of remediating contaminated sites, but its reliability depends on an improved understanding of governing mechanisms (22). One concern is that biodegradation of hydrophobic substrates is often limited by the rate of mass transfer from a nonaqueous phase (1, 3, 34). Removal of a contaminant from the aqueous phase through biodegradation can, however, improve dissolution of a pure substance or desorption from a nonaqueous phase by increasing the concentration gradient against which mass transfer occurs (3, 10, 33, 37). The rate of mass transfer, and hence the rate of biodegradation, is predicted to increase as the degrading organisms move closer to the contaminant source (3).
Chemotaxis by Pseudomonas putida strain G7 was shown recently to enhance the degradation of naphthalene diffusing from a naphthalene-saturated aqueous buffer contained in a capillary (17), consistent with theoretical predictions (2, 15) that bacterial chemotaxis towards nutrient sources can increase the rate of nutrient consumption. The effect of chemotaxis on naphthalene degradation was equivalent to increasing the concentration of nonchemotactic or nonmotile mutant strains by at least two orders of magnitude (27). Unlike the situation in a strictly aqueous system, however, bacteria do not have direct access to the substrate in a nonaqueous source. We therefore examined the effect of chemotaxis on the desorption and biodegradation of naphthalene initially dissolved in a model NAPL, 2,2,4,4,6,8,8-heptamethylnonane (HMN). The time course of naphthalene removal from an HMN droplet was monitored in static incubations with wild-type P. putida G7 and two mutant strains, one of which was motile but not chemotactic towards naphthalene (Che−) (9) and the other of which was nonmotile (Mot−) (17). Comparison of the wild type to the mutant strains allowed us to distinguish between the effects of biodegradation alone and those of biodegradation with chemotaxis.
MATERIALS AND METHODS
Media and chemicals.
Microbiological media were prepared as described previously (17). The experiments were conducted with phosphate buffer (pH 7) supplemented with chloramphenicol (10 μg/ml) as a growth inhibitor. Naphthalene (>99%), HMN, methanol (high-pressure liquid chromatography [HPLC] grade), and acetonitrile (HPLC grade) were obtained from Sigma-Aldrich (St. Louis, Mo.).
Culture conditions.
Wild-type P. putida G7 and a mutant strain that is nonchemotactic to naphthalene [P. putida G7.C1(pHG100)] were obtained from Caroline Harwood (University of Iowa). The nonmotile strain is a spontaneously generated mutant of wild-type P. putida G7 that was previously isolated in our lab (17). Bacterial suspensions were prepared as described previously (17). Briefly, batches of the bacterial cultures were grown by lightly scraping a frozen stock (−70°C) with a sterile wooden applicator stick and transferring the scraping to tryptone broth. For the nonchemotactic strain, 25 μg of tetracycline (Sigma-Aldrich)/ml was added to the tryptone broth to select for the desired mutant cells. After 24 h of growth at 25°C and 250 rpm in an incubator shaker, the cells were centrifuged for 1 min and resuspended in mineral salts medium. Aliquots of the resuspended cells were added to 20 ml of mineral salts medium containing 5 mM sodium salicylate (an inducer of naphthalene biodegradation and chemotaxis). The bacteria were grown to mid-exponential phase (optical density of 0.2 to 0.4 cm−1 at a wavelength of 590 nm, approximately 108 cells/ml) at 25°C and 250 rpm in an incubator shaker. The cell suspensions were then centrifuged at 2,800 × g for 3 min and resuspended in phosphate buffer. Dilutions to the desired cell concentration were done in phosphate buffer, and chloramphenicol (10 μg/liter) was added to inhibit growth.
Kinetic parameters for naphthalene degradation are similar for all three strains in well-mixed systems (17). To ensure that initial naphthalene degradation rates were identical for each strain in a given experiment, the inoculum sizes of the strains were normalized by the naphthalene degradation rate measured for each culture immediately prior to use in a given experiment. The maximum rate of naphthalene degradation was measured spectrophotometrically (17) in a sample of each strain at a cell concentration of approximately 106 CFU/ml and a naphthalene concentration of 1.2 mg/liter (one order of magnitude above the half-saturation concentration). If the rates of naphthalene removal were not equal among the strains, cultures with higher removal rates were diluted with phosphate buffer to provide equal rates for all three strains. The maximum amount of dilution was less than 20% of the final culture volume. The final inoculum size was quantified by plating on R2A agar (Difco, Detroit, Mich.).
Micropipette preparation.
Glass PCR micropipettes (5 μl) with stainless steel plungers (Drummond Scientific, Broomall, Pa.) were used to form a 1-μl droplet of HMN in the culture suspension. The micropipettes were prepared by flattening the tip of the stainless steel plunger with a file and flame polishing the tip of the glass micropipette body. Such preparation was necessary to form and retain the HMN droplet on the tip of the plunger.
Naphthalene removal from NAPL droplets.
Incubations were carried out in 1.5-ml crimp top vials (Laboratory Supply Distributors, Mt. Carmel, N.J.) that contained 1-ml aliquots of P. putida G7 suspension. A 0.5-ml layer of HMN was placed above the aqueous phase to absorb any naphthalene that was not degraded. A micropipette filled with 1 μl of HMN containing naphthalene was inserted though the septum (prepierced with an 18-gauge needle), and an HMN droplet was formed by pushing the plunger past the tip of the micropipette body. Control vials contained phosphate buffer only.
At designated time points, triplicate incubations were sacrificed by drawing the HMN droplets into the micropipettes and removing the micropipettes from the vials. The contents of each micropipette were expelled into a fixed volume of methanol, which was then vortexed for 10 s before being transferred into 4-ml HPLC autosampler vials (Laboratory Supply Distributors). The incubation vials were vortexed for 30 s before sampling of the HMN layer, and approximately 200 μl of the HMN layer was transferred to a 300-μl limited-volume insert (Laboratory Supply Distributors) inside a 4-ml HPLC vial. The HPLC vials were sealed with Teflon-lined septa and stored at −10°C until completion of the experiment.
HPLC analysis.
Naphthalene was analyzed with a Waters (Milford, Mass.) 600S system equipped with a Waters 717 autosampler and a Waters 996 photodiode array detector or a Waters 470 fluorescence detector. The methanol samples were analyzed with a Luna C-18 column (150 by 4.6 mm; 3 μm of medium; Phenomenex, Torrance, Calif.), and the HMN samples were analyzed with a Supelcosil LC-8 column (250 by 4.6 mm; 5 μm of medium; Supelco, Bellefonte, Pa.). Naphthalene peaks were detected at a wavelength of 220 nm with the photodiode array detector or at 280-nm excitation and 340-nm emission wavelengths with the fluorescence detector. The methanol samples were analyzed with a mobile phase of 85:15 (acetonitrile to water) run at 0.6 ml/min. The HMN samples were run with a gradient elution program. The initial mobile phase composition of 70:30 (acetonitrile to water) was held for 4 min at 0.9 ml/min, followed by ramping to 100% acetonitrile at 1.2 ml/min over 3 min, holding these conditions for 5 min before ramping to 70:30 (acetonitrile to water) over 6 min, and finally ramping to 0.9 ml/min over 7 min, resulting in a total run time of 25 min. For naphthalene amounts lower than the quantification limit, a value of one-half the quantification limit was assigned. The average initial amount of naphthalene in the HMN droplet was determined by using five different micropipettes to take five 1-μl samples of the HMN solution used for an experiment. Standard deviations of the initial amounts were within 5% of the mean.
Data analysis.
A full model of the experimental system requires numerical solution of unsteady-state material balance equations for naphthalene in both phases and for cells in the aqueous phase. To provide a simple comparison of rates of naphthalene removal under the various experimental conditions, apparent first-order rate coefficients (kapp) were obtained by fitting an exponential decay function to the experimental data representing the mass of naphthalene remaining in the HMN droplet versus time. The r2 values ranged from 0.8 to 0.99. Here kapp would be a function of the specific surface area of the HMN droplet, the diffusivity of naphthalene in water, any influence of biodegradation on the naphthalene concentration gradient in the aqueous phase, and the boundary conditions of the physical system.
Bioavailability number.
The bioavailability number (Bn) is a means of quantifying the potential rate of mass transfer relative to the potential rate of biodegradation (3) and was calculated for each experimental condition. We define Bn in these experiments as the ratio of the maximum potential rate of naphthalene desorption in the abiotic system to the maximum potential rate of biodegradation in the inoculated systems:
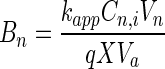 |
(1) |
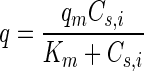 |
(2) |
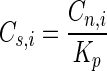 |
(3) |
where kapp is the apparent first-order rate coefficient for the abiotic controls, Cn,i is the initial naphthalene concentration in the HMN droplet, Vn is the volume of the HMN droplet, q is the specific rate of naphthalene degradation, X is the cell concentration, Va is the volume of the aqueous phase, qm is the maximum specific degradation rate, Cs,i is the saturation concentration of naphthalene in water in equilibrium with Cn,i, Km is the half-saturation coefficient for naphthalene degradation, and Kp is the equilibrium constant for naphthalene partitioning between HMN and water.
Microscopy.
Microscopic images of P. putida G7 at the HMN-water interface were taken at a magnification of ×200 by using bright field illumination. Images of the HMN-water interface were taken by filling a 10-μl capillary (Drummond Scientific) with a 1-cm plug of HMN containing 0.25 g of naphthalene/liter and placing it in a pool of wild-type P. putida G7 (108 CFU/ml) on a microscope slide. Digital photographs were taken after 60 min of incubation.
Images of the surface of an HMN droplet in contact with a bacterial suspension were taken by suspending a 0.5-μl droplet of HMN containing 0.25 g of naphthalene/liter from a coverslip into 400 μl of bacterial suspension (∼5 × 107 CFU/ml). The bacterial suspension was contained in a microscope chamber slide (Lab-Tek two-chamber slide; Nalge-Nunc, Rochester, N.Y.) that was modified by removing the plastic chamber, with the gasket left on the slide to support the coverslip. For the images of the HMN surface, the focal plane was at the HMN-water interface. The images of the bacterial suspension were at the same depth but away from the HMN droplet. Digital photographs were taken after incubating the slide assembly for 40 min. The contrast and brightness of the original images were adjusted using Jasc Paint Shop Pro (Jasc Software Inc., Eden Prairie, Minn.).
RESULTS
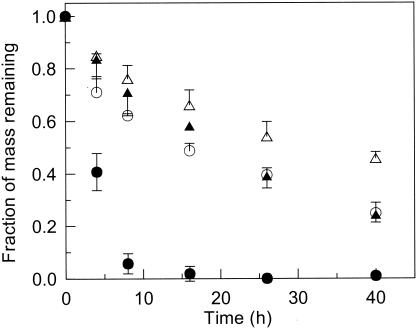
The time course of the removal of naphthalene from the HMN droplet was monitored in an experiment that involved an initial cell concentration of 8.6 × 105 CFU/ml in the bacterial suspension and an initial naphthalene concentration of 3.4 g/liter in the HMN droplet (Fig. 1). Wild-type P. putida G7 substantially increased the rate of naphthalene removal from the HMN droplet relative to that from the abiotic control and from incubations with the mutant strains. Naphthalene did not accumulate in the aqueous phase in any of the inoculated vials in this experiment, indicating that the mass of naphthalene lost from the HMN droplet equaled the mass consumed by the bacteria. Naphthalene removal by the two mutant strains was similar and exceeded that in the abiotic control, illustrating the effect of biodegradation alone on naphthalene desorption from the NAPL source.
FIG. 1.
Time courses of the removal of the initial naphthalene mass from the HMN droplet in parallel incubations of wild-type P. putida G7 (•), the Mot− mutant (▴), the Che− mutant (○), and an abiotic control (▵). The initial naphthalene concentration in the HMN droplet was 3.4 g/liter, and the cell concentration was 8.6 × 105 CFU/ml in the bacterial suspension. The average mass of naphthalene recovered from the abiotic controls after 40 h was 90% of the initial mass added to the HMN droplet. Error bars (shown in one direction only for the latter three sets) represent the standard deviations of the measurements from triplicate incubations.
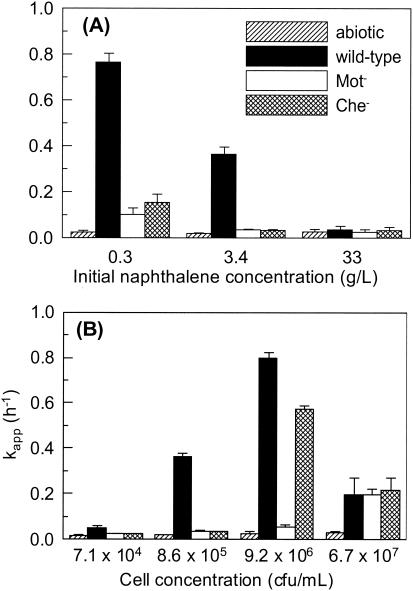
In general, the difference between the apparent first-order removal rate coefficients, kapp, for the inoculated systems and the abiotic controls increased with decreasing initial naphthalene concentrations at a fixed cell concentration (Fig. 2A) and with increasing cell concentrations at a fixed initial naphthalene concentration (Fig. 2B). If data for the Mot− mutant represent the effect of biodegradation alone on kapp, the effect of chemotaxis was an order of magnitude greater at the two lowest naphthalene concentrations and the two intermediate cell concentrations. Biodegradation had little to no effect on naphthalene desorption at the highest initial naphthalene concentration (33 g/liter) and the lowest cell concentration (7.1 × 104 CFU/ml); naphthalene recovery from the HMN layer under these conditions ranged from 4 to 25% of the initial mass added, indicating that the rate of mass transfer exceeded the rate of biodegradation. For comparison, in the case of the abiotic controls 42 to 50% of the initial naphthalene mass was recovered at the highest naphthalene concentration and the lowest cell concentration, demonstrating that biodegradation substantially reduced the amount of naphthalene recovered but did not affect naphthalene desorption.
FIG. 2.
Effects of initial naphthalene concentration (A) and cell concentration (B) on the apparent rate coefficient for naphthalene loss from the HMN droplet. The cell concentration in the bacterial suspension for the experiment with results shown in panel A was 8.6 × 105 CFU/ml, and the initial naphthalene concentration in the HMN droplet for the experiment with results shown in panel B was 3.4 g/liter. Error bars represent the 95% confidence intervals for kapp.
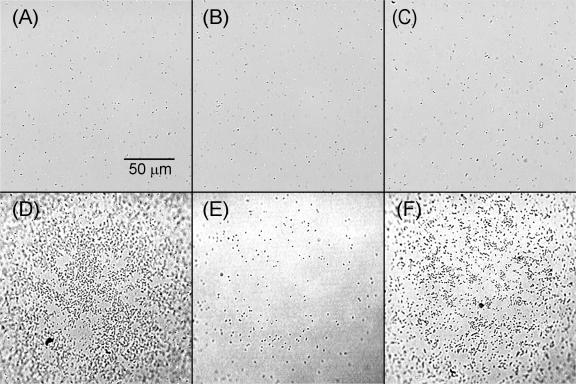
Chemotactic P. putida G7 cells accumulated near the NAPL-water interface in response to the presence of naphthalene (Fig. 3). At a high enough cell concentration, motility alone was sufficient to lead to cell accumulation at the interface, as the Che− strain accumulated at the NAPL-water interface much more than the Mot− strain and to nearly the same extent as the wild type under identical experimental conditions (Fig. 4). Accumulation of the Che− strain was caused by the adhesion of cells colliding with the HMN droplet, because it occurred whether naphthalene was present or not (data not shown). The bacteria we observed at the HMN-water interface were inferred to adhere because they were stationary, wiggling, or rotating in tight circles. Since the Mot− cells cannot swim, they would collide with the HMN droplet less frequently and therefore would accumulate to a lesser extent at a given cell concentration. Adhesion to the HMN droplet explains why the rate of naphthalene removal by the Che− strain was unexpectedly much higher than that by the Mot− strain and similar to that by the wild type at a cell concentration of 9.2 × 106 CFU/ml (Fig. 2B). This apparently anomalous result for the Che− strain was reproduced in two additional experiments with the same cell concentrations (data not shown).
FIG. 3.
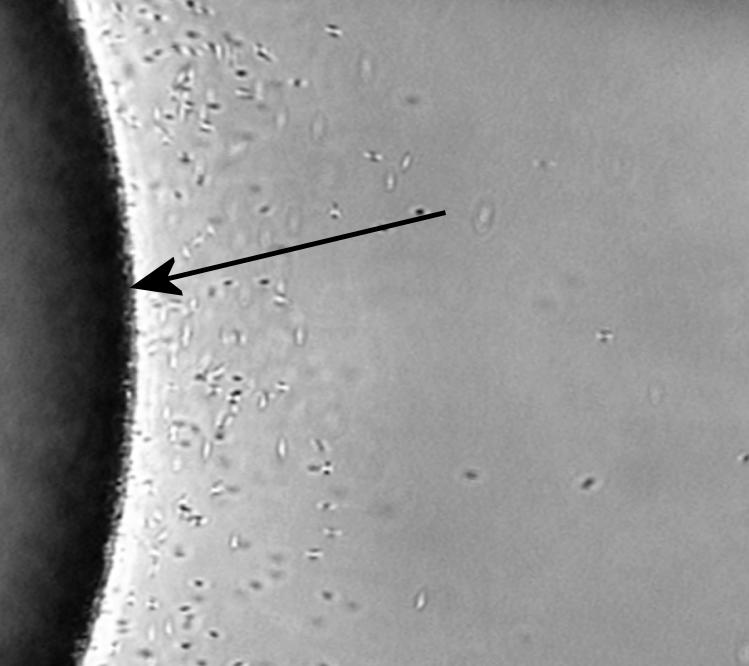
Accumulation of wild-type P. putida G7 near an HMN-water interface inside a capillary. The darker region at the left is HMN, with the HMN-water interface indicated by the arrow.
FIG. 4.
Bacterial accumulation on the surface of an HMN droplet. Distributions of cells in the bacterial suspension (A to C) are compared to cell distributions on the surface of the HMN droplet (D to F) for wild-type P. putida G7 (A and D), the Mot− mutant (B and E), and the Che− mutant (C and F). All images were taken at a cell concentration of 108 CFU/ml in the bacterial suspension.
DISCUSSION
Chemotaxis provides an increased cell density at or near an interface from which a chemical attractant desorbs or dissolves. Such an increase in cell density can lead to an increase in the degradation rate of the attractant near the interface, lowering the aqueous-phase concentration and thus increasing the concentration gradient against which mass transfer occurs. The increased concentration gradient in turn leads to an increased rate of attractant desorption or dissolution. Both the data on naphthalene removal and the microscopic observations in this study support such an effect of chemotaxis over a range of experimental conditions.
Motility-enhanced adhesion of cells at the interface can similarly enhance the rates of mass transfer and biodegradation, as was shown in the experiments with the Che− mutant (Fig. 2B). Others have shown that motility is an important factor in attachment to surfaces (14, 28, 35) and that such attachment can stimulate bacterial growth (32) and increase rates of biodegradation (10, 25, 36). On the contrary, still others have found that attachment in the form of a biofilm can increase mass transfer resistance (11, 19, 29) and that biofilm formation on surfaces can reduce overall degradation rates (19, 20). The role of adhesion in biodegradation will, therefore, depend on the specific conditions under which mass transfer and biodegradation occur.
The results generally supported expectations about the effects of biodegradation, with or without chemotaxis, on the removal of naphthalene from the HMN droplet. The value of kapp is expected to increase as the potential rate of biodegradation increases relative to the rate of desorption in the abiotic system. For example, changes in the rate of desorption in the abiotic system should be proportional to the change in naphthalene concentration in the HMN droplet, while the maximum potential rate of biodegradation should not change substantially with changes in the naphthalene concentration in the HMN droplet as long as the aqueous naphthalene concentration is near or greater than the half-saturation coefficient, Km. We consider the maximum potential rate of biodegradation to occur at the initial aqueous saturation concentrations of naphthalene, which were 0.12, 1.4, and 13 mg/liter for naphthalene concentrations of 0.3, 3.4, and 33 g/liter in the HMN droplet, respectively. The two highest naphthalene saturation concentrations were at least an order of magnitude above Km for degradation of naphthalene by P. putida G7 (0.13 mg/liter) (17), and the lowest concentration was close to Km. Thus, we expected that kapp would increase with increasing cell concentrations or with decreasing naphthalene concentrations in the HMN droplet under the range of conditions we evaluated.
The extent to which the rate of naphthalene removal may be limited by mass transfer or by biodegradation can be quantified with a bioavailability number, Bn (3). The bioavailability number was calculated for each experimental condition by using a measured Kp of 2,500 and parameter values for naphthalene degradation by P. putida G7 (17). The values of Bn varied from 5 × 10−4 to 0.4 over the range of experimental conditions. Under the conditions in which chemotaxis had a significant effect on kapp, Bn was <0.03. Under the conditions in which biodegradation had no effect on kapp, Bn was ≥0.3. It should be noted that the resistance to mass transfer in systems analogous to that used in this work is almost exclusively in the aqueous phase (26).
Chemotactic motion towards an attractant depends on the magnitude of the concentration gradient and on the concentration of the attractant relative to the dissociation constant for its chemoreceptor, Kd (4, 7, 18, 30), and thus is a complex function of the experimental conditions. If the attractant concentration is high enough, the chemoreceptors can become saturated and the bacteria cannot sense the concentration gradient. Saturation of the chemoreceptors could have occurred in our experimental system, but it is unlikely that it prevented a chemotactic response. In an earlier study (17), a chemotactic response was detected at an aqueous naphthalene concentration (28 mg/liter) much higher than the equilibrium naphthalene concentrations in our system.
The values of kapp for both the wild type and the Che− mutant decreased as the cell concentration increased from 9.2 × 106 to 6.7 × 107 CFU/ml (Fig. 2B). For the wild type, a higher initial rate of biodegradation at the higher cell concentration may have resulted in a concentration gradient of naphthalene that did not extend very far into the aqueous phase such that relatively few cells sensed the gradient. High cell concentrations have been observed to diminish chemotaxis in a strictly aqueous system (16). For the Che− strain, however, a higher cell concentration would be expected to increase kapp or (at worst) not affect it at all. It is more likely, therefore, that the rate of naphthalene removal was limited by a factor other than the cell concentration itself at the higher cell concentration. One possibility is oxygen limitation, since P. putida G7 degrades naphthalene aerobically. Increased oxygen consumption for endogenous respiration at the higher cell concentration could have reduced the amount of oxygen available for naphthalene degradation. Oxygen limitation also could have led to aerotactic movement of wild-type or Che− cells away from the NAPL source, since the only continuous source of oxygen was the atmosphere at the top of the incubation vials. Aerotaxis by P. putida G7 has not been documented, although the related strain P. putida PRS2000 is aerotactic (24). Additionally, we observed microscopically an accumulation of P. putida G7 cells near an air-water interface at high initial cell concentrations (data not shown).
Chemotaxis clearly is a mechanism by which bacteria can increase the availability of nutrients associated with nonaqueous sources, as are biosurfactant production (31) and biofilm formation (8). Adhesion to the NAPL source by a Che− mutant influenced naphthalene desorption and degradation to an extent similar to that of the influence of chemotaxis only at a relatively high cell concentration. At lower cell concentrations, chemotaxis was able to increase the rate of naphthalene degradation by an order of magnitude. The cell concentrations evaluated in this study cover a range of concentrations of culturable cells and total cell counts found in soil and aquifer environments (6). At cell concentrations equivalent to the highest concentration we studied, other factors such as oxygen limitation can govern biodegradation rates and thus can mitigate any positive influence of chemotaxis or other mechanisms of improving contaminant availability. This would be of less concern for chemicals that are metabolized anaerobically, with or without alternative electron acceptors.
Acknowledgments
We thank Robert Schoonhoven for assistance with the microscopy and Caroline Harwood (University of Iowa) for donating strains P. putida G7 and P. putida G7.C1(pHG100). We also thank Robert Bourret (UNC—Chapel Hill) and his research group for helpful suggestions and comments.
This work was supported by the National Science Foundation (grant BES-0121208) and the National Institute of Environmental Health Sciences (grant 5 P42 ES05948).
REFERENCES
- 1.Alexander, M. 2000. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ. Sci. Technol. 34:4259-4265. [Google Scholar]
- 2.Blackburn, N., and T. Fenchel. 1999. Influence of bacteria, diffusion and shear on micro-scale nutrient patches, and implications for bacterial chemotaxis. Mar. Ecol. Prog. Ser. 189:1-7. [Google Scholar]
- 3.Bosma, T. N. P., P. J. M. Middeldorp, G. Schraa, and A. J. B. Zehnder. 1997. Mass transfer limitation of biotransformation: quantifying bioavailability. Environ. Sci. Technol. 31:248-252. [Google Scholar]
- 4.Brown, D. A., and H. C. Berg. 1974. Temporal stimulation of chemotaxis in Escherichia coli. Proc. Natl. Acad. Sci. USA 71:1388-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brusseau, M. L., N. T. Nelson, M. Oostrom, Z. H. Zhang, G. R. Johnson, and T. W. Wietsma. 2000. Influence of heterogeneity and sampling method on aqueous concentrations associated with NAPL dissolution. Environ. Sci. Technol. 34:3657-3664. [Google Scholar]
- 6.Chapelle, F. H. 1993. Ground-water microbiology and geochemistry. Wiley, New York, N.Y.
- 7.Chen, K. C., R. M. Ford, and P. T. Cummings. 1998. Perturbation expansion of Alt's cell balance equations reduces to Segel's one-dimensional equations for shallow chemoattractant gradients. SIAM J. Appl. Math. 59:35-57. [Google Scholar]
- 8.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm, A. C., and C. S. Harwood. 1997. Chemotaxis of Pseudomonas spp. to the polyaromatic hydrocarbon naphthalene. Appl. Environ. Microbiol. 63:4111-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harms, H., and A. Zehnder. 1995. Bioavailability of sorbed 3-chlorodibenzofuran. Appl. Environ. Microbiol. 61:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden, P. A., J. R. Hunt, and M. K. Firestone. 1997. Toluene diffusion and reaction in unsaturated Pseudomonas putida biofilms. Biotechnol. Bioeng. 56:656-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi-Lotfabad, S., and M. R. Gray. 2000. Characterization of contaminated soils using confocal laser scanning microscopy and cryogenic-scanning electron microscopy. Environ. Sci. Technol. 34:3408-3414. [Google Scholar]
- 13.Khachikian, C., and T. C. Harmon. 2000. Nonaqueous phase liquid dissolution in porous media: current state of knowledge and research needs. Transp. Porous Media 38:3-28. [Google Scholar]
- 14.Kiorboe, T., H. P. Grossart, H. Ploug, and K. Tang. 2002. Mechanisms and rates of bacterial colonization of sinking aggregates. Appl. Environ. Microbiol. 68:3996-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiorboe, T., and G. A. Jackson. 2001. Marine snow, organic solute plumes, and optimal chemosensory behavior of bacteria. Limnol. Oceanogr. 46:1309-1318. [Google Scholar]
- 16.Marx, R. B., and M. D. Aitken. 1999. Quantification of chemotaxis to naphthalene by Pseudomonas putida G7. Appl. Environ. Microbiol. 65:2847-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx, R. B., and M. D. Aitken. 2000. Bacterial chemotaxis enhances naphthalene degradation in a heterogeneous aqueous system. Environ. Sci. Technol. 34:3379-3383. [Google Scholar]
- 18.Mesibov, R., G. W. Ordal, and J. Adler. 1973. The range of attractant concentrations for bacterial chemotaxis and the threshold and size of response over this range. J. Gen. Physiol. 62:203-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherji, S., and W. J. Weber. 1998. Mass transfer effects on microbial uptake of naphthalene from complex NAPLs. Biotechnol. Bioeng. 60:750-760. [PubMed] [Google Scholar]
- 20.Mulder, H., A. M. Breure, D. van Honschooten, J. T. C. Grotenhuis, J. G. van Andel, and W. H. Rulkens. 1998. Effect of biofilm formation by Pseudomonas 8909N on the bioavailability of solid naphthalene. Appl. Microbiol. Biotechnol. 50:277-283. [Google Scholar]
- 21.National Research Council Committee on Ground Water Cleanup Alternatives. 1994. Alternatives for ground water cleanup. National Academy Press, Washington, D.C.
- 22.National Research Council Committee on Intrinsic Remediation. 2000. Natural attenuation for groundwater remediation. National Academy Press, Washington, D.C.
- 23.National Research Council Committee on Technologies for Cleanup of Subsurface Contaminants in the DOE Weapons Complex. 1999. Groundwater and soil cleanup: improving management of persistent contaminants. National Academy Press, Washington, D.C.
- 24.Nichols, N. N., and C. S. Harwood. 2000. An aerotaxis transducer gene from Pseudomonas putida. FEMS Microbiol. Lett. 182:177-183. [DOI] [PubMed] [Google Scholar]
- 25.Ortegacalvo, J. J., and M. Alexander. 1994. Roles of bacterial attachment and spontaneous partitioning in the biodegradation of naphthalene initially present in nonaqueous-phase liquids. Appl. Environ. Microbiol. 60:2643-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz, E., M. Kraatz, and R. G. Luthy. 1999. Organic phase resistance to dissolution of polycyclic aromatic hydrocarbon compounds. Environ. Sci. Technol. 33:235-242. [Google Scholar]
- 27.Pedit, J. A., R. B. Marx, C. T. Miller, and M. D. Aitken. 2002. Quantitative analysis of experiments on bacterial chemotaxis to naphthalene. Biotechnol. Bioeng. 78:626-634. [DOI] [PubMed] [Google Scholar]
- 28.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen, K., and Z. Lewandowski. 1998. Microelectrode measurements of local mass transport rates in heterogeneous biofilms. Biotechnol. Bioeng. 59:302-309. [DOI] [PubMed] [Google Scholar]
- 30.Rivero, M. A., R. T. Tranquillo, H. M. Buettner, and D. A. Lauffenburger. 1989. Transport models for chemotactic cell populations based on individual cell behavior. Chem. Eng. Sci. 44:2881-2897. [Google Scholar]
- 31.Ron, E. Z., and E. Rosenberg. 2001. Natural roles of biosurfactants. Environ. Microbiol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg, M., and E. Rosenberg. 1981. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J. Bacteriol. 148:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seagren, E. A., B. E. Rittmann, and A. J. Valocchi. 2002. Bioenhancement of NAPL pool dissolution: experimental evaluation. J. Contam. Hydrol. 55:57-85. [DOI] [PubMed] [Google Scholar]
- 34.Simoni, S. F., H. Harms, and A. J. B. Zehnder. 2001. Factors affecting mass transfer limited biodegradation in saturated porous media. J. Contam. Hydrol. 50:99-120. [DOI] [PubMed] [Google Scholar]
- 35.Vande Broek, A., M. Lambrecht, and J. Vanderleyden. 1998. Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology (United Kingdom) 144:2599-2606. [DOI] [PubMed] [Google Scholar]
- 36.Woo, S. H., J. M. Park, and B. E. Rittmann. 2001. Evaluation of the interaction between biodegradation and sorption of phenanthrene in soil-slurry systems. Biotechnol. Bioeng. 73:12-24. [DOI] [PubMed] [Google Scholar]
- 37.Yang, Y., and P. L. McCarty. 2000. Biologically enhanced dissolution of tetrachloroethylene DNAPL. Environ. Sci. Technol. 34:2979-2984. [Google Scholar]