Abstract
Hereditary hemochromatosis (HH) is a common autosomal recessive disorder of iron metabolism that leads to excessive iron storage in the liver and other organs. Recently, between 83 and 100% of HH patients have been found to be homozygous for the same mutation in a novel major histocompatibility complex class I-like gene, called the HLA-H gene. The Cys-282 → Tyr mutation in HH patients would be expected to disrupt the function of the HLA-H gene product by altering a critical disulfide bridge. As a first step in understanding the function of the HLA-H gene product, we generated an antibody to a C-terminal peptide and used it for immunolocalization of the HLA-H protein in the gastrointestinal tract of Finnish and American subjects presumed not to have HH. Although staining for the HLA-H protein was seen in some epithelial cells in every segment of the alimentary canal, its cellular and subcellular expression in the small intestine were quite distinct from those seen in other segments. In contrast to the stomach and colon, where staining was polarized and restricted to the basolateral surfaces, and in contrast to the epithelial cells of the esophagus and submucosal leukocytes, which showed nonpolarized staining around the entire plasma membrane, the staining in small intestine was mainly intracellular and perinuclear, limited to cells in deep crypts. Prior genetic evidence suggested that a defective HLA-H protein is the molecular basis of HH. Here we show that the HLA-H protein not only varies in its pattern of expression along the cranial/caudal axis of the gastrointestinal tract but that it has a unique subcellular localization in the crypts of the small intestine in proximity to the presumed sites of iron absorption.
Hereditary hemochromatosis (HH) is the most common of the known autosomal recessive disorders in Caucasians. The carrier frequency has been estimated to be between 1 in 8 and 1 in 10 in North America and homozygosity for HH is ≈3–4 per 1000 (1–4). Thus, the incidence of HH is higher than the combined incidence of cystic fibrosis, phenylketonuria, and muscular dystrophy (5). The high frequency of this disorder and the challenge to understand it has led it to be called the disease of the 21st century (5).
HH is characterized by defective regulation of dietary iron absorption that leads to excessive iron accumulation in various organs including the liver, pancreas, and heart leading to hepatic cancer, liver failure, diabetes, and heart disease. The pathogenesis of HH is thought to involve a defect in the mechanisms controlling small intestinal iron absorption (6). Recently, Feder et al. (7) reported a mutation in a novel major histocompatibility complex class I-like gene to be present in 83% of HH patients. Two subsequent studies confirmed its high frequency, reporting 90.8% of French patients (8) and 100% of Australian patients (9) to be homozygous for this mutation, providing further support for this gene itself being the HH gene.
The HLA-H protein predicted from the cDNA sequence is comprised of 343 amino acids. Database comparisons revealed that the protein is most analogous to major histocompatibility complex class I molecules that contain an extracellular peptide-binding region (α1 and α2 domains), an immunoglobulin-like domain (α3), a transmembrane region, and a short cytoplasmic tail. By analogy with other class I proteins, HLA-H is presumed to contain intramolecular disulfide bridges that stabilize its tertiary structure. It has been suggested that one of these S—S bonds is required for appropriate intracellular processing and transport (10). Feder et al. (7) suggested that the Cys-282 → Tyr substitution in the HLA-H protein would disrupt the formation of the disulfide bridge between Cys-225 and Cys-282, thereby preventing the association of the HLA-H protein with β2-microglobulin, and eliminating the cell-surface presentation of the HLA-H protein. The functional importance of such an interplay in HH between β2-microglobulin and some class I-type HLA molecule was suggested by studies of β2-microglobulin-deficient mice, which develop progressive hepatic iron overload (11–13).
Northern blot experiments showed that HLA-H mRNA is widely expressed (7). A major transcript was seen in all tissues tested except for brain, with some suggestion of higher levels in liver and intestine, major sites of iron metabolism in the body. Although most iron absorption is thought to occur in the small intestine, the mechanisms involved in transferring iron across the microvillus and basolateral membranes of the enterocyte are poorly understood. The novel HLA-H protein could possibly be one link in the normally tightly regulated processes that take iron from the lumen of the gut to the plasma and prevent excessive absorption of iron when iron needs are met. As a first approach to understanding the function of the HLA-H protein, we generated a specific antibody to a C-terminal peptide predicted from the cDNA, and used it to define the localization of the protein in the normal gastrointestinal tract. Although positive staining for the HLA-H protein was seen in selected cells of all segments of the gastrointestinal tract, staining in the small intestine was not only intense but displayed unique cellular and subcellular localization.
MATERIALS AND METHODS
Production of Antibody.
A peptide corresponding to the 16 C-terminal amino acids predicted from the cDNA (7), chosen because it has only 4 amino acids, 1 amino acid, and 2 amino acids in common with the C terminus of HLA-A2, HLA-G, and human neonatal Fc receptor, respectively, was synthesized and coupled to pig thyroglobulin using a disuccinimidyl suberate bifunctional reagent (14). The C-terminal HLA-H peptide–thyroglobulin complex (300 μg protein) was injected subcutaneously into rabbits in complete Freund’s adjuvant. A second injection with incomplete Freund’s adjuvant containing 300 μg protein was given 4 weeks later. Antibody production, monitored by dot blot analysis, was evident in serum obtained 12 days after the second injection. After one further boost with 200 μg protein in incomplete Freund’s adjuvant, the rabbits were bled every 2 weeks.
Affinity-pure and peptide-specific IgG was isolated using a C-terminal HLA-H peptide–Affigel 10 affinity resin and stored in 50% glycerol at −20°C. Specificity of the antibody was established by demonstrating peptide-specific blocking of bands identified in tissue homogenates on Western blots in a manner similar to that previously described (15). The C-terminal HLA-H-specific IgG showed strong immunostaining of a 45- to 50-kDa protein in the tissue homogenates that also reacted with an anti-HLA-H antibody raised to a 15-amino acid peptide from the α3 loop in the extracellular domain of HLA-H. The C-terminal peptide antibody did not react with affinity-purified Fc receptor from human placenta. Its specificity was further established using COS-7 cell homogenate expressing HLA-H cDNA. The transfected cell homogenate showed a strong signal corresponding to a 45- to 50-kDa polypeptide, but no signal was seen in homogenates of COS-7 cells transfected with vector only, or with HLA-H cDNA that encoded truncated protein lacking the C-terminal amino acids.
Preparation of Samples and Immunohistochemistry.
The histological specimens from the human alimentary tract were obtained alongside routine histopathological specimens taken with informed consent during surgery. The brain cortex sample was obtained from autopsy material. Each tissue sample was divided into several small pieces, 5–10 mm thick. The specimens were fixed for 6 hr in Carnoy’s fluid (absolute ethanol/chloroform/glacial acetic acid, 6:3:1), dehydrated, and embedded in paraffin in a vacuum oven at 58°C; sections of 5 μm thickness were placed on microscope slides.
HLA-H protein was located by the biotin–streptavidin complex method. The steps in the staining procedure were as follows: (i) pretreatment of the sections for 40 min with cow colostrum diluted 1:10 in PBS and rinsing in PBS, (ii) incubation for 1 hr with the primary antibody (2 μg IgG/microscope slide) in 1% bovine serum albumin in PBS (BSA-PBS), (iii) treatment with 1:10 diluted cow colostrum for 40 min and rinsing in PBS, (iv) incubation for 1 hr with biotinylated swine anti-rabbit IgG (Sigma) diluted 1:800 in 1% BSA-PBS, (v) incubation for 30 min with peroxidase-conjugated streptavidin (Sigma) diluted 1:500 in PBS, and (vi) incubation for 1.5 min in diaminobenzidine solution containing 9 mg 3,3′-diaminobenzidine tetrahydrochloride (Sigma) in 15 ml PBS plus 5 μl 30% H2O2. The sections were washed in PBS after incubation steps ii, iv, and v. All the incubations and washings were carried out at room temperature. The stained sections were examined with Nikon Labophot 2 and Zeiss Axioplan microscopes.
RESULTS
The HLA-H protein was expressed in some epithelial cells throughout the alimentary canal from the esophagus to the rectum. It was also expressed in subepithelial leukocytes. Three distinctly different subcellular localizations were seen: (i) staining of the entire plasma membrane in nonpolarized epithelial cells and leukocytes, (ii) staining restricted to the basolateral membranes in most polarized epithelial cells, and (iii) a unique pattern of intracellular, perinuclear staining in the epithelium of the duodenum, jejunum, and ileum.
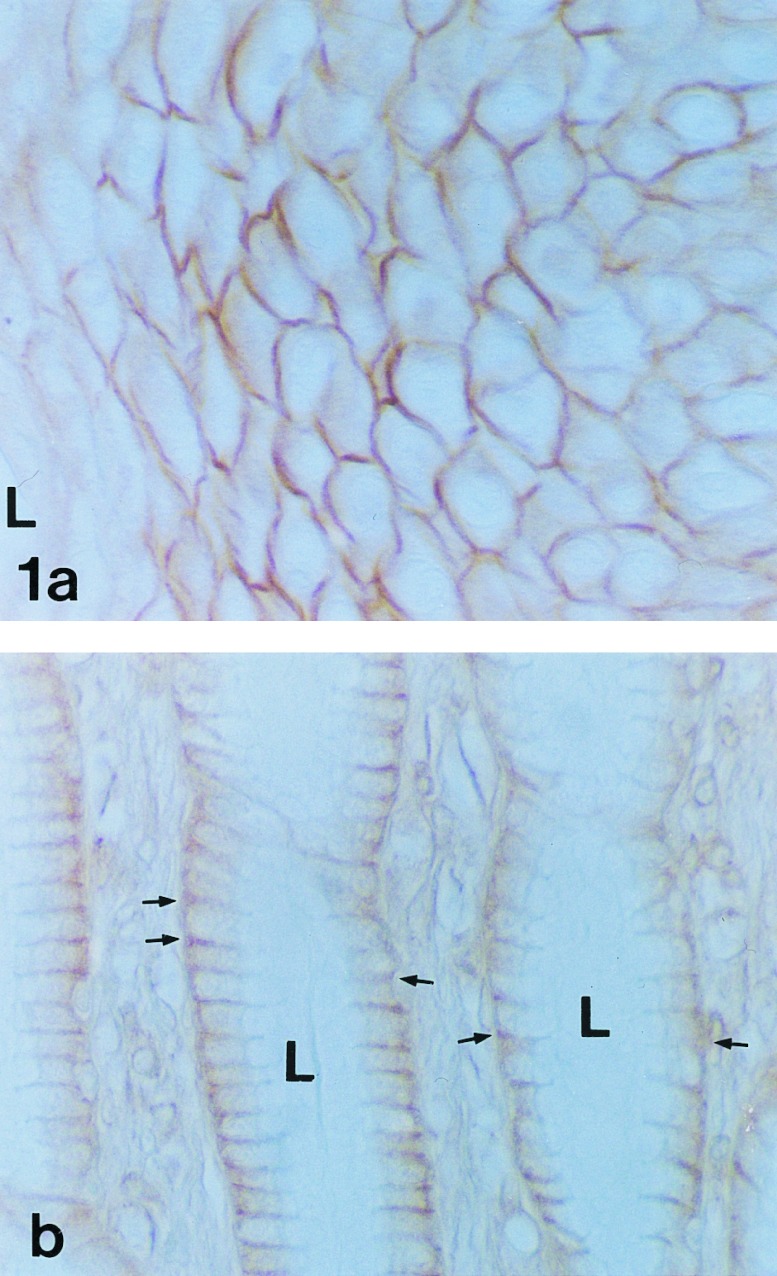
Fig. 1a shows HLA-H protein expression in the stratified squamous epithelial cells of the esophagus where the reaction product is distributed around the entire plasma membrane. No difference in the staining reaction was seen between the upper and lower segments of the esophagus (data not shown). By contrast, expression in the stomach is polarized as is evident in Fig. 1b. The positive reaction in gastric epithelial cells was restricted to the basolateral plasma membrane. The signal was generally more abundant in the pyloric antrum than in the body of the stomach (data not shown). The most intense reactions were present in the gastric pit and neck regions of the mucosa.
Figure 1.
Immunohistochemical demonstration of HLA-H protein in human esophagus (a) and pyloric antrum (b). In esophagus, the positive immunoreaction labels the entire plasma membrane of the stratified squamous epithelial cells. The luminal surface (L) is at the left. Only a partial thickness of the epithelium is shown. In contrast to the staining in esophagus, the polarized epithelial cells of the neck of the pyloric antrum show positive staining only in the basolateral plasma membrane (arrows). The apical plasma membranes facing the lumen (L) are not stained. (×400.)
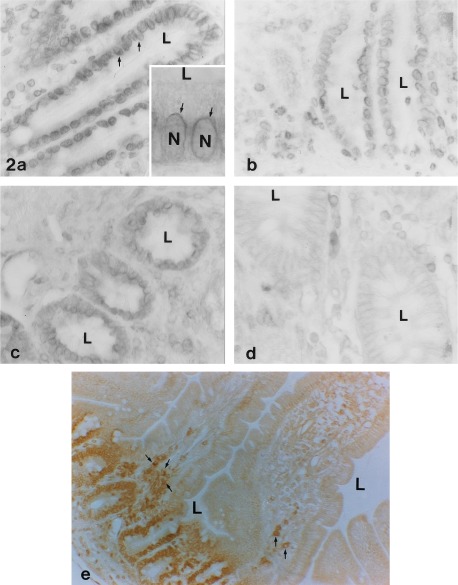
The staining pattern for the HLA-H protein changed dramatically in the small intestine. Not only was staining generally more intense in the duodenum, jejunum, and ileum (strongest in the duodenum) than that seen in stomach, the subcellular localization of the staining was quite different, being primarily intracellular and perinuclear. Fig. 2 a–c shows the strong perinuclear reaction, which was seen in all samples collected from the duodenum (Fig. 2a), jejunum (Fig. 2b), and ileum (Fig. 2c). Samples from eight unrelated subjects were examined, and all showed the same findings in the small intestine. Neither apical nor basolateral surfaces showed much reaction in any of the samples. The positive staining was most intense in the epithelial cells in crypts while the surface epithelial cells in upper portions and tips of the villi did not exhibit immunoreactivity (Fig. 2e). In contrast to the staining seen in the epithelial cells in the crypts, no staining was seen in the Brunner’s glands of the duodenum (see below, Fig. 4d).
Figure 2.
HLA-H protein immunostaining in small intestine and colon. Distinct perinuclear signals are seen in the absorptive epithelium of duodenum (arrows in a and Inset). The Inset in a is a higher magnification photo showing staining around nuclei (N). Similar perinuclear staining is seen in jejunum (b and e) and ileum (c). In all of these segments, the reaction is localized to the intestinal crypts. This is seen most clearly in a lower magnification view of jejunum (e). In ascending colon (d), the signal is weaker and limited to the basolateral plasma membrane of the epithelial cells. Subepithelial leukocytes also show positive immunoreaction (see arrows in e). L, lumen. (a–d, ×400; a Inset, ×800; e, ×200.)
Figure 4.
Specificity of HLA-H protein immunostaining demonstrated in pyloric antrum (a–c) and duodenum (d–f). Sections a and d were stained using anti-HLA-H protein antibody. The positive immunoreaction is seen in the surface epithelium of pyloric antrum, the cryptal epithelium of duodenum, and subepithelial leukocytes (small arrowheads). The positive reaction was blocked in both tissues by addition of the C-terminal peptide (50 μg/microscope slide) (b and e). Sections c and f show control staining with 1:100 diluted normal rabbit serum. Small arrowheads, leukocytes; large arrowheads, Brunner’s glands. (×200.)
The cecum, all segments of the colon, and the rectum were positive for HLA-H protein. Although it was expressed primarily in the deep crypts in the large intestine (Fig. 2d), the staining differed from that in the small intestine in that it was much weaker, and was confined to basolateral surfaces in the large intestine.
In the liver, staining for HLA-H protein was prominent in the basolateral plasma membranes of the bile ductular epithelium (Fig. 3a). In addition, a definite positive staining was seen in the sinusoidal lining cells. Staining for the HLA-H protein was strong in the epithelial cells of the gallbladder where (like the stomach and large intestine) the signal was primarily restricted to the basolateral plasma membrane (Fig. 3b). Although a positive signal was seen on a Northern blot with mRNA from pancreas (7), no immunoreactivity was seen in the cells of the exocrine or endocrine elements of the pancreas (data not shown).
Figure 3.
Immunoperoxidase staining of HLA-H protein in liver (a) and gallbladder (b). Basolateral membrane-associated immunoreaction product is seen in the bile ductular epithelial cells of the liver (large arrows). Staining is also present in the sinusoidal lining cells (arrowheads). In the gallbladder, positive staining is localized to the basolateral plasma membrane of the surface epithelial cells (small arrows). L, lumen. (a, ×200; b, ×400.)
Fig. 4 provides examples of controls for the specificity of the antibody for the HLA-H protein. Antibody-stained sections of pyloric antrum (Fig. 4a) showing basolateral staining of epithelial cells and nonpolarized staining around the entire plasma membrane in subepithelial leukocytes (Fig. 4a, arrowheads) and of duodenum (Fig. 4d) showing perinuclear staining were compared with sections exposed to antibody in the presence of blocking peptide (Fig. 4 b and e) and to nonimmune serum in place of antibody (Fig. 4 c and f). The positive staining seen in Fig. 4 a and d was blocked by the added peptide and no staining was seen with the nonimmune serum.
As additional controls, we examined tissues where expression of the HLA-H protein was not expected. Feder et al. (7) reported that no HLA-H mRNA was detected by Northern blots in brain. Using immunohistochemistry, cortical samples from brain were also negative for staining for the HLA-H protein (data not shown). Feder et al. (7) found very little HLA-H mRNA in lymphoblasts. The immunohistochemistry agrees with this finding, since lymphoid tissue in the pharyngeal tonsil was found to be largely negative for HLA-H staining (data not shown). Only occasional macrophages and polymorphonuclear phagocytes present in the sample showed a positive signal.
DISCUSSION
HH is a common autosomal recessive disorder characterized by increased intestinal iron absorption (or failure to down-regulate iron absorption) resulting in iron-overloading of the liver and other tissues (5, 6). Iron absorption normally occurs mainly in the duodenum and jejunum, but other parts of the gut have some absorptive capacity (5, 16). Four distinct steps can be distinguished in iron absorption: (i) binding and transport of iron across the luminal plasma membrane of the enterocyte, (ii) transport through the cytoplasm, (iii) transport across the basolateral membrane, and (iv) transport through the interstitial space to the submucosal capillaries. Iron transport in plasma is mainly served by transferrin, which carries iron from sites of release to sites of utilization and storage. Iron is stored intracellularly in the proteins ferritin and hemosiderin (16). Normally, most of the storage iron is in the form of ferritin and is mainly distributed in the liver, spleen, bone marrow, and muscle. In iron overload, the proportion of stored iron in the form of hemosiderin increases relative to that in ferritin (16).
One reason that the pathogenesis of HH is obscure is that the normal mechanisms involved in transporting iron through the enterocyte are poorly understood. Several early reports suggested that enterocytes absorb luminal iron by receptor-mediated endocytosis involving transferrin (17–20). However, recent studies have indicated that transferrin is not important for the first steps of iron absorption, even though it regulates the distribution of absorbed iron (21). This conclusion is also supported by the observations that patients with atransferrinemia develop iron overload rather than iron deficiency (22). Furthermore, several studies have shown that transferrin is not expressed by small intestinal cells and that the transferrin receptor is localized only in the basolateral plasma membrane of the cryptal epithelium (21, 23, 24).
Recently, Feder et al. (7) described a mutation, present in most HH patients, in a gene that is homologous to the major histocompatibility complex class I proteins and the human neonatal Fc receptor. This finding was surprising because the HLA-H protein does not resemble other iron-binding proteins. Although it is not obvious how the HLA-H protein might play a role in regulating iron absorption, the HLA-H protein might be indirectly involved through an interaction with another protein that itself binds iron and either acts as a ligand for the HLA-H protein or is regulated by the HLA-H protein.
Previous studies have demonstrated several candidate proteins, other than transferrin, which may participate in iron absorption through the luminal brush border and cytoplasm of the enterocyte. Melanotransferrin (p97) is an iron-binding membrane glycoprotein with 39% homology to transferrin (25) that is anchored to the plasma membrane through a glycosyl phosphatidylinositol moiety and is highly expressed in melanoma cells (26, 27). In normal tissues, melanotransferrin is expressed in umbilical cord, sweat gland ducts, liver sinusoidal lining cells, and apical brush border of epithelial cells in the fetal intestine (28–30). Another protein potentially involved in iron transport is a 54-kDa protein that has been purified from human intestinal microvillus membranes (31). Antibodies against this 54-kDa protein inhibit Fe(III) uptake by microvillus membrane vesicles from the duodenum by more than 50%. Furthermore, preliminary data indicated that this protein is highly expressed in duodenum and liver of patients with HH (6). Other intestinal iron-binding proteins include transmembrane β3-integrin (240 kDa), cytosolic mobilferrin (56 kDa), and cytosolic paraferritin (520-kDa protein complex) (6). Apparently, none of these is the same as the 54-kDa iron-binding protein located in the luminal plasma membrane of the enterocyte. Although the physiological function of none of these proteins has been established, their presence in the intestinal mucosa and their iron-binding capacity makes each of them a candidate to play some role in iron absorption in the gut. How the HLA-H protein might be related to one or more of these proteins remains to be established.
As a first step in understanding the physiological function of the HLA-H protein we raised a C-terminal peptide-specific antibody, which allowed us to study the distribution and cellular localization of the HLA-H protein. Immunohistochemistry indicated that, while it is widely expressed in the gastrointestinal tract, the most abundant staining occurs in the crypts of the small intestine, where it has a distinct and provocative subcellular localization.
On the other hand, its expression on basolateral surfaces of some epithelial cells in stomach, colon, and the biliary tract, and on sinusoidal lining cells of liver, raises the possibility that the HLA-H protein may have a different function at these sites. Perhaps its normal function in these sites is to serve as a barrier to iron transport, and loss of this function is a contributing factor in HH. It will now be of great interest to determine whether and how the level of expression and the subcellular distribution of the HLA-H protein in the gastrointestinal tract and liver are affected by the Cys-282 → Tyr mutation in HH patients as well as by other conditions associated with iron overload or iron deficiency.
Acknowledgments
This work was supported by Grant DK41816 from the U.S. Public Health Service to B.R.B., Grants DK40163 and GM34182 to W.S.S., and the Sigrid Juselius Foundation to S.P.
ABBREVIATION
- HH
hereditary hemochromatosis
References
- 1.Cartwright G E, Edwards C Q, Kravitz K, Skolnick M, Amos D B, Johnson A, Buskjaer L. N Engl J Med. 1979;301:175–179. doi: 10.1056/NEJM197907263010402. [DOI] [PubMed] [Google Scholar]
- 2.Borwein S T, Ghent C N, Flanagan P R, Chamberlain M J, Valberg L S. Clin Invest Med. 1983;6:171–179. [PubMed] [Google Scholar]
- 3.Edwards C Q, Griffen L M, Goldgar D, Drummond C, Skolnick M H, Kushner J P. N Engl J Med. 1988;318:1355–1362. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- 4.Bacon B R, Tavill A S. In: Hepatology: A Textbook of Liver Diseases. Zakim D, Boyer T D, editors. Philadelphia: Saunders; 1996. pp. 1439–1472. [Google Scholar]
- 5.Barton J C, Bertoli L F. Nat Med. 1996;2:394–395. doi: 10.1038/nm0496-394. [DOI] [PubMed] [Google Scholar]
- 6.Bonkovsky H L, Ponka P, Bacon B R, Drysdale J, Grace N D, Tavill A S. Hepatology. 1996;24:718–729. doi: 10.1002/hep.510240341. [DOI] [PubMed] [Google Scholar]
- 7.Feder J N, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy D A, et al. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 8.Jouanolle A M, Gandon G, Jézéquel P, Blayau M, Campion M L, Yaouanq J, Mosser J, Fergelot P, Chauvel B, Bouric P, Carn G, Andrieux N, Gicquel I, Le Gall J-Y, David V. Nat Genet. 1996;14:251–252. doi: 10.1038/ng1196-251. [DOI] [PubMed] [Google Scholar]
- 9.Jazwinska E C, Cullen L M, Busfield F, Pyper W R, Webb S I, Powell L W, Morris C P, Walsh T P. Nat Genet. 1996;14:249–251. doi: 10.1038/ng1196-249. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki J-I, Appella E, Ozato K. Proc Natl Acad Sci USA. 1986;83:757–761. doi: 10.1073/pnas.83.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sousa M, Reimao R, Lacerda R, Hugo P, Kaufmann S H E, Porto G. Immunol Lett. 1994;39:105–111. doi: 10.1016/0165-2478(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 12.Porto G, Reimao R, Goncalves C, Vicente C, Justica B, De Sousa M. Eur J Haematol. 1994;52:283–290. doi: 10.1111/j.1600-0609.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg B E, Voland J R. Proc Natl Acad Sci USA. 1996;93:1529–1534. doi: 10.1073/pnas.93.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waheed A, Hille A, Junghans U, von Figura K. Biochemistry. 1990;29:2449–2455. doi: 10.1021/bi00462a003. [DOI] [PubMed] [Google Scholar]
- 15.Parkkila S, Parkkila A-K, Juvonen T, Waheed A, Sly W S, Saarnio J, Kaunisto K, Kellokumpu S, Rajaniemi H. Hepatology. 1996;24:1104–1108. doi: 10.1002/hep.510240521. [DOI] [PubMed] [Google Scholar]
- 16.Bacon B R, Brown K E. In: Liver and Biliary Diseases. 2nd Ed. Kaplowitz N, editor. Baltimore: Williams & Wilkins; 1996. pp. 349–362. [Google Scholar]
- 17.Huebers H, Huebers E, Rummel W, Crichton R R. Eur J Biochem. 1976;66:447–455. doi: 10.1111/j.1432-1033.1976.tb10569.x. [DOI] [PubMed] [Google Scholar]
- 18.Bleil J D, Bretscher M S. EMBO J. 1982;1:351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huebers H A, Huebers E, Csiba E, Rummel W, Finch C A. Blood. 1983;61:283–290. [PubMed] [Google Scholar]
- 20.Dautry-Varsat A, Ciechanover A, Lodish H F. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson G J, Powell L W, Halliday J W. Gastroenterology. 1990;98:576–585. doi: 10.1016/0016-5085(90)90276-7. [DOI] [PubMed] [Google Scholar]
- 22.Goya N, Miyazaki S, Kodate S, Ushio B. Blood. 1972;40:239–245. [PubMed] [Google Scholar]
- 23.Banerjee D, Flanagan P R, Cluett J, Valberg L S. Gastroenterology. 1986;91:861–869. doi: 10.1016/0016-5085(86)90687-6. [DOI] [PubMed] [Google Scholar]
- 24.Levine D S, Woods J W. J Histochem Cytochem. 1990;38:851–858. doi: 10.1177/38.6.2186090. [DOI] [PubMed] [Google Scholar]
- 25.Rose T M, Plowman G D, Teplow D B, Dreyer W J, Hellstrom K E, Brown J P. Proc Natl Acad Sci USA. 1986;83:1261–1265. doi: 10.1073/pnas.83.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danielsen E M, van Deurs B. J Cell Biol. 1995;131:939–950. doi: 10.1083/jcb.131.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennard M L, Richardson D R, Gabathuler R, Ponka P, Jefferies W A. EMBO J. 1995;14:4178–4186. doi: 10.1002/j.1460-2075.1995.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown J P, Woodbury R G, Hart C E, Hellstrom I, Hellstrom K E. Proc Natl Acad Sci USA. 1981;78:539–543. doi: 10.1073/pnas.78.1.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Real F X, Furukawa K S, Mattes M J, Gusik S A, Cordon-Cardo C, Oettgen H F, Old L J, Lloyd K O. Proc Natl Acad Sci USA. 1988;85:3965–3969. doi: 10.1073/pnas.85.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciot R, De Vos R, van Eyken P, van der Steen K, Moerman P, Desmet V J. Liver. 1989;9:110–119. doi: 10.1111/j.1600-0676.1989.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 31.Teichmann R, Stremmel W. J Clin Invest. 1990;86:2145–2153. doi: 10.1172/JCI114953. [DOI] [PMC free article] [PubMed] [Google Scholar]