Abstract
Stable carbon isotope fractionations between dissolved inorganic carbon and lipid biomarkers suggest photoautotrophy by Chloroflexus-like organisms in sulfidic and nonsulfidic Yellowstone hot springs. Where co-occurring, cyanobacteria appear to cross-feed Chloroflexus-like organisms supporting photoheterotrophy as well, although the relatively small 13C fractionation associated with cyanobacterial sugar biosynthesis may sometimes obscure this process.
Chloroflexus aurantiacus and its phylogenetic relatives, which comprise a deeply branching kingdom-level lineage in the domain Bacteria (24), are major components of photosynthetic microbial mats in both sulfidic and nonsulfidic hot springs in Yellowstone National Park, Wyo. (3, 4, 5, 12, 23, 47). C. aurantiacus, the most studied representative of the green nonsulfur bacteria available in pure cultures, can grow heterotrophically by aerobic respiration, photoheterotrophically (using light to incorporate prereduced organic compounds), and photoautotrophically (using light to fix inorganic carbon) (26). Photoautotrophic metabolism by an obligately phototrophic relative of C. aurantiacus, the predominant phototroph in sulfidic hot-spring microbial mats, has been reported (12). However, based on culture (25, 26) and radiolabeling studies (2, 32), it has been hypothesized (44) that Chloroflexus-like organisms are mainly photoheterotrophic in mats where they live together with cyanobacteria. In such mats cyanobacteria are thought to be the main primary producers, which cross-feed reduced organic compounds to Chloroflexus-like organisms, thus supporting their photoheterotrophic metabolism. We exploited the distinctive lipid biomarkers of cyanobacteria and Chloroflexus-like organisms to test this hypothesis (39). If Chloroflexus-like organisms were purely photoheterotrophic in cyanobacterial mats, their biomarkers should have a δ13C signature, similar to biomarkers of cyanobacteria. Lipids derived from cyanobacteria in hot-spring mats were found to have δ13C values typical of the −20 to −25‰ fractionations relative to the inorganic carbon source expected of the Calvin cycle (27, 31). However, long-chain polyunsaturated alkenes (e.g., the C31:3 alkene hentriacontatriene) and C30-37 wax esters that are typical of Chloroflexus (17, 35, 40) and mats containing Chloroflexus relatives (9, 35, 37, 39) were approximately 10 to 15‰ enriched in 13C relative to cyanobacterial lipids (37, 39). Such values are expected based on autotrophic metabolism by Chloroflexus, which is known to use an inorganic carbon fixation pathway, the 3-hydroxypropionate pathway (15), that imparts an unusually heavy δ13C signature to both biomass and lipids (16, 40). Thus, the enrichment in 13C of Chloroflexus lipids relative to cyanobacterial lipids in microbial mats pointed towards autotrophic growth of Chloroflexus-like organisms in hot-spring microbial mats (39).
Here, we examine an alternative explanation—Chloroflexus-like biomarkers could have heavy δ13C signatures because they are cross-fed isotopically heavy fixed organic matter from cyanobacteria. Radiolabeling studies have shown that light-driven CO2 fixation in hot-spring cyanobacterial mats leads mainly to production of polysaccharides (18, 22), presumably by cyanobacteria, and the polysaccharides are fermented during darkness to short-chain fatty acids known to be photoassimilated by Chloroflexus-like organisms (2, 23, 32). It has been shown that sugars may be significantly enriched in 13C relative to lipids in cyanobacteria and other Calvin cycle photoautotrophs (7, 43). Hence, we now have significantly expanded our previous study by investigating not only the δ13C signatures of lipid biomarkers and biomass but also sugars and dissolved inorganic carbon (DIC) species to enable determination of isotopic fractionations. Furthermore, we included more representatives of mats where cyanobacteria and Chloroflexus-like organisms live together in different environments, thereby enabling us to better observe differences in isotopic fractionation patterns.
MATERIALS AND METHODS
Samples were taken from five different hot-spring microbial mats containing Chloroflexus-like bacteria with and without cyanobacteria located in Yellowstone National Park, Wyo. (Table 1). Samples for lipid analysis were frozen in the field and were kept frozen until lyophilization and lipid extraction. Samples for microscopy were stored on ice and directly analyzed after returning from the field. The presence of cyanobacteria and Chloroflexus-like bacteria was determined by phase contrast and autofluorescence microscopy. Water samples for sulfide analysis were collected from above each mat, preserved in zinc acetate, and analyzed by the method of Cline (6). Inorganic carbon in water overflowing mats was trapped as BaCO3 by increasing the pH of 200 ml of spring water to approximately pH 11 by adding a saturated NaOH solution (pH 13) and solid BaCl2 (36). From the stable carbon isotopic composition of the BaCO3, the isotopic composition of the CO2 in the spring water was calculated using the temperature-dependent isotopic equilibrium equation of Mook et al. (21).
TABLE 1.
General description of mat samples
| Mat samples | Geothermal area | Temp (°C) | pH | [S2−] (μM) | Chloroflexus-like | Cyanobacteria | Sampling dated |
|---|---|---|---|---|---|---|---|
| NMA sourcea | Mammoth Terraces | 58.9 | 6.1 | 133 | + | − | 05-12-1995 |
| NMA downstreama | Mammoth Terraces | 56.3 | 6.4 | 19 | + | + | 05-12-1995 |
| Tangerine Springb | Mammoth Terraces | 60 | 6.4 | 40 | + | + | 26-08-1999 |
| Mushroom Springc | Lower Geyser Basin | 64 | 8.3 | BDe | + | + | 25-08-1997 |
| Octopus Springc | Lower Geyser Basin | 58-64 | 8.3 | BD | + | + | 27-08-1997 |
Lipids were extracted, derivatized, and analyzed by using gas chromatography, gas chromatography-mass spectrometry, and isotope-ratio-monitoring gas chromatography-mass spectrometry (33). Cell-associated sugars were analyzed by using approximately 20 to 160 mg of microbial mat residue after lipid extraction. This material was hydrolyzed, and sugar monomers were derivatized and analyzed by gas chromatography, gas chromatography-mass spectrometry, and isotope-ratio-monitoring gas chromatography-mass spectrometry (42). Stable carbon isotopic compositions of the bulk cell material and BaCO3 were determined by automated on-line combustion (Carlo Erba CN analyser 1502 series) followed by conventional isotope ratio-mass spectrometry (Fisons optima [11]). The stable carbon isotope compositions are reported in the delta notation relative to the Vienna PeeDee Belemnite 13C standard.
RESULTS AND DISCUSSION
The mat samples studied are compared in Table 1. The New Mound Annex (NMA) mat, found in a sulfidic, high-carbonate hot spring at Mammoth Terraces, is comprised of Chloroflexus without cyanobacteria (39). The sulfide is thought to poison cyanobacteria but is utilized by Chloroflexus spp. in a photoautotrophic metabolism (12). All other mats are comprised of both cyanobacteria (Synechococcus) and Chloroflexus-like filamentous bacteria. The Tangerine Spring mat, like the downstream NMA mat (39), occurs in a slightly acidic, high-carbonate spring in the Mammoth Terraces group, where sulfide levels have been reduced due to its use by Chloroflexus spp. and other upstream sulfide-utilizing organisms. The Octopus Spring and Mushroom Spring mats occur in low-sulfide, alkaline silica-rich springs of the Lower Geyser Basin.
Lipid and sugar composition.
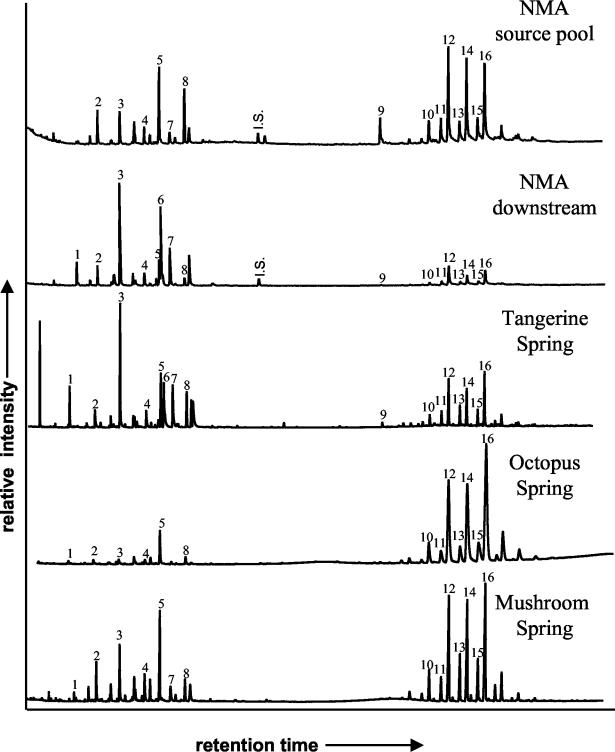
The lipid compositions of the five mat samples are compared in Fig. 1 and Table 2, which also includes information for cultures of C. aurantiacus and its phylogenetic relative, Roseiflexus castenholzii (13), for reference. Lipids characteristic of Chloroflexus-like organisms were abundant in all mats. For instance, wax esters ranging from C31 to C37 were among the predominant lipids in all mats, with small-scale variation in distribution pattern among mat samples. Differences in the carbon skeletons of the wax esters between the environmental samples and the cultures may reflect physiological differences between cultivated and natural populations. For instance, the different mat systems do not contain monounsaturated wax esters as does C. aurantiacus but rather contain iso-branched wax esters (35, 39). Also, the mats contain C31 to C37 wax esters, whereas R. castenholzii produces C37 to C40 wax esters (41). Long-chain (C29-32) alkenes, predominantly hentriacontatriene (C31:3) (38), were abundant in mats in Mammoth Terraces springs but were present at only trace levels in mats from the Lower Geyser Basin. This may reflect the predominance of Chloroflexus spp. in Mammoth Terraces (46) and more distantly related Roseiflexus-like organisms in springs of the Lower Geyser Basin (23, 30), since the latter organism lacks hentriacontatriene (41). C17 n-alkane, a biomarker for cyanobacteria (34), was found in all mats containing cyanobacteria but was absent from the NMA source mat. More common lipids, such as C15-18 fatty acids and C17-18 alcohols, were also detected in all mats.
FIG. 1.
Partial gas chromatograms of the total lipid extracts from the investigated microbial mats. 1, C17 n-alkane; 2, C15 fatty acid; 3, C16 fatty acid; 4, C17 fatty acid; 5, C17 alkanol; 6, C18:1 fatty acid; 7, C18 fatty acid; 8, C18 alkanol; 9, C31:3 alkene; 10, C31 wax ester; 11, C32 iso-wax ester; 12, C32 wax ester; 13, C33 iso-wax ester; 14, C33 wax ester; 15, C34 iso-wax ester; 16, C34 wax ester; I.S., internal standard.
TABLE 2.
Relative abundance of different lipids within mat samples
| Category or compound | Peak no.a | Relative abundanceb
|
||||||
|---|---|---|---|---|---|---|---|---|
| NMA sourcec | NMA downstreamd | Tangerine Spring | Octopus Spring | Mushroom Spring | C. aurantiacusd | R. castenholziie | ||
| Chloroflexus biomarkers | ||||||||
| C31:3 alkene | 9 | ++ | + | + | Trace | Trace | ++ | − |
| n-C31 wax ester | 10 | ++ | + | + | ++ | ++ | − | |
| iso-C31 wax ester | + | + | + | + | + | |||
| n-C32 wax ester | 12 | +++ | ++ | ++ | +++ | +++ | + | |
| iso-C32 wax ester | 11 | + | + | + | + | + | ||
| C32:1 wax ester | + | |||||||
| n-C33 wax ester | 14 | +++ | ++ | ++ | +++ | +++ | + | |
| iso-C33 wax ester | 13 | + | + | + | + | + | ||
| n-C34 wax ester | 16 | +++ | ++ | ++ | +++ | +++ | ++ | |
| iso-C34 wax ester | 15 | + | + | + | + | + | ||
| C34:1 wax ester | + | |||||||
| n-C35 wax ester | + | + | + | ++ | ++ | ++ | ||
| n-C36 wax ester | + | − | + | + | + | ++ | ||
| C36:1 wax ester | + | |||||||
| n-C37 wax ester | + | − | + | + | + | + | + | |
| n-C38 wax ester | ++ | |||||||
| n-C39 wax ester | + | |||||||
| n-C40 wax ester | ++ | |||||||
| Cyanobacterial biomarker | ||||||||
| C17n-alkane | 1 | − | ++ | ++ | + | + | − | − |
| Nondiagnostic lipids | ||||||||
| C15 fatty acid | 2 | ++ | ++ | + | + | ++ | + | − |
| C16 fatty acid | 3 | ++ | +++ | +++ | + | ++ | +++ | − |
| C17 fatty acid | 4 | + | + | + | + | ++ | +++ | − |
| C17 alkanol | 5 | +++ | ++ | ++ | ++ | +++ | + | − |
| C18:1 fatty acid | 6 | − | +++ | ++ | − | − | +++ | − |
| C18 fatty acid | 7 | + | ++ | ++ | + | + | +++ | − |
| C18 alkanol | 8 | +++ | + | ++ | + | ++ | ++ | − |
The sugar fractions of all mats contained arabinose, xylose, rhamnose, and glucose, the latter being dominant (approximately 30 to 80% of the sugar fraction). The sugar distribution was similar to that reported for C. aurantiacus (40).
Stable carbon isotopic compositions.
The isotopic compositions of DIC, bulk biomass, lipids, and sugars for all mats are reported in Table 3. The values for DIC species determined for Tangerine Spring was within the range previously reported for other Mammoth Terraces hot springs (19). The values for Mushroom Spring were somewhat lower. Isotopic composition of bulk biomass ranged from −13 to −17‰, except for the NMA downstream and Tangarine Spring mats, which were isotopically lighter (−24 and −25‰, respectively). Isotopic compositions of specific lipids ranged from −8.9 to −36.3‰. Compounds known from or possibly contributed by cyanobacteria (e.g., C17 n-alkane and C16 and C18 fatty acids, respectively) were isotopically lighter (−21.3 to −36.3‰) than biomarkers of Chloroflexus-like organisms (C31:3 and wax esters; −8.9 to −27.0‰). The isotopic composition of glucose ranged from −5.1 to −21.8‰. Isotopic compositions in cyanobacterial mats were generally more depleted in 13C in springs from Mammoth Terraces (Tangerine and NMA downstream) than in springs from the Lower Geyser Basin (Octopus Spring and Mushroom Spring).
TABLE 3.
Stable carbon isotopic compositions of bicarbonate, CO2 (calculated from bicarbonate), bulk biomass, Chloroflexus and cyanobacterial biomarkers, nondiagnostic lipids, and glucose in mat samples expressed in per mille relative to the PeeDee Belemnite standard
| Category or compound | Peak no.a | δ13C values of samples
|
|||||
|---|---|---|---|---|---|---|---|
| NMA sourceb | NMA downstreamb | Tangerine Spring | Octopus Spring | Mushroom Spring | C. aurantiacusc | ||
| Bicarbonate | −2 to −4d | −2 to −4d | −3.3 | ∼6.8e | −6.8 | 0 | |
| CO2f | ∼9 | ∼9 | ∼9 | ∼12 | ∼11 | ||
| Bulk biomass | −14.9 | −23.5 | −24.8 | −16.9 | −13.2 | −13.0 | |
| Chloroflexus biomarkers | |||||||
| C31:3 alkene | 9 | −8.9 | −15.1 | −20.0 | −18.9g | NMh | −12.5 |
| C32 wax ester | 12 | −17.7 | −22.7 | −26.0 | −18.3 | −16.5 | NM |
| C33 wax ester | 14 | −17.9 | −24.1 | −27.0 | −18.5 | −16.4 | −15.0 |
| C34 wax ester | 16 | −17.4 | −23.4 | −26.7 | −18.5 | −16.5 | −14.4 |
| Cyanobacterial biomarker | |||||||
| C17 n-alkane | 1 | −36.3 | −36.3 | −34.1 | −29.6 | ||
| General lipids | |||||||
| C15 fatty acid | 2 | −19.4 | −23.4 | −27.1 | −20.2 | −17.5 | NM |
| C16 fatty acid | 3 | −19.3 | −34.5 | −35.3 | −21.3 | −21.3 | −13.8 |
| C17 fatty acid | 4 | −18.9 | −22.1 | −27.6 | −18.7 | −16.8 | −14.8 |
| C17 alkanol | 5 | −18.0 | −22.0 | −27.9 | −19.4 | −15.5 | NM |
| C18:1 fatty acid | 6 | −32.7 | −35.5 | −12.5 | |||
| C18 fatty acid | 7 | −16.0 | −33.9 | −35.1 | NM | −24.4 | −13.6 |
| C18 alkanol | 8 | −16.1 | −24.3 | −29.1 | −22.2 | −21.5 | −12.5 |
| Sugar | |||||||
| Glucose | −5.1 | −21.8 | −20.0 | −11.0 | −11.5 | −5.7 | |
See Figure 1.
Previously reported by van der Meer et al. (39), except for the glucose isotope values.
From van der Meer et al. (40). Isotope values for organic compounds are reported relative to bicarbonate.
Madigan et al. (19).
Assuming similar δ13C value as for Mushroom Spring.
Calculated based on temperature-dependent isotope equilibrium equation (21).
Partial coelution.
NM, not measured.
Comparison of 13C fractionation in mats and cultures.
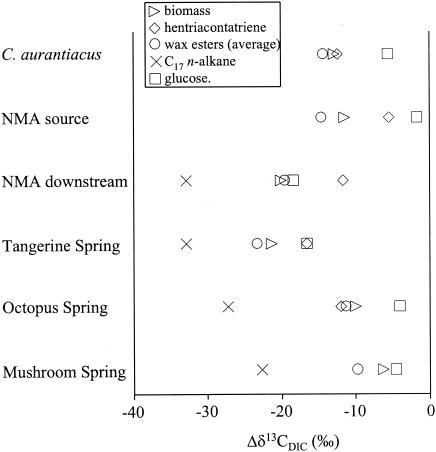
Isotopic fractionations between bicarbonate, the principle DIC species, and bulk biomass, lipids, and glucose for all mat samples and C. aurantiacus are compared in Fig. 2. The 13C fractionations between biomass, lipids, and glucose relative to DIC are similar for the photoautotrophically grown C. aurantiacus culture and the Chloroflexus spp.-dominated NMA source pool microbial mat (Fig. 2). The smallest 13C fractionation relative to DIC (Δδ13CDIC) was observed for glucose, followed by hentriacontatriene, the bulk cell material, the fatty acids, and wax esters. This confirms earlier observations of Chloroflexus photoautotrophy in these high-sulfide, Chloroflexus-dominated microbial mats (12, 39) and shows that carbon isotopic observations made in culture experiments can be extended to environmental settings.
FIG. 2.
Depletions in 13C of biomass, key biomarkers, and glucose relative to DIC. ▹, biomass; ◊, hentriacontatriene; ○, wax esters (average); ×, C17 n-alkane; □, glucose.
Chloroflexus biomarkers showed much larger fractionation relative to DIC in the two high-carbonate mats (NMA downstream and Tangerine Spring) where they live together with cyanobacteria (Fig. 2). This indicates that Chloroflexus-like organisms are not purely autotrophic in these cyanobacterial mats. Some isotopically lighter carbon must be obtained from an alternative source. The large Δδ13CDIC for the cyanobacterial biomarker C17 n-alkane suggests cyanobacteria as a possible source for this light carbon. We considered whether cyanobacterial glucose synthesis and fermentation coupled with cross-feeding could explain the observed isotopic fractionation patterns. The Δδ13CDIC of glucose in these two high-carbonate mats is smaller than that observed for the cyanobacterial biomarker, C17 n-alkane, consistent with the possibility that cyanobacterial glucose biosynthesis imparts a heavier isotopic signature than lipid biosynthesis (7, 43). However, even in the unlikely event that all of the glucose detected was from cyanobacteria and Chloroflexus derived all of its carbon from cyanobacterial sugar fermentation, the δ13C value for all Chloroflexus biomarkers should be lower than the δ13C of glucose, due to isotopic effects of subsequent lipid biosynthesis pathways (1, 14, 20). The fact that the δ13C value of the C31:3 alkane is higher than (NMA downstream) or similar to (Tangarine Spring) that of glucose and that the δ13C value of wax esters (NMA downstream) is similar to that of glucose therefore suggests that cyanobacterial cross-feeding alone is unlikely to explain the observed results. Apparently, photoautotrophy is also occurring in Chloroflexus spp. in these mats. This could reflect either the inputs of separate heterotrophic and autotrophic Chloroflexus populations or mixotrophic carbon metabolism in a single Chloroflexus population. Since both cyanobacteria and Chloroflexus-like organisms contribute organic compounds to the mats, the δ13C values for the bulk biomass and glucose must be intermediate to cyanobacterial and autotrophic Chloroflexus isotope signatures.
Relative to the Mammoth Terraces cyanobacterial mats, the two mats in alkaline siliceous springs show smaller Δδ13CDIC values (Fig. 2). The fractionation pattern of the bulk biomass, glucose, Chloroflexus biomarkers, and more general lipids resembles that of the autotrophically grown C. aurantiacus culture and the NMA source pool mat, suggesting the possibility of photoautotrophic metabolism by Chloroflexus relatives. However, in these mats the δ13C values of glucose are sufficiently heavy to support the hypothesis that cyanobacterial sugar biosynthesis imparts a heavier isotopic signature to sugars than to lipids; cross-feeding of sugar fermentation products could then impart the heavier signatures of Chloroflexus biomarkers. A complicating factor in Octopus Spring and Mushroom Spring could be the effect of CO2 limitation on stable carbon isotope fractionation by cyanobacteria in these much more alkaline and lower-DIC settings (8). The Mammoth Terraces mats occur in carbonate-depositing springs that are high in DIC and, at pH 6.4, are poised near the pKa of H2CO3/HCO3− (i.e., CO2 is readily available). In contrast, the mats in alkaline silicious springs of the Lower Geyser Basin have midday pHs as high as 9.4, approaching the pKa of HCO3−/CO32− (29). CO2 limitation in these alkaline silicious hot springs may decrease the degree of isotopic fractionation by cyanobacteria, resulting in higher δ13C values for all organic compounds produced by cyanobacteria, including the C17 n-alkane and glucose. However, the very large differentials in δ13C values of C17 n-alkane and glucose (18 to 22.4‰) exceed those observed so far in Calvin cycle organisms (1 to 16‰ [43]). This large isotopic difference between the cyanobacterial biomarker and glucose, especially in combination with very small or no isotopic fractionation between CO2 and glucose, makes it unlikely that all of the glucose is derived from cyanobacteria even when the possible effect of CO2 limitation on the stable carbon isotope ratios of cyanobacterial products is considered. The high abundance of Chloroflexus biomarkers (i.e., wax esters) relative to cyanobacterial biomarkers (i.e., C17 n-alkane) (Fig. 1) and the heavier isotopic signatures of C16 and C18 fatty acids (Table 3), which could be contributed by either type of phototroph (10, 17, 35, 40), indeed suggest that a large fraction of the total biomass might be Chloroflexus derived. This might be due to our analysis of thicker mat samples (i.e., >1 cm for Lower Geyser Basin mats versus 1 to 2 mm for Mammoth Terraces mats) and the persistence of Chloroflexus carbohydrates in deeper layers of the mats in alkaline siliceous springs (39).
By comparing isotopic compositions of compound classes from replicate cyanobacterial mats from different hot-spring settings, we were able to observe that both autotrophic and heterotrophic carbon metabolisms are employed by Chloroflexus-like bacteria. Heavier isotopic signatures of Chloroflexus-like bacteria may be due in part to their unique autotrophic biochemistry and in part to the differences in isotopic fractionation in sugar and lipid biosynthetic pathways of cyanobacteria. Further work will be necessary, however, in order to observe the degree to which cyanobacterial sugar biosynthesis affects isotopic compositions and, thus, the relative importance of heterotrophy (via cross-feeding from cyanobacteria) and autotrophy in the carbon metabolism of Chloroflexus-like bacteria in these mats. This is especially true of mats in alkaline siliceous springs, where CO2 limitation effects make resolution of the two types of carbon metabolism more difficult.
Acknowledgments
We thank M. M. Bateson and B. Lindstrom for help with field work and R. Pancost, B. E. van Dongen, W. I. C. Rijpstra, M. Baas, R. Kloosterhuis, and J. Ossebaar for analytical assistance. We thank The U.S. National Park Service for permission to conduct research in Yellowstone National Park.
This study was supported by U.S. National Aeronautics and Space Administration grants NAGW-2764 and NAG5-3652 and a PIONIER grant awarded to J. S. Sinninghe Damsté by The Netherlands Organization for Scientific Research (NWO). We also thank NWO for supporting travel of M. T. J. van der Meer. We thank Shell International Petroleum Maatschappij BV for financial support for the GC-irMS facility.
Footnotes
NIOZ contribution no. 3630; journal series no. 2003-27, Montana Agricultural Experiment Station, Montana State University—Bozeman.
REFERENCES
- 1.Abraham, W. R., C. Hesse, and O. Pelz. 1998. Ratios of carbon isotopes in microbial lipids as an indicator of substrate usage. Appl. Environ. Microbiol. 64:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, K. L., T. A. Tayne, and D. M. Ward. 1987. Formation and fate of fermentation products in hot-spring cyanobacterial mats. Appl. Environ. Microbiol. 53:2343-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauld, J., and T. D. Brock. 1973. Ecological studies of Chloroflexis, a gliding photosynthetic bacterium. Arch. Microbiol. 92:267-284. [Google Scholar]
- 4.Boomer, S. M., D. P. Lodge, B. E. Dutton, and B. I. Pierson. 2002. Molecular characterization of novel red green nonsulfur bacteria from five distinct hot spring communities in Yellowstone National Park. Appl. Environ. Microbiol. 68:346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castenholz, R. W. 1973. The possible photosynthetic use of sulfide by the filamentous phototrophic bacteria of hot springs. Limnol. Oceanogr. 18:863-876. [Google Scholar]
- 6.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 7.Deines, P. 1980. The isotopic composition of reduced organic carbon, p. 329-406. In P. Fritz and J. C. Fontes (ed.), Handbook of environmental isotope geochemistry. Elsevier Scientific Publishing Company, Amsterdam, The Netherlands.
- 8.DesMarais, D. J., J. Bauld, A. C. Palmisano, R. E. Summons, and D. M. Ward. 1992. The biogeochemistry of carbon in modern microbial mats, p. 299-308. In J. W. Schopf and C. Klein (ed.), The proterozoic biosphere: a multidisciplinary study. Cambridge University Press, Cambridge, United Kingdom.
- 9.Dobson, G., D. M. Ward, N. R. Robinson, and G. Eglinton. 1988. Biogeochemistry of hot spring environments: free lipids of a cyanobacterial mat. Chem. Geol. 68:155-179. [Google Scholar]
- 10.Fork, D. C., N. Murata, and N. Sato. 1979. Effect of growth temperature on the lipid and fatty acid composition, and the dependence on temperature of light-induced redox reactions of cytochrome f and of light energy redistribution in the thermophilic blue-green alga Synechococcus lividus. Plant Physiol. 63:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry, B., W. Brand, F. J. Mensch, K. Tholke, and R. Garritt. 1992. Automated analysis system for coupled δ13C and δ15N measurements. Anal. Chem. 64:288-291. [Google Scholar]
- 12.Giovannoni, S. J., N. P. Revsbech, D. M. Ward, and R. W. Castenholz. 1987. Obligately phototrophic Chloroflexus: primary production in anaerobic hot spring microbial mats. Arch. Microbiol. 147:80-87. [Google Scholar]
- 13.Hanada, S., S. Takaichi, K. Matsuura, and K. Nakamura. 2002. Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium which lacks chlorosomes. Int. J. Syst. Evol. Microbiol. 52:187-193. [DOI] [PubMed] [Google Scholar]
- 14.Hayes, J. M. 2001. Fractionation of carbon and hydrogen isotopes in biosynthetic processes, p. 225-277. In J. W. Valley and D. Cole (ed.), Stable isotope geochemistry. Reviews in mineralogy and geochemistry, vol. 43. Mineralogical Society of America, Washington, D.C.
- 15.Herter, S., G. Fuchs, A. Bacher, and W. Eisenreich. 2002. A bicyclic autotrophic CO2 fixation pathway in Chloroflexus aurantiacus. J. Biol. Chem. 277:20277-20283. [DOI] [PubMed] [Google Scholar]
- 16.Holo, H., and R. Sirevåg. 1986. Autotrophic growth and CO2 fixation of Chloroflexus aurantiacus. Arch. Microbiol. 145:173-180. [Google Scholar]
- 17.Knudsen, E., E. Jantzen, K. Bryn, J. G. Ormerod, and R. Sirevåg. 1982. Quantitative and structural characteristics of lipids in Chlorobium and Chloroflexus. Arch. Microbiol. 132:149-154. [Google Scholar]
- 18.Konopka, A. 1992. Accumulation and utilization of polysaccharide by hot-spring phototrophs during a light-dark transition. FEMS Microb. Ecol. 102:27-32. [Google Scholar]
- 19.Madigan, M. T., R. Takigiku, R. G. Lee, H. Gest, and J. M. Hayes. 1989. Carbon isotope fractionation by thermophilic phototrophic sulfur bacteria: evidence for autotrophic growth in natural populations. Appl. Environ. Microbiol. 55:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monson, K. D., and J. M. Hayes. 1982. Carbon isotopic fractionation in the biosynthesis of bacterial fatty acids. Ozonolysis of unsaturated fatty acids as a means of determining the intramolecular distribution of carbon isotopes. Geochim. Cosmochim. Acta 46:139-149. [Google Scholar]
- 21.Mook, W. G., J. C. Bommerson, and W. H. Staberman. 1974. Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sci. Lett. 22:169-176. [Google Scholar]
- 22.Nold, S. C., and D. M. Ward. 1996. Photosynthate partitioning and fermentation in hot spring microbial mat communities. Appl. Environ. Microbiol. 62:4598-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nübel, U., M. M. Bateson, V. Vandieken, M. Kühl, and D. M. Ward. 2002. Microscopic examination of distribution and phenotypic properties of phylogenetically diverse Chloroflexaceae-related bacteria in hot spring microbial mats. Appl. Environ. Microbiol. 68:4593-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyaizu, H., B. Debrunner-Vossbrinck, L. Mandelco, J. A. Studier, and C. R. Woese. 1987. The green non-sulfur bacteria: a deep branching in the eubacterial line of descent. Syst. Appl. Microbiol. 9:47-53. [DOI] [PubMed] [Google Scholar]
- 25.Pierson, B. K., and R. W. Castenholz. 1974. Studies of pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium. Arch. Mikrobiol. 100:283-305. [DOI] [PubMed] [Google Scholar]
- 26.Pierson, B. K. and R. W. Castenholz. 1992. The family Chloroflexaceae, p. 3754-3774. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 27.Popp, B. N., E. A. Laws, R. R. Bridigare, J. E. Dore, K. L. Hanson, and S. G. Wakeham. 1998. Effect of phytoplankton cell geometry on carbon isotopic fractionation. Geochim. Cosmochim. Acta 62:69-77. [Google Scholar]
- 28.Ramsing, N. B., M. J. Ferris, and D. M. Ward. 2000. Highly ordered vertical structure of Synechococcus populations within the one-millimeter-thick photic zone of a hot spring cyanobacterial mat. Appl. Environ. Microbiol. 66:1038-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revsbech, N. P., and D. M. Ward. 1984. Microelectrode studies of interstitial water chemistry and photosynthetic activity in a hot spring microbial mat. Appl. Environ. Microbiol. 48:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruff-Roberts, A. L., J. G. Kuenen, and D. M. Ward. 1994. Distribution of cultivated and uncultivated cyanobacteria and Chloroflexus-like bacteria in hot spring microbial mats. Appl. Environ. Microbiol. 60:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakata, S., J. M. Hayes, A. R. McTaggart, R. A. Evans, K. J. Leckrone, and R. K. Togasaki. 1997. Carbon isotopic fractionation associated with lipid biosynthesis by a cyanobacterium: relevance for interpretation of biomarker records. Geochim. Cosmochim. Acta 61:5379-5389. [DOI] [PubMed] [Google Scholar]
- 32.Sandbeck, K. A., and D. M. Ward. 1981. Fate of immediate methane precursors in low sulfate hot spring algal-bacterial mats. Appl. Environ. Microbiol. 41:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schouten, S., W. C. M. Klein Breteler,P. Blokker, N. Schogt, W. I. C. Rijpstra, K. Grice, M. Baas, and J. S. Sinninghe Damsté. 1998. Biosynthetic effects on the stable carbon isotopic composition of algal lipids: implications for deciphering the carbon isotopic biomarker record. Geochim. Cosmochim. Acta 62:1397-1406. [Google Scholar]
- 34.Shiea, J., S. C. Brassell, and D. M. Ward. 1990. Mid-chain branched mono- and dimethyl alkanes in hot spring cyanobacterial mats: a direct biogenic source for branched alkanes in ancient sediments? Org. Geochem. 15:223-231. [Google Scholar]
- 35.Shiea, J., S. C. Brassell, and D. M. Ward. 1991. Comparative analysis of extractable lipids in hot spring microbial mats and their component photosynthetic bacteria. Org. Geochem. 17:309-319. [Google Scholar]
- 36.Simon, H., and H. G. Floss. (1967). Bestimmung der Isotopenverteilung in markierten Verbindungen. Springer, Berlin, Germany.
- 37.Summons, R. E., L. L. Jahnke, and B. R. T. Simoneit. 1996. Lipid biomarkers for bacterial ecosystems: studies of cultured organisms, hydrothermal environments and ancient sediments, p. 174-194. In G. R. Block and J. A. Goode (ed.), Evolution of hydrothermal ecosystems on Earth (and Mars?). Ciba Foundation Symposium no. 202. Wiley, Chichester, United Kingdom. [DOI] [PubMed]
- 38.Van der Meer, M. T. J., S. Schouten, D. M. Ward, J. A. J. Geenevasen, and J. S. Sinninghe Damsté. 1999. All-cis hentriaconta-9,15,22-triene in microbial mats formed by the phototrophic prokaryote Chloroflexus. Org. Geochem. 30:1585-1587. [DOI] [PubMed] [Google Scholar]
- 39.Van der Meer, M. T. J., S. Schouten, J. W. de Leeuw, and D. M. Ward. 2000. Autotrophy of green non-sulphur bacteria in hot spring microbial mats: biological explanations for isotopically heavy organic carbon in the geological record. Env. Microbiol. 2:428-435. [DOI] [PubMed] [Google Scholar]
- 40.Van der Meer, M. T. J., S. Schouten, B. E. van Dongen, W. I. C. Rijpstra, G. Fuchs, J. S. Sinninghe Damsté, J. W. de Leeuw, and D. M. Ward. 2001. Biosynthetic controls on the 13C-contents of organic components in the photoautotrophic bacterium Chloroflexus aurantiacus. J. Biol. Chem. 276:10971-10976. [PubMed] [Google Scholar]
- 41.Van der Meer, M. T. J., S. Schouten, S. Hanada, E. C. Hopmans, S. Sinninghe Damsté, and D. M. Ward. 2002. Alkane-1,2-diol-based glycosides and fatty glycosides and wax esters in Roseiflexus castenholzii and hot spring microbial mats. Arch. Microbiol. 178:229-237. [DOI] [PubMed] [Google Scholar]
- 42.Van Dongen, B. E., S. Schouten, and J. S. Sinninghe Damsté. 2001. Gas chromatography/combustion/isotope-ratio-monitoring mass spectrometric analysis of methylboronic derivatives of monosaccharides: a new method for determining natural 13C abundances of carbohydrates. Rapid Commun. Mass Spectrom. 15:496-500. [DOI] [PubMed] [Google Scholar]
- 43.Van Dongen, B. E., S. Schouten, and J. S. Sinninghe Damsté. 2002. Carbon isotopic variability in algal and terrestrial carbohydrates. Mar. Ecol. Prog. Ser. 232:83-92. [Google Scholar]
- 44.Ward, D. M., T. A. Tayne, K. L. Anderson, and M. M. Bateson. 1987. Community structure, and interactions among community members in hot spring cyanobacterial mats. Symp. Soc. Gen. Microbiol. 41:179-210. [Google Scholar]
- 45.Ward, D. M., R. Weller, J. Shiea, R. W. Castenholz, and Y. Cohen. 1989. Hot spring microbial mats: anoxygenic and oxygenic mats of possible evolutionary significance, p. 3-15. In Y. Cohen and E. Rosenberg (ed.), Microbial mats: physiological ecology of benthic microbial communities. American Society for Microbiology, Washington, D.C.
- 46.Ward, D. M., C. M. Santegoeds, S. C. Nold, N. B. Ramsing, M. J. Ferris, and M. M. Bateson. 1997. Biodiversity within hot spring microbial mat communities: molecular monitoring of enrichment cultures. Antonie Leeuwenhoek 71:143-150. [DOI] [PubMed] [Google Scholar]
- 47.Ward, D. M., M. J. Ferris, S. C. Nold, and M. M. Bateson. 1998. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 62:1353-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]