Abstract
Vibrio cholerae is both an intestinal pathogen and a microbe in the estuarine community. To persist in the estuarine environment, V. cholerae must adjust to changes in ionic composition and osmolarity. These changes in the aquatic environment have been correlated with cholera epidemics. In this work, we study the response of V. cholerae to increases in environmental osmolarity. Optimal growth of V. cholerae in minimal medium requires supplementation with 200 mM NaCl and KCl. However, when the NaCl concentration is increased beyond 200 mM, a proportionate delay in growth is observed. During this delay in growth, osmotic equilibrium is reached by cytoplasmic accumulation of small, uncharged solutes that are compatible with growth. We show that synthesis of the compatible solute ectoine and transport of the compatible solute glycine betaine impact the length of the osmoadaptive growth delay. We also demonstrate that high-osmolarity-adapted V. cholerae displays a growth advantage when competed against unadapted cells in high-osmolarity medium. In contrast, low-osmolarity-adapted V. cholerae displays no growth advantage when competed against high-osmolarity-adapted cells in low-osmolarity medium. These results may have implications for V. cholerae population dynamics when seawater and freshwater and their attendant microbes mix.
For prokaryotes and eukaryotes inhabiting diverse terrestrial environments, loss of osmotic equilibrium is an ever-present threat. Thus, the survival strategy of most organisms includes a mechanism for osmoadaptation. Most organisms accumulate intracellular solutes or osmolytes in response to increasing environmental osmolarity. In bacteria, charged and/or uncharged osmolytes may be accumulated (40).
Transport of charged osmolytes such as K+ or Na+ from the environment is one strategy by which bacteria adapt to elevated external osmolarity (40). These charged osmolytes, however, alter the chemical environment of the cytoplasm, and in order to function efficiently, bacterial housekeeping processes must be specifically adapted to high-ionic-strength environments. Bacteria that function best with elevated cytoplasmic ionic strength are known as halophiles, while nonhalophiles function poorly when their cytoplasmic ionic strength is elevated. The optimal environmental osmolarity for growth differs widely among halophiles. Most halophiles, however, are restricted to hyperosmolar environments.
When transferred from an environment of low osmolarity to one of high osmolarity, an influx of K+ or Na+ is observed for both halophiles and nonhalophiles. If this influx of ions results in a suboptimal cytoplasmic ionic strength, a delay in growth is observed. Nonhalophiles replace K+ with uncharged solutes that are compatible with cellular function (9, 37). In contrast, halophiles maintain high concentrations of cytoplasmic K+ following osmotic shock (21). However, when a halophile's cytoplasmic ionic strength exceeds that optimal for growth, these bacteria also accumulate compatible intracellular solutes to replace the excess ionic osmolytes (21).
Common compatible solutes include amino acids such as glutamate and proline, amino acid derivatives such as glycine betaine and ectoine (1,4,5,6-tetrahydro-2-methyl pyrimidine-4-carboxylate), and sugars such as trehalose and mannitol (8, 9, 20, 41, 45). Many of these compatible solutes can be synthesized, imported, or exported by various bacteria. The pathway for ectoine synthesis has been determined for several moderate halophiles (5, 11, 22, 26, 31). In all instances studied, the pathway for ectoine synthesis is similar and utilizes the products of the ectA, ectB, and ectC genes as follows. In a pathway that is common to ectoine, lysine, threonine, and methionine biosynthesis, aspartate is converted to β-aspartyl phosphate by the action of aspartokinase. Aspartate semialdehyde dehydrogenase then converts β-aspartyl phosphate to l-aspartate-β-semialdehyde, which may be funneled into the following ectoine synthesis pathway: l-aspartate-β-semialdehyde is converted to l-2,4 diaminobutyric acid by the protein encoded by the ectA gene, l-2,4-diaminobutyric acid acetyltransferase. This is converted to Nγ-acetyl-l-2,4-diaminobutyric acid by the product of the ectB gene, diaminobutyrate-pyruvate aminotransferase. Finally, ectoine is synthesized by ectoine synthase, the protein product of the ectC gene (26, 30). Because glutamate is an excellent substrate for aspartate synthesis and proline may serve as a substrate for glutamate synthesis, both proline and glutamate are capable of fueling ectoine synthesis (30).
In many organisms, including Escherichia coli, enzymes encoded by the betAB genes carry out the synthesis of glycine betaine from choline (3, 7, 23, 34). Transport of glycine betaine is also widespread among gram-negative and gram-positive bacteria (4, 6, 19, 27, 29, 32, 33).
While ample evidence exists that bacterial osmoadaptation plays an important role in environmental survival, there has also been some suggestion that osmoadaptive processes play a role in the virulence of intestinal pathogens. Researchers demonstrated that the 50% lethal dose of Aeromonas hydrophila was decreased by 1 log if the bacterium was prepared by growth in Luria-Bertani (LB) broth supplemented with 300 mM NaCl prior to inoculation into fish or mice (1). Furthermore, when multiple osmolyte transport genes of Listeria monocytogenes were interrupted, colonization of the liver and spleen was compromised in a mouse model of infection (46).
Vibrio cholerae is an intestinal pathogen and a natural inhabitant of estuarine environments. As a member of the Vibrio genus, V. cholerae is classified as a halophile. However, it is reported to require as little as 5 to 15 mM NaCl for optimal growth (15, 36). Growth of V. cholerae in microcosms of various salinities has been studied previously (17, 18, 38, 39, 42). Under the conditions employed in these experiments, media with 25% salinity yielded the highest growth. There has been one study of osmoadaptation by Vibrio costicola, a distant relative of V. cholerae. In this study, ectoine was identified as the primary osmolyte of V. costicola cultured in medium containing 600 mM NaCl or more. However, if glycine betaine was present in the growth medium, this was accumulated preferentially (35).
As is evident above, very little is known about the response of V. cholerae to osmotic stress. However, this is likely to be a critical factor in the survival of V. cholerae in both the estuary and the intestine. Our intent in this work was to delineate the osmoadaptive strategies of V. cholerae in media of various osmolarities and to position ectoine synthesis within this framework.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The V. cholerae O139 strain MO10, a clinical isolate from India, was used in all experiments (44). For competition experiments, a derivative of MO10 (PW354) was used which contains a small insertion in the lacZ gene. The salt base of the minimal media used in this study was as follows: 10 mM KCl, 0.8 mM MgSO4 · 7H2O, 0.3 mM CaCl2 · 2H2O, 0.6 μM FeSO4 · 7H2O, 0.06 mM KH2PO4. NaCl was added in various amounts as detailed in the text. The following mixture of amino acids was used: 0.6 mM alanine, 0.2 mM arginine, 0.03 mM cysteine, 0.3 mM glycine (Bio-Rad), 0.2 mM histidine, 0.5 mM isoleucine, 0.8 mM leucine, 0.7 mM lysine (Fisher Scientific), 0.1 mM methionine, 0.2 mM phenylalanine, 0.4 mM serine, 0.3 mM threonine, 0.04 mM tyrosine, and 0.7 mM valine. This amino acid mixture served as both a carbon and nitrogen source for the growing cells. The pH of the mixture was adjusted to 7.4 with KOH. Where noted, this osmolarity medium (OM) was supplemented with proline, glutamate, ectoine, or glycine betaine. All components were purchased from Sigma unless otherwise noted. Osmolarities of OM supplemented with 5 mM NaCl and 500 mM NaCl were measured on a freezing-point osmometer (model 3300, Advanced Instruments).
Construction of the ectA deletion mutant.
The ectA gene was located in the V. cholerae genome at locus VCA0825. A mutant harboring a deletion of the ectA gene was constructed as previously described (13). Briefly, a 392-bp fragment including the stop codon of ectA and a 393-bp fragment located 1 bp upstream of the start codon of ectA were amplified by the PCR using the primer pairs PectA1 (AAC TTT AAC GCT GCT TCG)-PectA2 (TTA CGA GCG GCC GCA ATC ATC GAA CTG ACC TAA AGG A) and PectA3 (TGC GGC CGC TCG TAA CAA GGC GTA CGG TAA ACA TC)-PectA4 (CGC TTC AAA CCT CTG CTA AA), respectively. These two fragments were joined using the SOE technique, resulting in the construction of a fragment with a 511-bp deletion in the ectA gene (16, 24). The fragment containing the deletion was ligated into pWM91, and this plasmid was used to create a deletion in the ectA gene of wild-type V. cholerae strain MO10 by double homologous recombination and sucrose selection as previously described (10, 28). Because the ect operon promoter was preserved and no additional translation initiation sites were created, the deletion should not produce polar effects on downstream genes.
Measurements of growth following osmotic shock.
Precultures of either wild-type V. cholerae or the ΔectA mutant were grown to stationary phase at 27°C in OM supplemented with 5 mM NaCl. These cultures were subsequently diluted in a ratio of 1:100 into the wells of a microtiter dish filled with fresh growth medium containing various concentrations of NaCl and organic supplements, as indicated. The microtiter dishes were incubated at 27°C, and optical densities were measured at various times using a model 680 microplate reader (Bio-Rad). Where indicated, viable cell counts were measured as follows. Cultures were periodically sampled. Samples were diluted in LB broth and then plated on LB agar. After a 24-h incubation at 27°C, the number of cells present in each dilution was determined, and an approximate number of CFU per milliliter was calculated. All measurements were done in triplicate. Standard deviations were calculated based on three values and are displayed as error bars in the figures.
Preparation of cell extracts and NMR analysis.
Wild-type V. cholerae was incubated with shaking at 37°C in OM supplemented with either 5 or 500 mM NaCl. When stationary phase was reached, cells were pelleted gently, and the spent growth medium was removed. Cell pellets were subjected to three freeze-thaw cycles to enhance lysis. Pellets were then resuspended in 750 μl of ethanol. Debris was removed by centrifugation, and the ethanol extract was transferred to a clean tube. The ethanol was subsequently removed from the extracted material by evaporation under vacuum (Speedvac DNA A-110; Savant). The resulting pellet was resuspended in 800 μl of D2O (Aldrich). After removal of insoluble material by centrifugation, the solution was transferred to a 5-mm nuclear magnetic resonance (NMR) tube for analysis. One-dimensional, double-filtered quantum coherence and heteronuclear multiple-quantum coherence experiments were performed on an AMX500 spectrometer (Bruker).
Competition experiments.
For competition experiments, wild-type V. cholerae and PW354, a strain harboring a small insertion in the lacZ gene, were grown for 48 h in OM supplemented with either 5 or 500 mM NaCl. At this point, strains were mixed in approximately equal numbers at a 1:100 dilution in OM supplemented with either 5 or 500 mM NaCl. Cultures were allowed to grow for 24 h at 27°C. Appropriate dilutions of the initial and final cultures were spread on agar plates containing 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)/ml (New England Biolabs), and blue and white colonies were enumerated. A competitive index was calculated by dividing the ratio of high- osmolarity-adapted cells to low-osmolarity-adapted cells after 24 h of coculture by the ratio of high-osmolarity-adapted cells to low-osmolarity-adapted cells at the initiation of coculture. Competition experiments were conducted in triplicate, and standard deviations were calculated from these values.
RESULTS AND DISCUSSION
Development of a minimal medium for the study of osmoadaptation.
We were interested in studying the osmoadaptive response of V. cholerae and relevant mutants. We presumed that the ΔectA mutant, but not wild-type V. cholerae, would have a growth defect in high-osmolarity medium. However, when grown in LB broth supplemented with 500 mM NaCl, wild-type V. cholerae and a ΔectA mutant grew equally well. We hypothesized that various components of LB broth were providing redundant pathways of osmoadaptation to V. cholerae. Thus, we developed OM, a defined medium containing all the inorganic components required for growth as well as a mixture of purified amino acids similar to that found in Casamino Acids but excluding glutamate, proline, and aspartate. Through the use of this medium, we hoped to limit the number of osmoadaptation pathways available to V. cholerae with the goal of isolating the various pathways of osmoadaptation and examining them independently. Osmolarities of OM supplemented with 5 and 500 mM NaCl were measured and found to be 96 and 1,040 mosM, respectively. V. cholerae has been found in both fresh and estuarine environments. While the osmolarities of these environments are variable, the osmolarity of riverine water may be as low as 4 mosM, increasing as it nears the sea, where osmolarities are as high as 1,000 mosM. Thus, the osmolarities of the media used in these experiments include a range of osmolarities that V. cholerae would experience in its natural aquatic habitats.
Growth of wild-type V. cholerae in OM is optimized by moderate NaCl supplementation.
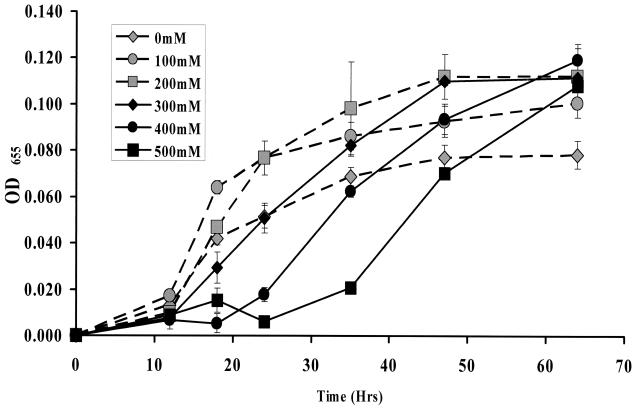
We hypothesized that V. cholerae might exhibit a growth delay when exposed to sufficiently high concentrations of NaCl. To test this, we prepared wild-type V. cholerae cells by growth in OM supplemented with 5 mM NaCl. These cells were then transferred to OM supplemented with a variety of NaCl concentrations. Growth curves of wild-type V. cholerae in OM supplemented with various amounts of NaCl could be grouped in the following way. In OM supplemented with 200 mM NaCl or less, growth rates and final cell densities increased with increasing NaCl concentration (Fig. 1). In OM supplemented with greater than 200 mM NaCl, an initial growth delay was observed. The length of this growth delay increased as the concentration of NaCl in the medium was increased. Interestingly, in spite of an initial delay in growth, the final density of cells grown in OM supplemented with NaCl concentrations above 200 mM remained higher than that of cells grown in unsupplemented OM. Colony counts of cultures demonstrated that transfer of V. cholerae from low-osmolarity medium to OM supplemented with 500 mM NaCl did not result in loss of viability. In contrast, cells transferred from low-osmolarity medium to OM supplemented with 1 M NaCl never recovered from the initial growth delay. Plating of these cultures demonstrated that cells had lost viability (data not shown).
FIG. 1.
Growth of wild-type V. cholerae (MO10) following transfer from OM supplemented with 5 mM NaCl to OM supplemented with various NaCl concentrations. NaCl concentrations for various growth curves are noted in the key. Dashed lines connecting gray symbols illustrate growth of V. cholerae in OM supplemented with NaCl concentrations at or below 200 mM, while solid lines connecting black symbols represent growth in OM supplemented with NaCl concentrations above 200 mM NaCl. OD655, optical density at 655 nm.
K+ is required for optimal growth of V. cholerae in the presence of NaCl.
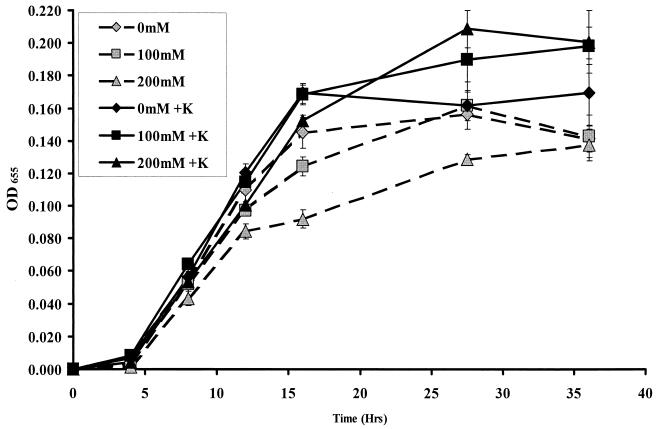
In the absence of supplementation, V. cholerae was able to grow in a 1% vitamin assay Casamino Acids solution in distilled H2O (CAA medium). Because elemental analysis demonstrated less than 100 μM K+ in this medium, it was used to assess the role of K+ in V. cholerae osmoadaptation. For these experiments, we cultured V. cholerae in CAA medium supplemented with various amounts of NaCl and either 0 or 10 mM KCl. As shown in Fig. 2, V. cholerae growth was similar in CAA medium, CAA medium supplemented with 10 mM KCl alone, and CAA medium supplemented with 100 mM NaCl alone. V. cholerae growth was negatively impacted by supplementation of CAA medium with 200 mM NaCl alone. However, if CAA medium was supplemented with both 200 mM NaCl and 10 mM KCl, growth rates and maximum cell densities increased considerably. Thus, supplementation of CAA medium with 200 mM NaCl yielded optimal conditions for V. cholerae growth only if KCl was also present.
FIG. 2.
Growth of wild-type V. cholerae in CAA medium supplemented with a variety of NaCl concentrations, as noted in the key. Gray symbols connected by dashed lines represent growth of V. cholerae in CAA medium without KCl. Black symbols connected by solid lines represent growth of V. cholerae in CAA medium supplemented with 10 mM KCl. OD655, optical density at 655 nm.
The osmoadaptive response of the ΔectA mutant is defective.
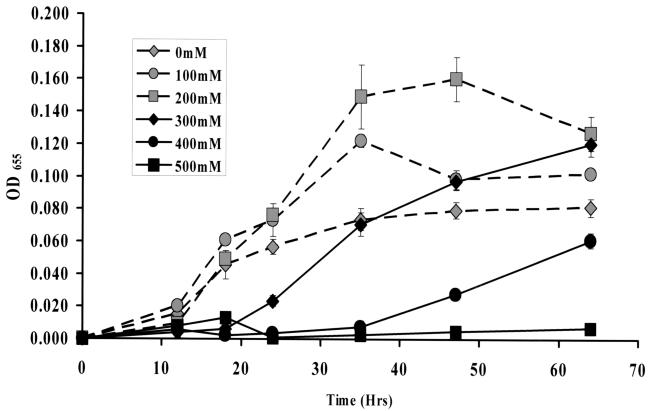
By searching the published V. cholerae genome sequence, we identified a locus on the second chromosome of V. cholerae (VCA0825) that was annotated as ectA (14). In V. cholerae, ectA is the first gene in a four-gene operon also harboring ectB (VCA0824), ectC (VCA0823), and a gene encoding a putative aspartokinase (VC0822). The enzymes encoded by these four genes are predicted to play a role in ectoine synthesis. Using the sequences of the E. coli bet genes to search the V. cholerae genome sequence, we were unable to locate bet gene homologs in the V. cholerae genome. Furthermore, addition of choline to OM supplemented with 500 mM NaCl had no impact on growth of V. cholerae (data not shown). Thus, we hypothesized that while V. cholerae was unable to synthesize glycine betaine from choline, ectoine synthesis might play a role in V. cholerae osmoadaptation. To examine the role of ectoine in V. cholerae osmoadaptation, we constructed a strain containing a deletion of the ectA gene, VCA0825. We hypothesized that a mutation in the ectA gene would impair the ability of V. cholerae to respond to increases in external osmolarity. To test this hypothesis, we grew the ΔectA mutant in OM supplemented with 5 mM NaCl and then transferred the cells to OM supplemented with a variety of NaCl concentrations. As was observed for wild-type V. cholerae, growth of the ΔectA mutant in OM supplemented with NaCl could be divided into two categories (Fig. 3). In OM supplemented with concentrations of NaCl below or equal to 200 mM, the growth rate and maximum cell density of V. cholerae improved with increasing NaCl concentration. Above 200 mM NaCl, an initial growth delay was observed which increased proportionally with increasing NaCl concentration. However, the growth delay of the ΔectA mutant was much more pronounced than that of wild-type V. cholerae (Fig. 1 and 3). In fact, in OM supplemented with 500 mM NaCl, little growth of the ΔectA mutant was observed over the course of the 3-day experiment.
FIG. 3.
Transfer of a V. cholerae ΔectA mutant from OM supplemented with 5 mM NaCl to OM supplemented with a variety of NaCl concentrations. Dashed lines connecting gray symbols illustrate growth of V. cholerae in OM supplemented with NaCl concentrations at or below 200 mM, while solid lines connecting black symbols represent growth in OM supplemented with NaCl concentrations above 200 mM NaCl. OD655, optical density at 655 nm.
We first questioned whether survival of the ΔectA mutant cells in high-osmolarity environments was impaired. To test this, we prepared ΔectA mutant cells in OM supplemented with 5 mM NaCl and then transferred these cells into OM supplemented with 500 mM NaCl. By testing daily viable counts over the course of several days, we excluded cell death as the basis for the lack of growth of the ΔectA mutant in OM supplemented with 500 mM NaCl (results not shown). Because no loss in viability was observed, we hypothesized that the growth delay of the ΔectA mutant might extend beyond 3 days. In fact, when the ΔectA mutant was grown in OM supplemented with 500 mM NaCl, the optical density of the culture increased after 4 days and reached a maximum optical density at 655 nm of 0.086 by day 10 (data not shown).
Ectoine synthesis is induced by growth in high-osmolarity medium.
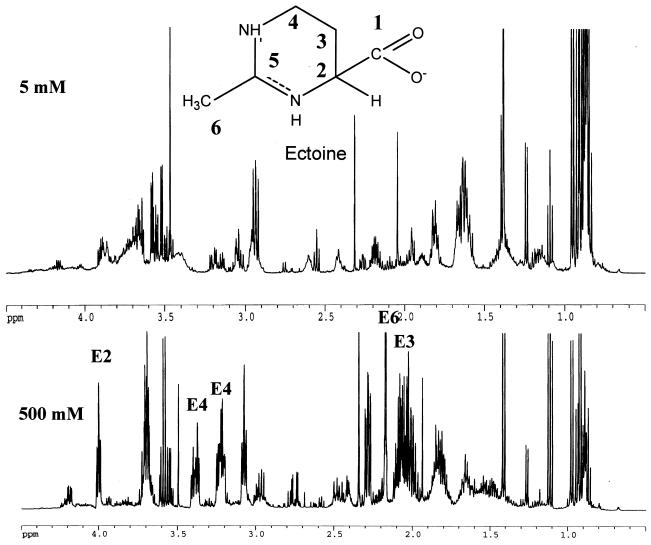
Because the V. cholerae ΔectA mutant displayed a significant growth defect in OM supplemented with 500 mM NaCl, we suspected that ectoine might be synthesized by V. cholerae in response to increased external osmolarity. To test this hypothesis, we made ethanol extracts of V. cholerae cells grown in OM supplemented with 5 and 500 mM NaCl. The one-dimensional 1H-NMR spectra of these extracts are shown in Fig. 4. The peaks corresponding to the various hydrogen atoms of ectoine are labeled. The two-dimensional double-filtered quantum coherence and heteronuclear multiple-quantum coherence spectra of these extracts were also measured and compared with the spectrum of purified ectoine (data not shown). This comparison supported the spectral assignments shown in Fig. 4. Although there were some similarities between the spectra derived from cells grown in low- and high-osmolarity media, ectoine-specific peaks were clearly missing from the spectrum of the cell extract derived from V. cholerae grown in OM supplemented with 5 mM NaCl.
FIG. 4.
One-dimensional 1H-NMR spectra of cell extracts derived from cells grown in OM supplemented with 5 and 500 mM NaCl. Peaks derived from ectoine are labeled with E plus the number of the carbon atom to which the hydrogen atoms are attached. The structure of ectoine is included for reference.
Proline and glutamate are substrates for ectoine synthesis.
The medium used in these experiments was designed to exclude the common osmolytes proline and glutamate. In order to determine the effect of proline and glutamate supplementation on growth of V. cholerae in high-osmolarity medium, we grew wild-type V. cholerae or a ΔectA mutant in OM supplemented with 5 mM NaCl and then transferred these cultures to OM supplemented with either 5 or 500 mM NaCl and various concentrations of proline or glutamate. After 24 h of growth in each of these media, we measured the optical density of wild-type V. cholerae and ΔectA mutant cultures. Interestingly, addition of 500 μM proline or glutamate dramatically improved growth of wild-type V. cholerae in OM supplemented with 500 mM NaCl (Table 1). Proline and glutamate had very little impact on the growth of V. cholerae in OM supplemented with 5 mM NaCl, suggesting that these amino acids were not merely providing nutrition to the growing cells. In contrast, addition of proline and glutamate had only modest effects on growth of the ΔectA mutant in OM supplemented with 500 mM NaCl. Thus, we hypothesized that in the face of osmotic stress, V. cholerae utilized proline and glutamate primarily as substrates for ectoine synthesis. If proline or glutamate did serve as an osmolyte for V. cholerae, one would predict that cells grown in OM supplemented with 500 mM NaCl and either proline or glutamate would accumulate intracellular proline or glutamate, respectively. To determine whether this was the case, we performed 1H-NMR on extracts of wild-type V. cholerae cells grown in OM supplemented with 500 mM NaCl and proline or glutamate. As expected, these spectra were very similar to that of wild-type V. cholerae grown in OM supplemented only with 500 mM NaCl, suggesting that ectoine, rather than proline or glutamate, was accumulating in these cells (data not shown). This confirms our hypothesis that at this concentration, proline and glutamate are not osmolytes themselves, but rather serve primarily as substrates for ectoine synthesis.
TABLE 1.
Twenty-four-hour growth densities of wild-type V. cholerae and a ΔectA mutant in OM supplemented with 5 or 500 mM NaCl and no amino acid, 500 μM proline, or 500 μM glutamate
| [NaCl], mM (amino acid) | Density (OD655) of:
|
|
|---|---|---|
| Wild type | ΔectA mutant | |
| 5 (none) | 0.055 ± 0.009 | 0.053 ± 0.009 |
| 500 (none) | 0.005 ± 0.002 | 0.005 ± 0.001 |
| 5 (proline) | 0.086 ± 0.003 | 0.095 ± 0.003 |
| 500 (proline) | 0.080 ± 0.001 | 0.020 ± 0.004 |
| 5 (glutamate) | 0.081 ± 0.013 | 0.079 ± 0.005 |
| 500 (glutamate) | 0.072 ± 0.003 | 0.005 ± 0.003 |
Exogenous glycine betaine, but not ectoine, accelerates osmoadaptation by V. cholerae.
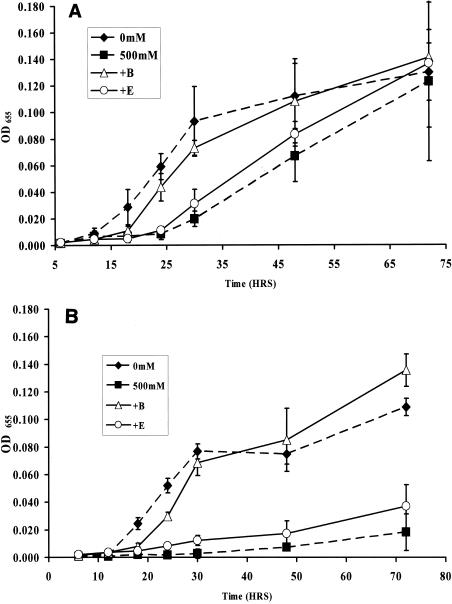
A survey of the V. cholerae genome revealed a homolog of opuD, the glycine-betaine transporter of Bacillus species, but no homolog of the ectoine transport system, TeaABC of Halomonas elongata (19, 43). This suggested to us that V. cholerae might transport glycine betaine, but not ectoine, for use as an osmolyte. To confirm this, we grew wild-type V. cholerae and a ΔectA mutant in OM supplemented with 5 mM NaCl and then transferred these cultures to OM supplemented with 500 mM NaCl and either glycine betaine or ectoine. As predicted, when exogenous glycine betaine was provided, the growth of wild-type V. cholerae and that of a ΔectA mutant in OM supplemented with 5 and 500 mM NaCl were indistinguishable (Fig. 5). In contrast, supplementation of high-osmolarity medium with ectoine had no effect on the growth of either wild-type V. cholerae or the ΔectA mutant (Fig. 5).
FIG. 5.
Growth of V. cholerae after transfer from OM supplemented with 5 mM NaCl to OM supplemented with either 5 mM NaCl or 500 mM NaCl and 250 μM ectoine or glycine betaine. Dashed lines and solid symbols represent growth measurements in OM without osmolytes. Solid lines and open symbols represent growth measurements in OM supplemented with osmolytes. Shown are growth of wild-type V. cholerae (MO10) (A) and a ΔectA mutant (PW411) (B).
Adaptation to high external osmolarity confers a growth advantage in high-osmolarity medium but no disadvantage in low-osmolarity medium.
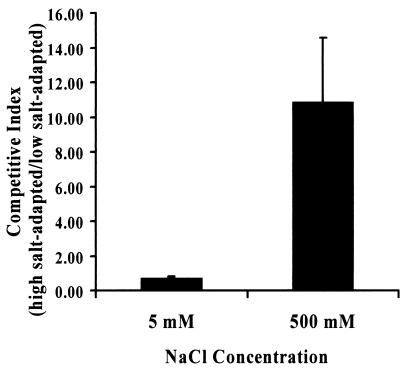
V. cholerae grows well in both low- and high-osmolarity media. However, our results suggested that transitions from low-osmolarity to high-osmolarity medium would result in a growth delay. We postulated that if bacteria adapted to low- and high-osmolarity environments were cocultured, bacteria adapted to low-osmolarity medium would display a growth advantage in low-osmolarity medium, while bacteria adapted to high-osmolarity medium would display a growth advantage in high-osmolarity medium. To test this, we adapted two V. cholerae strains, one of which possessed an interrupted lacZ gene, to OM supplemented with either 5 or 500 mM NaCl. We combined these two strains in approximately equal numbers in OM supplemented with either 5 or 500 mM NaCl. A competitive index was calculated after 24 h of coculture. As shown in Fig. 6, high- and low-osmolarity-adapted V. cholerae grew equally well in OM supplemented with 5 mM NaCl. However, in OM supplemented with 500 mM NaCl, high-osmolarity-adapted cells had a significant advantage. Competition experiments conducted in OM supplemented with 500 mM NaCl yielded the same results regardless of whether wild-type V. cholerae or the lacZ mutant was used as the high-osmolarity-adapted strain.
FIG. 6.
Competition indices of low- and high-osmolarity-adapted V. cholerae in OM supplemented with 5 or 500 mM NaCl.
Potential roles for V. cholerae osmoadaptation in the epidemiology of cholera.
Several studies have suggested that the salinity of riverine environments is a factor in the incidence of cholera. Measurements of salinity over time in the Hoogly River in Calcutta and the Thames River in London demonstrate a correlation between increased salinity and cholera (46). In the Indian subcontinent, cholera epidemics are correlated with monsoon (2). Monsoon brings heavy rain, wind, and flooding. Modeling studies of the Bay of Bengal suggest that monsoon winds force seawater into the bay, resulting in an increase in sea surface height in the bay (12). Interestingly, increases in sea surface height, rather than heavy rainfall or flooding, are most strongly correlated with cholera epidemics (2, 12, 25). Increases in sea surface height are also likely to result in increases in the salinity of nearby riverine environments as the seawater is forced into estuaries. Thus, a significant body of work points to a correlation between increased salinity and the incidence of cholera.
It has been postulated that the correlation of increased sea surface height and riverine salinity with cholera epidemics reflects transport of V. cholerae-laden seawater into freshwater beds where contact with humans is more likely (25). Based on the earlier studies and those presented here, we would like to propose three additional hypotheses. First of all, we have confirmed that V. cholerae is a versatile halophile that is able to persist and even grow, albeit slowly, in environments with low salinity. Thus, if V. cholerae were a continuous inhabitant of a particular freshwater environment, a small increase in the salinity of this environment might enhance the growth of V. cholerae dramatically. Increased numbers of V. cholerae in the freshwater environment would increase the likelihood of ingesting an infectious dose, resulting in a correlation between increased salinity and increased incidence of cholera. Secondly, our results suggest that osmotic upshock may result in an initial growth delay. If ingested from freshwater, V. cholerae may experience osmotic shock upon reaching the high-osmolarity environment of the intestinal lumen. This growth delay might decrease the infectivity of V. cholerae. However, if the bacteria are ingested from a higher-salinity environment, they may be better prepared for the high osmolarity of the intestine. Lastly, we have shown that V. cholerae adapted to high-osmolarity environments grow as well as or outcompete V. cholerae adapted to low-osmolarity environments. This suggests that if mixing of freshwater and seawater occurs, versatile halophiles such as V. cholerae arriving from the sea may outcompete freshwater-adapted bacteria in the estuarine environment. This would result in higher numbers of V. cholerae in the aquatic environment. One or more of these forces may be at play in the epidemiology of cholera epidemics. Environmental studies are planned to evaluate the relevance of these hypotheses.
Acknowledgments
We acknowledge Mary Roberts for helpful discussions and advice. NMR experiments were performed at the Tufts University Biological NMR Center with the expert help of James Sudmeier. We also thank Anne Kane of the GRASP Center and her staff for their patience with the development of the various growth media and their expert preparation of many reagents, which greatly accelerated the course of these experiments.
This work was supported by NIH grant R01 AI50032 to P.I.W. and also by a pilot project grant from the New England Medical Center GRASP Center (NIH/NIDDK P30 DK34928).
REFERENCES
- 1.Aguilar, A., S. Merino, X. Rubires, and J. M. Tomas. 1997. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O:34 strains grown at 37°C. Infect Immun. 65:1245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, M., M. Emch, J. P. Donnay, M. Yunus, and R. B. Sack. 2002. The spatial epidemiology of cholera in an endemic area of Bangladesh. Soc. Sci. Med. 55:1015-1024. [DOI] [PubMed] [Google Scholar]
- 3.Boch, J., B. Kempf, and E. Bremer. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boscari, A., K. Mandon, L. Dupont, M. C. Poggi, and D. Le Rudulier. 2002. BetS is a major glycine betaine/proline betaine transporter required for early osmotic adjustment in Sinorhizobium meliloti. J. Bacteriol. 184:2654-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canovas, D., C. Vargas, M. I. Calderon, A. Ventosa, and J. J. Nieto. 1998. Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst. Appl. Microbiol. 21:487-497. [DOI] [PubMed] [Google Scholar]
- 6.Canovas, D., C. Vargas, L. N. Csonka, A. Ventosa, and J. J. Nieto. 1996. Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J. Bacteriol. 178:7221-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canovas, D., C. Vargas, S. Kneip, M. J. Moron, A. Ventosa, E. Bremer, and J. J. Nieto. 2000. Genes for the synthesis of the osmoprotectant glycine betaine from choline in the moderately halophilic bacterium Halomonas elongata DSM 3043, USA. Microbiology 146:455-463. [DOI] [PubMed] [Google Scholar]
- 8.Ciulla, R. A., and M. F. Roberts. 1999. Effects of osmotic stress on Methanococcus thermolithotrophicus: 13C-edited 1H-NMR studies of osmolyte turnover. Biochim. Biophys. Acta 1427:193-204. [DOI] [PubMed] [Google Scholar]
- 9.Dinnbier, U., E. Limpinsel, R. Schmid, and E. P. Bakker. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 150:348-357. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 58:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galinski, E. A., H. P. Pfeiffer, and H. G. Truper. 1985. 1, 4, 5, 6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 149:135-139. [DOI] [PubMed] [Google Scholar]
- 12.Han, W. Q., and P. J. Webster. 2002. Forcing mechanisms of sea level interannual variability in the Bay of Bengal. J. Physical Oceanogr. 32:216-239. [Google Scholar]
- 13.Haugo, A. J., and P. I. Watnick. 2002. Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt, J. G. (ed.). 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 16.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 17.Huq, A., P. A. West, E. B. Small, M. I. Huq, and R. R. Colwell. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl. Environ. Microbiol. 48:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaper, J., H. Lockman, R. R. Colwell, and S. W. Joseph. 1979. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 37:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kets, E. P., E. A. Galinski, M. de Wit, J. A. de Bont, and H. J. Heipieper. 1996. Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J. Bacteriol. 178:6665-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraegeloh, A., and H. J. Kunte. 2002. Novel insights into the role of potassium for osmoregulation in Halomonas elongata. Extremophiles 6:453-462. [DOI] [PubMed] [Google Scholar]
- 22.Kuhlmann, A. U., and E. Bremer. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamark, T., I. Kaasen, M. W. Eshoo, P. Falkenberg, J. McDougall, and A. R. Strom. 1991. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol. Microbiol. 5:1049-1064. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre, B., P. Formstecher, and P. Lefebvre. 1995. Improvement of the gene splicing overlap (SOE) method. BioTechniques 19:186-188. [PubMed] [Google Scholar]
- 25.Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A. S. G. Faruque, and R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA. 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis, P., and E. A. Galinski. 1997. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143(Pt. 4):1141-1149. [DOI] [PubMed] [Google Scholar]
- 27.Mendum, M. L., and L. T. Smith. 2002. Characterization of glycine betaine porter I from Listeria monocytogenes and its roles in salt and chill tolerance. Appl. Environ. Microbiol. 68:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ a for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 29.Nau-Wagner, G., J. Boch, J. A. Le Good, and E. Bremer. 1999. High-affinity transport of choline-O-sulfate and its use as a compatible solute in Bacillus subtilis. Appl. Environ. Microbiol. 65:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neidhardt, F. C., et al. (ed.). 1996. Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 31.Ono, H., K. Sawada, N. Khunajakr, T. Tao, M. Yamamoto, M. Hiramoto, A. Shinmyo, M. Takano, and Y. Murooka. 1999. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J. Bacteriol. 181:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perroud, B., and D. Le Rudulier. 1985. Glycine betaine transport in Escherichia coli: osmotic modulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter, H., B. Weil, A. Burkovski, R. Kramer, and S. Morbach. 1998. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J. Bacteriol. 180:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pocard, J. A., N. Vincent, E. Boncompagni, L. T. Smith, M. C. Poggi, and D. Le Rudulier. 1997. Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiology 143(Pt. 4):1369-1379. [DOI] [PubMed] [Google Scholar]
- 35.Regev, R., I. Peri, H. Gilboa, and Y. Avi-Dor. 1990. 13C NMR study of the interrelation between synthesis and uptake of compatible solutes in two moderately halophilic eubacteria, bacterium Ba1 and Vibro costicola. Arch. Biochem. Biophys. 278:106-112. [DOI] [PubMed] [Google Scholar]
- 36.Reichelt, J. L., and P. Baumann. 1974. Effect of sodium chloride on growth of heterotrophic marine bacteria. Arch. Microbiol. 97:329-345. [DOI] [PubMed] [Google Scholar]
- 37.Roesser, M., and V. Muller. 2001. Osmoadaptation in bacteria and archaea: common principles and differences. Environ. Microbiol. 3:743-754. [DOI] [PubMed] [Google Scholar]
- 38.Singleton, F. L., R. Attwell, S. Jangi, and R. R. Colwell. 1982. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 44:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singleton, F. L., R. W. Attwell, M. S. Jangi, and R. R. Colwell. 1982. Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl. Environ. Microbiol. 43:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sleator, R. D., and C. Hill. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 41.Talibart, R., M. Jebbar, G. Gouesbet, S. Himdi-Kabbab, H. Wroblewski, C. Blanco, and T. Bernard. 1994. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J. Bacteriol. 176:5210-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamplin, M. L., and R. R. Colwell. 1986. Effects of microcosm salinity and organic substrate concentration on production of Vibrio cholerae enterotoxin. Appl. Environ. Microbiol. 52:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tetsch, L., and H. J. Kunte. 2002. The substrate-binding protein TeaA of the osmoregulated ectoine transporter TeaABC from Halomonas elongata: purification and characterization of recombinant TeaA. FEMS Microbiol. Lett. 211:213-218. [DOI] [PubMed] [Google Scholar]
- 44.Waldor, M. K., and J. J. Mekalanos. 1994. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J. Infect. Dis. 170:278-283. [DOI] [PubMed] [Google Scholar]
- 45.Welsh, D. T., R. H. Reed, and R. A. Herbert. 1991. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J. Gen. Microbiol. 137(Pt 4):745-750. [DOI] [PubMed] [Google Scholar]
- 46.Wemekamp-Kamphuis, H. H., J. A. Wouters, R. D. Sleator, C. G. Gahan, C. Hill, and T. Abee. 2002. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl. Environ. Microbiol. 68:4710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]