Abstract
Thirty-two strains of nonflocculating bacteria isolated from sewage-activated sludge were tested by a spectrophotometric assay for their ability to coaggregate with one other in two-membered systems. Among these strains, eight showed significant (74 to 99%) coaggregation with Acinetobacter johnsonii S35 while only four strains coaggregated, to a lesser extent (43 to 65%), with Acinetobacter junii S33. The extent and pattern of coaggregation as well as the aggregate size showed good correlation with cellular characteristics of the coaggregating partners. These strains were identified by sequencing of full-length 16S rRNA genes. A. johnsonii S35 could coaggregate with strains of several genera, such as Oligotropha carboxidovorans, Microbacterium esteraromaticum, and Xanthomonas spp. The role of Acinetobacter isolates as bridging organisms in multigeneric coaggregates is indicated. This investigation revealed the role of much-neglected nonflocculating bacteria in floc formation in activated sludge.
The importance of bacterial aggregation as a primary step towards biofilm development, host colonization, and flocculation of activated sludge, etc., has long been realized. Efficient aggregation and proper settling of flocs is important for the generation of good-quality effluent in the activated sludge process. However, attention is focused mainly on floc-forming bacteria, such as Kluyvera cryocrescens, Pseudomonas sp., and Flavobacterium sp., etc. (7, 8, 21, 22). The presence of nonflocculating bacteria is often considered a nuisance responsible for increased turbidity and poor quality of the treated effluent. As a result, it is difficult to contemplate the role of nonflocculating bacteria in floc formation.
The ability of bacterial cells to recognize and communicate with one other, leading to coaggregation, is extensively investigated with regard to oral biofilms. A large amount of literature exists on the types and mechanisms of interactions in bacterial tooth plaques (5, 10), in addition to some reports on coaggregation of organisms in the urinogenital tract (16) and chicken crops (24). Relatively few studies of coaggregation between aquatic biofilm bacteria have been reported (3, 17). Although environmental factors such as substrate gradient, chemical and/or physical stress, and predation are known to trigger bacterial aggregation in activated sludge systems (1), specific cell-cell interactions and coaggregation among pure cultures of nonflocculating sludge bacteria have never been investigated.
This study explores and describes the possibility of intergeneric coaggregation among bacteria isolated from a municipal sewage treatment plant. A systematic study has been conducted to identify the coaggregating pairs among 32 strains of nonflocculating bacteria. The time courses and the aggregate sizes and structures have been examined in cases of efficient coaggregations. A correlation between coaggregation and the cellular characteristics of the partners has also been demonstrated.
MATERIALS AND METHODS
Bacterial isolates.
Fifty-two bacterial strains were isolated by ultrasonic treatment of a sludge sample obtained from the Kawada Sewage Treatment Plant, Utsunomiya, Japan. These strains were characterized by Gram staining and standard biochemical tests. Flocculation characteristics of the isolates were examined using polypeptone medium as described before (7). Isolates were identified based on sequencing of the full-length 16S rRNA genes through National Collections of Industrial, Food and Marine Bacteria—Japan.
Medium and growth conditions.
Bacterial isolates were cultured at 25°C by using polypeptone liquid medium. The medium consisted of the following (per liter of distilled water): polypeptone, 10 g; (NH4)2SO4, 1 g; NaNO3, 0.5 g; NaCl, 0.1 g; MgSO4 · 7H2O, 0.2 g; CaCl2 · 2H2O, 0.05 g; FeCl3 · 6H2O, 0.01 g; and K2HPO4, 1 g. The pH before sterilization was adjusted to 7.0. The bacterial growth was monitored by the measurement of the optical density at 660 nm (OD660) in a 10-mm-path-length cuvette with the use of a spectrophotometer (Hitachi model no. 101).
Hydrophobicity assessment.
Surface hydrophobicities of the bacterial strains were examined by the microbial adhesion to hydrocarbon assay. This test, originally reported by Rosenberg et al. (18), was modified and used as follows. The samples were washed twice with 3 mM NaCl containing 0.5 mM CaCl2 and suspended in the same solution. A 5-ml aliquot of the washed cell suspension and 5 ml of p-xylene were transferred into a 20-ml round-bottomed test tube. The mixture was shaken 20 times and allowed to stand for several minutes. Adhesion of the test samples to p-xylene was examined qualitatively, and adsorption percentage was calculated based on the measurement of the OD660s of the cell suspension before and after the assay.
Preparation of bacterial suspensions for coaggregation assay.
Round flat-bottomed Sakaguchi flasks (500 ml) containing 250 ml of liquid polypeptone medium were inoculated with pure bacterial strains grown on solid medium (NY agar [polypeptone, 5 g/liter; meat extract, 3 g/liter; yeast extract, 5 g/liter; agar, 20 g/liter] or B1 agar [NY agar lacking yeast extract but containing 3 g of NaCl/liter]). Liquid cultures were incubated at 25°C and 300 rpm for suitable periods of time depending upon the growth rates of the bacterial strains to be used. Cells were harvested in the late exponential growth phase by high-speed centrifugation (11,000 × g for 10 min), washed twice in 3 mM NaCl containing 0.5 mM CaCl2, and suspended in the same solution. Subsequently, the cell suspension was centrifuged at 650 × g for 2 min and the remaining cell suspension was used for aggregation experiments. The OD660 of the cell suspension was measured and readjusted to 0.3 by using the same solution (3 mM NaCl containing 0.5 mM CaCl2). Finally, the pH of the cell suspension (OD660 = 0.3) was adjusted to 7.0.
Coaggregation assay.
The cell suspensions (OD660 = 0.3; pH = 7) of each of the pure bacterial partners were prepared separately as described above. Equal volumes (100 ml each) of the suspensions of coaggregating partners were mixed in 300-ml beakers and immediately incubated at 20°C and 300 rpm. Pure bacterial suspensions (200 ml each) were incubated under similar conditions as controls to check self-flocculation. Several 10-ml aliquots of coaggregating mixtures were withdrawn at regular intervals over the course of a 360-min duration, and the aggregation indices were measured by two different assays (with and without a centrifugation step). In the first assay, samples were simply allowed to stand for 5 to 60 min. The OD660s of the carefully pipetted supernatant (upper layer) were measured after 5, 10, 30, and 60 min and used to calculate the aggregation indices. In the alternate assay, samples were immediately centrifuged at 650 × g for 2 min and the OD660 of the supernatant was measured. The aggregation index was calculated as follows:
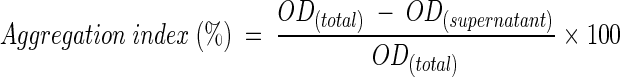 |
Phase-contrast microscopy and SEM.
The sizes and shapes of coaggregates were observed by phase-contrast microscopy with an optical microscope (Olympus BX50 F4). The samples for scanning electron microscopy (SEM) were washed and hardened by suspension in 2.5% (wt/vol) glutaraldehyde solution for 1 h. Subsequently, the samples were dehydrated in series of 20, 40, 60, 80, and 99.5% ethanol solution for 30 min each. Finally, the pellet was suspended in 3-methylbutyl acetate for 12 h, after which critical point drying was performed using a critical point drier (Hitachi HCP-2). Then the samples were fixed and scanned with a scanning electron microscope (Hitachi S-4500).
Nucleotide sequence accession numbers.
DDBJ accession numbers of 16S rRNA gene sequences determined in this study are shown in Table 1.
TABLE 1.
Characterization and identification of bacterial isolates by sequencing of full-length 16S rRNA genes
| Isolate | Accession no. of sequence determined in this study | % Identity of base sequences | Proposed identity | Result of gram staining | Shape | Motility | Color | Presence of oxidase | Presence of catalase |
|---|---|---|---|---|---|---|---|---|---|
| S11 | AB101445 | 92.87 | Xanthomonas sp. | − | Rod | − | Cream | + | + |
| S23 | AB099659 | 99.19 | O. carboxidovorans | − | Twisted rod | + | White | + | + |
| S28 | AB099660 | 99.19 | O. carboxidovorans | − | Twisted rod | + | White | + | + |
| S29 | AB099658 | 99.74 | M. esteraromaticum | + | Rod curved to V | + | Yellow | − | + |
| S33 | AB10144 | 99.97 | A. junii | − | Rod | − | White | − | + |
| S35 | AB099655 | 99.51 | A. johnsonii | − | Rod | − | White | − | + |
| S38 | AB099656 | 99.41 | M. esteraromaticum | + | Rod | − | Yellow | − | + |
| S51 | AB099657 | 99.77 | M. esteraromaticum | + | Rod | + | Yellow | − | + |
| S53 | AB101447 | 95.13 | Xanthomonas sp. | − | Rod | − | Cream | + | + |
| S54 | AB101446 | 92.99 | Xanthomonas sp. | − | Rod | − | Cream | + | + |
RESULTS
Characterization and identification of bacterial isolates by sequencing of full-length 16S rRNA genes.
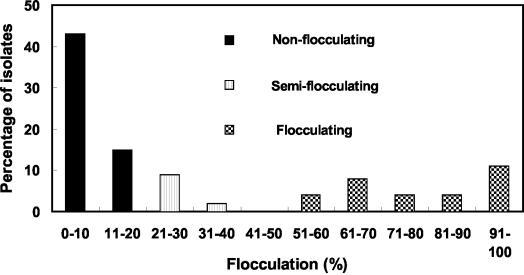
The flocculation characteristics of 52 strains of bacteria isolated from activated sludge are summarized in Fig. 1. About 31% of the bacterial isolates were good floc formers (aggregation index of 50% or more), while 58% were nonflocculating bacteria (aggregation index of 20% or less) and 11% were partially flocculating bacteria (aggregation index of 20 to 50%). Relative abundance of nonflocculating bacteria in this sewage sludge prompted further studies on the role of these bacteria in aggregation. A preliminary survey of adsorption of the nonflocculating bacteria to octyl-Sepharose CL-4B indicated that some of the strains were hydrophobic. Acinetobacter johnsonii S35, Acinetobacter junii S33, and Oligotropha carboxidovorans S23 isolates that exhibited high or moderate hydrophobicities were then picked up as core bacteria for investigation of the possibility of their coaggregation with remaining nonflocculating bacteria. Overall, about 100 bacterial pairs were tested and the results indicated that A. johnsonii S35 had the ability to coaggregate with the highest number of bacterial strains.
FIG. 1.
Flocculation characteristics of the sewage sludge isolates.
All those strains that coaggregated with A. johnsonii S35 were identified by sequencing of full-length 16S rRNA genes. The characteristics, DDBJ accession numbers, and percent identities of the base sequences with the closest sequences in the database (proposed identities) are shown in Table 1. Identification revealed that all the partner strains belonged to one of three groups: Oligotropha carboxidovorans (S23 and S28), Microbacterium esteraromaticum (S29, S38, and S51), and Xanthomonas spp. (S11, S53, and S54). Due to relatively lower sequence homology (93 to 95%), strains belonging to the Xanthomonas genus could not be identified to the species level. Further, three strains of M. esteraromaticum did not show identical morphologies. S29 cells were long rods, often curved into a V, while S38 and S51 cells were short rods.
Identification of coaggregating pairs.
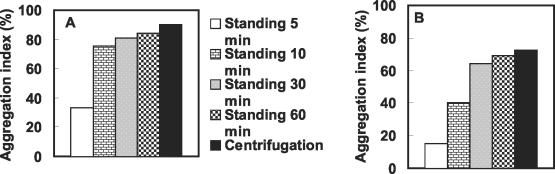
In sewage treatment plants, 30-min-settled sludge volume is used as an index to evaluate settleability of sludge. Therefore, in the present study, the extent of coaggregation among different pairs was initially measured by using either settling (5 to 60 min) or centrifugation of the coaggregating mixtures. Comparison of the aggregation indices recorded by the two assays is shown in Fig. 2. Letting the samples stand for 30 to 60 min before measurement of ODs gave aggregation indices similar to those obtained after centrifugation, with both larger (Fig. 2A) and smaller (Fig. 2B) coaggregates. Therefore, the assay involving the centrifugation step was used as the standard assay throughout this study to obtain quick results.
FIG. 2.
Comparison of results from the two assays (with and without a centrifugation step) for measurement of aggregation indices of larger coaggregates (110 μm) of A. johnsonii S35 and M. esteraromaticum S29 (A) and smaller coaggregates (50 μm) of A. johnsonii S35 and M. esteraromaticum S51 (B).
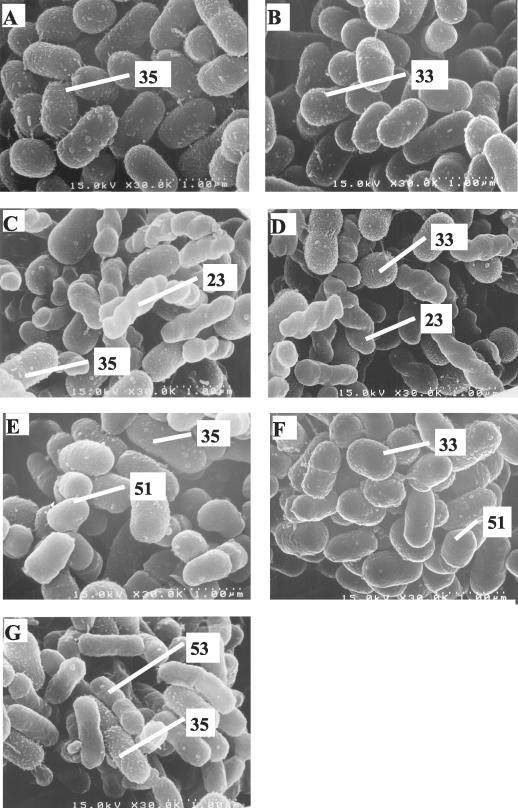
O. carboxidovorans S23, A. junii S33, and A. johnsonii S35 were initially used as the core bacteria in this study to test coaggregation with 29 other strains, and the results are shown in Table 2. The data for 22 strains that did not show coaggregation with any of the core strains are not shown here. O. carboxidovorans S23 coaggregated with only two strains and hence was no more anticipated to be a core bacterium. A. johnsonii S35 showed coaggregation with 8 of 31 strains. As shown in Table 2, aggregation indices during coaggregation of A. johnsonii S35 varied from 73 to 98% depending upon the partner bacterium. Moreover, strains of the same species showed various coaggregation abilities, indicating that the extent of coaggregation was strain specific. A. junii S33 also coaggregated to a lesser extent with four partner strains of A. johnsonii S35. Aggregate sizes assessed using phase-contrast microscopy (micrographs not shown) indicated that these also varied depending upon the bacterial combination involved. A. johnsonii S35 coaggregates were generally larger (50 μm to over 100 μm) than A. junii S33 coaggregates (20 to 30 μm). The scanning electron micrographs of A. johnsonii S35 (Fig. 3A) and A. junii S33 (Fig. 3B) show that both these strains had protrusions on the cell surfaces, on the basis of which they could be readily distinguished from partner strains in the scanning electron micrographs of coaggregates (Fig. 3C to G).
TABLE 2.
Aggregation indices during coaggregation of O. carboxidovorans S23, A. junii S33, and A. johnsonii S35 with other nonflocculating bacteria
| Strain | Cell length (μm) | Aggregation index (%) at 360 min of:
|
||
|---|---|---|---|---|
| O. carboxidovorans S23 | A. junii S33 | A. johnsonii S35 | ||
| A. junii S33 | 1.00 | 62.3 | 17.3 | |
| A. johnsonii S35 | 1.05 | 98.5 | 17.3 | |
| Xanthomonas sp. strain S11 | 1.25 | 15.3 | 18.9 | 90.7 |
| O. carboxidovorans S23 | 1.50 | 62.3 | 98.5 | |
| O. carboxidovorans S28 | 1.50 | 14.7 | 65.2 | 98.3 |
| M. esteraromaticum S29 | 0.9-1.0 | 28.3 | 17.3 | 91.8 |
| M. esteraromaticum S38 | 0.75 | 30.0 | 48.3 | 78.7 |
| M. esteraromaticum S51 | 0.75 | 19.7 | 43.7 | 73.3 |
| Xanthomonas sp. strain S53 | 1.25 | 14.7 | 29.5 | 91.7 |
| Xanthomonas sp. strain S54 | 1.25 | 25.0 | 29.5 | 72.7 |
FIG. 3.
Scanning electron micrographs of the coaggregates. (A) A. johnsonii S35. (B) A. junii S33. (C) A. johnsonii S35 and O. carboxidovorans S23. (D) A. junii S33 and O. carboxidovorans S23. (E) A. johnsonii S35 and M. esteraromaticum S51. (F) A. junii S33 and M. esteraromaticum S51. (G) A. johnsonii S35 and Xanthomonas sp. strain S53.
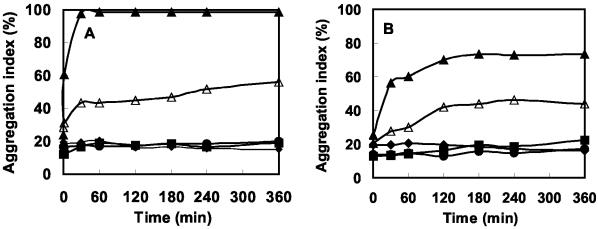
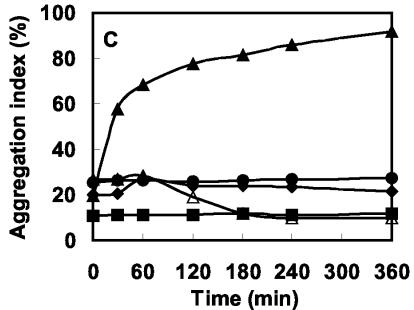
Time course of coaggregation.
Coaggregation patterns of all the efficient pairs were examined in detail, and the time course of coaggregation by one representative strain of each genus is presented (Fig. 4A to C). The results were quite reproducible for most of the coaggregating pairs, and representative values from three or more experiments are reported here. Broadly, two types of patterns could be demonstrated. In the first case, significant coaggregation occurred as soon as the bacterial suspensions were mixed and instant formation of significantly large aggregates could be seen with the naked eye. Such a pattern was observed in the case of coaggregation of A. johnsonii S35 with O. carboxidovorans strains (Fig. 4A). On the other hand, during coaggregation of A. johnsonii S35 with M. esteraromaticum or Xanthomonas spp., aggregation indices gradually increased with time (Fig. 4B and C). In no case did the aggregation indices decline with time, indicating that the aggregates were stable and that no deflocculation occurred during the process. The time courses of coaggregation between A. junii S33 and its partners are also plotted in Fig. 4. A. junii S33 did not coaggregate with any of the strains of Xanthomonas spp.
FIG. 4.
Time courses of coaggregation of A. johnsonii S35 and A. junii S33 with O. carboxidovorans S23 (A), M. esteraromaticum S51 (B), and Xanthomonas sp. strain S53 (C). Symbols: •, A. johnsonii S35; ♦, A. junii S33; ▪, partner strain; ▴, A. johnsonii S35 and partner strain; ▵, A. junii S33 and partner strain.
Correlation between cellular characteristics and coaggregation.
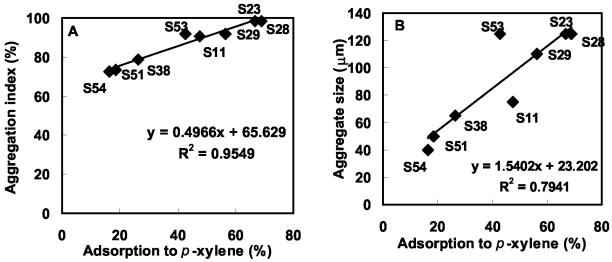
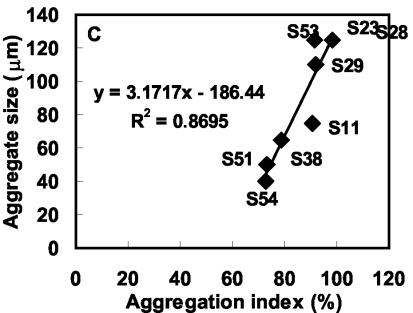
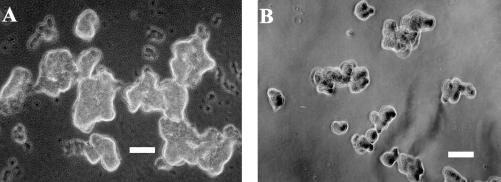
The correlation between aggregation index, aggregate size, and hydrophobicity (percent adsorption to p-xylene) during coaggregation of A. johnsonii S35 with other nonflocculating bacteria is shown in Fig. 5. Positive correlation between cell surface hydrophobicities of the strains and aggregation indices was observed (Fig. 5A). Hydrophobicity could be well correlated with aggregate size except in the cases of Xanthomonas spp. strains S53 and S11 (Fig. 5B). Similarly, in the plot of the correlation between aggregation index and aggregate size, both these strains of Xanthomonas spp. (S53 and S11) were the outliers (Fig. 5C). After 360 min of coaggregation, Xanthomonas sp. strain S53 coaggregates were much larger (125 μm) (Fig. 6A) than Xanthomonas sp. strain S11 coaggregates (75 μm) (Fig. 6B). In the case of Xanthomonas sp. strain S53, the coaggregate size increased between 60 min (80 μm) and 240 min (125 μm), indicating that the smaller coaggregates had joined to form larger ones. On the other hand, in the case of Xanthomonas sp. strain S11, the same coaggregate size was retained until the end point of 360 min. However, the reason for such different aggregate structures of the two strains of Xanthomonas spp., which have similar hydrophobicities and aggregation indices, could not be elucidated. Thus, the aggregation index during coaggregation of A. johnsonii with M. esteraromaticum or O. carboxidovorans strains was better correlated with hydrophobicity and aggregate size.
FIG. 5.
Correlation during coaggregation of A. johnsonii S35 with other nonflocculating bacteria. (A) Cell surface hydrophobicity (percent adsorption to p-xylene) and aggregation index. (B) Cell surface hydrophobicity and aggregate size. (C) Aggregation index and aggregate size.
FIG. 6.
Phase-contrast micrographs of the coaggregates. (A) A. johnsonii S35 and Xanthomonas sp. strain S53. (B) A. johnsonii S35 and Xanthomonas sp. strain S11. Bar, 50 μm.
DISCUSSION
Factors determining coaggregation behavior.
A preliminary report (3) on coaggregation among aquatic biofilm bacteria speculated that the extent of coaggregation assessed by aggregation score is also a measure of sizes or densities of aggregates. Based on phase-contrast microscopic examination, we found a definite and positive relationship between the aggregation index and aggregate size. Further, the aggregation index and aggregate size appeared to depend upon the cellular characteristics of the participating bacteria. A. johnsonii S35 was found to be more hydrophobic (showing 85% adsorption to p-xylene) than A. junii S33 (63%), and this finding could well explain the higher coaggregation scores of the former with the same partner bacteria. Among the partners, O. carboxidovorans S23 (66%) and O. carboxidovorans S28 (69%) were the most hydrophobic, followed by M. esteraromaticum S29 (56%), while hydrophobicities of the rest of the coaggregating strains ranged from 16 to 47%. It is interesting that although M. esteraromaticum S29 (aggregation index = 92%) and M. esteraromaticum S51 (aggregation index = 73%) were identified to the same species level, their coaggregation scores with A. johnsonii S35 varied reasonably due to differences in cell surface properties. Thus, differences in hydrophobicities and cell morphologies of the two strains of M. esteraromaticum led to varied aggregation indices and aggregate sizes. Cell surface hydrophobicity is known to be a dominant characteristic promoting bacterial adhesion (15), and hence the role of hydrophobic interactions during coaggregation of A. johnsonii S35 and A. junii S33 with other bacteria cannot be ignored. However, further studies essential to elucidate the exact mechanism of coaggregation are in progress. Although no direct correlation could be observed between the cell length and the aggregation index, often strains with longer cells had higher aggregation indices. Further, our results show that the coaggregation pairings of bacteria belonging to the same genera were quite similar, while the extents (aggregation indices) and patterns of coaggregation varied. Such differences in coaggregation behaviors of Prevotella nigrescens and Prevotella intermedia with Actinomyces naeslundii strains have been previously demonstrated in the case of oral pathogens (5).
Ecological significance of multigeneric coaggregations.
All the eight strains showing good coaggregation ability with A. johnsonii S35 have been identified. Although several of the strains belong to the same species or genera, the results show that A. johnsonii S35 is an efficient coaggregator which can coaggregate with bacteria belonging to various groups. In addition, A. junii S33 can also coaggregate with some of the common partners. These results suggest the possibility of formation of multigeneric coaggregates with the Acinetobacter isolates as bridging organisms, similar to the role proposed for Fusobacterium and Prevotella in dental plaques (9, 10). Such bridging organisms are believed to carry complementary receptors recognized by functionally similar adhesins on cells from distinct genera (11). Unlike other strains, A. johnsonii S35 had the striking combination of low zeta potential (−5.6 ± 1.3 mV) and high hydrophobicity (85%). In their studies involving adhesion of 23 bacterial strains (from a culture collection) to sulfated polystyrene, van Loosdrecht et al. (25) found that none of the hydrophobic bacteria had low electrophoretic mobility. They suggested that hydrophobic bacteria with low electrokinetic potential are expected to adhere very strongly to other surfaces as well as to one other and hence have fewer chances of being isolated. However, Zita and Hermansson (27) reported that adhesion of Escherichia coli strains or sludge isolates to activated sludge flocs can be better correlated with the surface hydrophobicity than with the cell surface charge. Possibly the combination of high surface hydrophobicity and low zeta potential is responsible for coaggregation of A. johnsonii S35 with several strains and for its role as a bridging organism. We have earlier observed that A. johnsonii S35 can also adhere to the activated sludge flocs with much higher efficiency than the other nonflocculating bacteria. Some protrusions on the surfaces of A. johnsonii S35 and A. junii S33 cells could also be observed through SEM (Fig. 3A and B). There is a possibility that such surface structures mediate the process and participate in coaggregation. However, further studies are required to ascertain the structure and role of these protrusions in coaggregation.
A role for Acinetobacter spp. in bioaccumulation of phosphate and heavy metals along with bioemulsan and biodispersan production has been reported (2, 23). The wide metabolic versatility of these species makes them a prospective candidate for bioremediation of a range of recalcitrant aromatic contaminants (4), including benzene, toluene, and xylene. Acinetobacter spp. strains have been isolated from diverse environments, such as a deep radioactive liquid waste repository (14), oil-contaminated soil (19), and even Siberian tundra soils (20), indicating their universal existence in natural environments. However, this is the first report concerning coaggregation capabilities of Acinetobacter spp. Coaggregate formation will have various advantages for sludge bacteria, such as protection against washout and protozoan predation, resistance to toxins, and better provisions for carbon and energy sources (1). Consequently, the potential ecological significance of various coaggregations involving A. johnsonii S35 or A. junii S33, although unknown at present, may hold great promises with respect to effluent treatment. However, recent studies employing in situ identification methods argue the dominant presence of Acinetobacter species in activated sludge (12, 26). Very few reports indicate the presence of strains belonging to the Xanthomonas group and Microbacterium spp. in activated sludge or drain biofilms (6, 13). Therefore, our future studies will be focused on application of fluorescence in situ hybridization to in situ observation of coaggregating bacterial species and coaggregates in activated sludge to confirm the natural occurrence and significance of these coaggregations in sewage treatment plants.
Through this investigation, we have exposed the occurrence of intergeneric coaggregation among nonflocculating sludge bacteria. We have demonstrated that a positive correlation exists between extent of coaggregation, coaggregate size, and the cell surface characteristics of the partners. The results of our ongoing studies indicate potential similarity of patterns and mechanisms of these coaggregations to those of oral pathogens and aquatic bacteria. Examination of the remaining groups of sludge bacteria for their coaggregation ability will further increase the potential significance of the highly specific coaggregations described here.
Acknowledgments
This work was partially supported by the Japan Society for the Promotion of Science (identification no. 02377).
REFERENCES
- 1.Bossier, P., and W. Verstraete. 1996. Triggers for microbial aggregation in activated sludge? Appl. Microbiol. Biotechnol. 45:1-6. [Google Scholar]
- 2.Boswell, C. D., R. E. Dick, H. Eccles, and L. E. Macaskie. 2001. Phosphate uptake and release by Acinetobacter johnsonii in continuous culture and coupling of phosphate release to heavy metal accumulation. J. Ind. Microbiol. Biotechnol. 26:333-340. [DOI] [PubMed] [Google Scholar]
- 3.Buswell, C. M., Y. M. Herlihy, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1997. Coaggregation amongst aquatic biofilm bacteria. J. Appl. Microbiol. 83:477-484. [Google Scholar]
- 4.Choi, S. C., and Y. S. Oh. 2002. Simultaneous removal of benzene, toluene and xylenes mixture by a constructed microbial consortium during biofiltration. Biotechnol. Lett. 24:1269-1275. [Google Scholar]
- 5.Cookson, A. L., P. S. Handley, A. E. Jacob, G. K. Watson, and C. Allison. 1995. Coaggregation between Prevotella nigrescens and Prevotella intermedia with Actinomyces naeslundii strains. FEMS Microbiol. Lett. 132:291-296. [Google Scholar]
- 6.Hollender, J., U. Dreyer, L. Kornberger, P. Kampfer, and W. Dott. 2002. Selective enrichment and characterization of a phosphorus-removing bacterial consortium from activated sludge. Appl. Microbiol. Biotechnol. 58:106-111. [DOI] [PubMed] [Google Scholar]
- 7.Kakii, K., E. Sugahara, T. Shirakashi, and M. Kuriyama. 1986. Isolation and characterization of a Ca++-dependent floc-forming bacterium. J. Ferment. Technol. 64:57-62. [Google Scholar]
- 8.Kakii, K., M. Hasumi, T. Shirakashi, and M. Kuriyama. 1990. Involvement of Ca2+ in the flocculation of Kluyvera cryocrescens KA-103. J. Ferment. Bioeng. 69:224-227. [Google Scholar]
- 9.Kolenbrander, P. E. 1989. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. CRC Crit. Rev. Microbiol. 17:137-158. [DOI] [PubMed] [Google Scholar]
- 10.Kolenbrander, P. E., R. N. Andersen, and L. V. Holdeman. 1985. Coaggregation of oral bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect. Immun. 48:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolenbrander, P. E., R. N. Andersen, D. L. Clemans, C. J. Whittaker, and C. M. Klier. 1999. Potential role of functionally similar coaggregation mediators in bacterial succession, p. 171-186. In H. N. Newman and M. Wilson (ed.), Dental plaque revisited: oral biofilms in health and disease. Bioline, Cardiff, United Kingdom.
- 12.Manz, W., M. Wagner, R. Amann, and K. H. Schleifer. 1994. In situ characterization of the microbial consortia active in two wastewater treatment plants. Water Res. 28:1715-1723. [Google Scholar]
- 13.Mcbain, A. J., R. G. Bartolo, C. E. Catrenich, D. Charbonneau, R. G. Ledder, A. H. Rickard, S. A. Symmons, and P. Gilbert. 2003. Microbial characterization of biofilms in domestic drains and the establishment of stable biofilm microcosms. Appl. Environ. Microbiol. 69:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazina, T. N., I. M. Kosareva, A. S. Davidov, T. P. Tourova, E. V. Novikova, R. R. Khafizov, and A. B. Poltaraus. 2000. Physicochemical and microbiological characteristics of groundwater from observation wells of a deep radioactive liquid waste repository. Microbiology 69:105-112. [PubMed] [Google Scholar]
- 15.Olofsson A.-C., A. Zita, and M. Hermansson. 1998. Floc stability and adhesion of green-fluorescent-protein-marked bacteria to flocs in activated sludge. Microbiology 144:519-528. [DOI] [PubMed] [Google Scholar]
- 16.Reid, G., J. A. McGroarty, R. Angotti, and R. L. Cook. 1990. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can. J. Microbiol. 34:344-351. [DOI] [PubMed] [Google Scholar]
- 17.Rickard, A. H., A. L. Stephen, C. M. Buswell, N. J. High, and P. S. Handley. 2002. Phylogenetic relationships and coaggregation ability of freshwater biofilm bacteria. Appl. Environ. Microbiol. 68:3644-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg, M., D. Gutnik, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 19.Saddoun, I. 2002. Isolation and characterization of bacteria from crude petroleum oil contaminated soil and their potential to degrade diesel fuel. J. Basic Microbiol. 42:420-428. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki, T., T. Nakayama, T. Kurihara, T. Nishino, and N. Esaki. 2001. Cold-active lipolytic activity of psychrotrophic Acinetobacter sp. strain no. 6. J. Biosci. Bioeng. 92:144-148. [DOI] [PubMed] [Google Scholar]
- 21.Tago, Y., and K. Aida. 1977. Extracellular mucopolysaccharide closely related to bacterial floc formation. Appl. Environ. Microbiol. 34:308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tezuka, Y. 1969. Cation-dependent flocculation in a Flavobacterium species predominant in activated sludge. Appl. Microbiol. 17:222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towner, K. J. 1999. The genus Acinetobacter, p. 3137-3143. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 24.Vandervoorde, L., H. Christiaens, and W. Verstraete. 1992. Prevalence of coaggregation reactions among chicken lactobacilli. J. Appl. Bacteriol. 72:214-219. [DOI] [PubMed] [Google Scholar]
- 25.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman, J., W. Ludwig, and K. H. Schleifer. 2001. DNA polynucleotide probes generated from representatives of the genus Acinetobacter and their application in fluorescence in situ hybridization of environmental samples. Syst. Appl. Microbiol. 24:238-244. [DOI] [PubMed] [Google Scholar]
- 27.Zita, A., and M. Hermansson. 1997. Effects of bacterial cell surface structure and hydrophobicity on attachment to activated sludge flocs. Appl. Environ. Microbiol. 63:1168-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]