Abstract
The free-living amoeboflagellate Naegleria fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), a rapidly fatal disease of the central nervous system. In the United States, the disease is generally acquired while swimming and diving in freshwater lakes and ponds. In addition to swimming, exposure to N. fowleri and the associated disease can occur by total submersion in bathwater or small backyard wading pools. In the present study, swipe samples and residual pipe water from homes in Arizona were examined for N. fowleri by nested PCR due to the death of two previously healthy children from PAM. Since neither child had a history of swimming in a freshwater lake or pond prior to the onset of disease symptoms, the domestic water supply was the suspected source of infection. Of 19 samples collected from bathroom and kitchen pipes and sink traps, 17 samples were positive for N. fowleri by PCR. A sample from a Micro-Wynd II filter was obtained by passing water from bathtubs through the filter. Organisms attached to the filter also tested positive by PCR. The two samples that tested negative for N. fowleri were one that was obtained from a kitchen sink trap and a swipe sample from the garbage disposal of one home.
The genus Naegleria is composed of a group of free-living amoeboflagellates that are distributed worldwide. Although several species have been identified, only one, Naegleria fowleri, has been associated with human disease. N. fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), a fatal disease of the central nervous system (8, 26, 29, 30, 44). N. fowleri has been isolated from a variety of water sources, including domestic water supplies (1-3, 8, 10, 16), recreational water facilities (6, 7, 9, 11, 19, 20, 43, 45, 46), and thermally polluted water from industrial sources (13, 21, 36, 42). The presence of N. fowleri in environmental water has been linked to temperature, pH, coliforms, and the amount of organic matter present (17, 18, 36). Iron and iron-containing compounds in water favor growth of N. fowleri (22).
Although PAM is not common, cases of the disease have been reported in almost every continent. Indoor swimming pools have been the source of PAM in Czechoslovakia (9). In Mexico, five cases of PAM were associated with swimming in shallow water in an artificial canal (23). In South Australia, cases of PAM have occurred through the domestic water supply. These cases occurred during the summer months in children submerged in bathtubs and wading pools (1-3, 16, 31). In Great Britain, PAM was acquired from mud puddles in which children played after a heavy rainstorm (4). The majority of cases of PAM have been reported in the United States, and these cases have occurred in previously healthy young adults and children associated with water sports (7, 12, 14, 15, 19, 28-30, 40, 45). The presence of N. fowleri in the environment may present a risk to human health. Indeed, there has been an increase in the number of cases of PAM reported in recent years (25).
The correct identification of N. fowleri is difficult because several genera of amoebae found in the same ecological habitat are morphologically similar (33, 39). Furthermore, pathogenic N. fowleri and nonpathogenic Naegleria lovaniensis are antigenically related (39). Therefore, a sensitive and specific nested-PCR assay was developed in our laboratory to identify N. fowleri in water and soil samples (35; R. MacLean, D. J. Richardson, R. LePardo, and F. Marciano-Cabral, submitted for publication). The nested-PCR assay has been used previously to document the occurrence of N. fowleri in environmental samples collected in Connecticut and Virginia (MacLean et al., submitted).
In the present study, 19 samples were collected from sources in homes associated with the deaths of two children from PAM in Arizona. Samples were cultured on nonnutrient agar containing Escherichia coli or in liquid medium and tested for N. fowleri by nested PCR. Thermotolerant amoeboflagellates were observed by light microscopy in liquid cultures. N. fowleri was detected by nested PCR in swipe samples from sink traps, in residual pipe water, and from a Micro-Wynd filter which was used to filter bathtub water in the homes of victims of PAM.
MATERIALS AND METHODS
Study area and collection sites.
Swipe samples from sink traps and residual water present in pipes were collected from the homes associated with two cases of PAM and from the home of an adjacent neighbor. The domestic water was supplied by a private water company in Arizona directly from a well or a holding tank, depending on the demand in the system. Disinfection of the water supply by methods of chlorination, ozone or UV light treatment, or filtration did not occur at the time of the incidents. Swipe samples from the victims' homes were obtained by passing sterile cotton gauze Mirasorb sponges through the kitchen and bathroom sink traps. Sink traps were removed, and the residual water (∼150 to 350 ml) was collected into one or two sterile 250-ml containers. The cotton gauze was placed in one of the containers, and both were topped off with Page's amoeba saline (29, 33). A soil sample from outside one home with water leakage was collected and placed in amoeba saline. Also, a sample from a Micro-Wynd II filter (Cuno, Incorporated, Meriden, Conn.) was obtained by collecting approximately 60.8 liters (16 gallons) of water into the bathtubs of each individual's home. Using a positive-pressure displacement pump, the collected water was passed through a 1-μm-pore-size polypropylene Micro-Wynd II filter (grade Y) and recycled back into the tub. All of the household pump fittings and hoses used were standard. The pump flow rate was approximately 16 gal per min at a pressure of 2.2 lb/in2 and was operated for approximately 3 min so that the water was cycled at least three times. The Micro-Wynd II filter was then placed in a container in sterile Page's amoeba saline.
Processing of samples.
Nineteen samples were collected, transported to the laboratory, and processed within 1 week of collection. All 19 samples were dispensed into individual 75-cm2 tissue culture flasks in 10-ml volumes in duplicate and placed either at 44 or 37°C. The samples in tissue culture flasks were observed daily for the presence of amoebae by light microscopy and were kept for PCR. A third set of samples was prepared by dispensing 10 ml of fluid into centrifuge tubes and subjecting the samples to centrifugation for 10 min at 5,000 × g. The supernatant was discarded, and the pellet was suspended in 1 ml of Page's amoeba saline and placed onto a plate of nonnutrient agar spread with heat-killed E. coli. The plates were incubated at 44°C for 48 h to isolate thermotolerant amoebae. The plates were observed for the presence of plaques produced by amoebae clearing the bacteria. Amoebae were subcultured to new plates by cutting a small portion of the agar from each plaque and placing the agar square onto new plates containing nonnutrient agar with heat-killed E. coli to avoid overgrowth of fungi. After 48 h of incubation, the plates were sealed with Parafilm and stored at room temperature for later use. The original 19 samples containing swipes or water were stored at 37°C for 3 months to promote growth of the amoebae, and portions were prepared for PCR as needed.
Preparation of samples for nested-PCR analysis.
Samples maintained in tissue culture flasks in which cysts or trophozoites were observed were kept in continuous culture in liquid medium by alternating American Type Culture Collection (ATCC) medium 802 with Page's amoeba saline to hinder growth of bacteria and fungi present in the samples. Samples were assayed by PCR beginning 10 days after collection and at intervals for 3 months. Tissue culture flasks maintained at 37°C containing amoebae were prepared by scraping the flask with a sterile cell scraper and centrifuging the contents at 5,000 × g for 5 min. The supernatant was removed, and the pellet was suspended in 100 μl of PCR-grade water and tested for N. fowleri by nested PCR. Repeat PCR assays were performed at various intervals during a 3-month period following continuous culture of the amoebae in liquid medium kept at 37°C. Cultures were observed by light microscopy for the presence of trophozoites, cysts, and flagellates. Cultures were photographed with an Olympus Ck2 microscope with a computer attachment.
Test for flagellates.
A test for flagellates was performed on three select samples that had sufficient numbers of amoebae present in the culture. Samples placed in ATCC medium 802 for 24 h were transferred to sterile distilled water and placed in a shaker incubator. Samples were examined at 15-min intervals by light microscopy for transformation of amoebae to flagellates (29).
PCR analysis.
PCR was performed by the method of Reveiller et al. (35) by amplifying a portion of a gene unique to N. fowleri. Samples were subjected to PCR amplification without prior genomic DNA extraction. Cell suspensions were used as the source of genomic DNA rather than purified genomic DNA. The forward primer, Mp2Cl5.for (5′-TCTAGAGATCCAACCAATGG-3′) and the reverse primer, Mp2Cl5.rev (5′-ATTCTATTCACTCCACAATCC-3′), were used to amplify a 166-bp fragment of the Mp2Cl5 gene. PCR was performed in a 50-μl volume consisting of 1× Taq DNA polymerase buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2), a 0.2 mM concentration of each deoxynucleoside triphosphate, 0.6 μM primer, and 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer, Branchburg, N.J.). The positive control consisted of 10 ng of plasmid DNA purified from E. coli clone Mp2Cl5. The negative control consisted of PCR-grade water lacking the DNA template. Thirty-three microliters of the samples was used in the first round of PCR amplification. The standard temperature program was 5 min at 95°C for one cycle and 1 min at 95°C, 1 min at 65°C, and 2 min at 72°C for 35 cycles. To increase the sensitivity of the assay, nested primers, Mp2Cl5.for-in (5′-GTACATTGTTTTTATTAATTTCC-3′) and Mp2Cl5.rev-in (5-GTCTTTGTGAAAACATCACC-3′), which amplified a 110-bp fragment of Mp2Cl5, were used in a second round of PCR. PCR was also performed with a 50-μl volume consisting of 1× Taq DNA polymerase buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2), a 0.2 mM concentration of each deoxynucleoside triphosphate, 0.5 μM primer, and 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer). Two microliters of the PCR product from the first round of PCR was used in the second PCR. The positive control consisted of 1 μl of the first PCR product of the positive control diluted 50 times in PCR-grade water as the DNA template. The negative control consisted of PCR-grade water lacking the DNA template. The standard temperature program was 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C for 35 cycles. Amplified PCR products from the samples were demonstrated either on a 1.5% GenePure agarose gel (ISC BioExpress, Kaysville, Utah) stained with ethidium bromide or on a 4% NuSieve 3:1 agarose gel (BioWhittaker Molecular Applications, Rockland, Maine) stained with ethidium bromide.
Western immunoblot analysis.
Select domestic samples from Arizona cultured in liquid medium were harvested by centrifugation and placed in lysis buffer consisting of 50 mM Tris-HCl (pH 7.4), 1 mM phenylmethylsulfonyl fluoride, 2.1 mM pepstatin A, and 1.5 mM leupeptin. Samples were subjected to polyacrylamide gel electrophoresis (4% stacking gel and 12% separating gel) using a Protean Slab II unit (Bio-Rad, Richmond, Calif.). Samples were transferred to a nitrocellulose membrane overnight using a Trans-Blot cell unit. The nitrocellulose membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 5% nonfat milk for 1 h. Membranes containing the samples were incubated with rabbit polyclonal antiserum to whole-cell lysates of N. fowleri or Acanthamoeba castellanii or with monoclonal antibody 5D12 (Indicia, Oullins, France) to N. fowleri (34, 38). The primary antibodies were preabsorbed three times with heat-killed E. coli for 2 h at 37°C. Membranes were incubated in secondary antibody, which consisted of peroxidase-conjugated goat anti-rabbit immunoglobulin G or rabbit anti-mouse immunoglobulin G (Sigma Co., St. Louis, Mo.) for 1 h. Blots were washed with Tris-buffered saline Tween and then developed by enhanced chemiluminescence (Western blotting detection kit; Amersham Co., Piscataway, N.J.).
Cloning and sequencing of a PCR-positive sample.
Cloning of the PCR product from sample 4 (swipe sample from a bathroom sink trap) was performed with a TOPO TA cloning kit (Invitrogen, San Diego, Calif.) according to the manufacturer's instructions. Briefly, fresh PCR product was ligated into a pCR 2.1 TOPO vector, heat shocked into a competent E. coli TOP10 strain, and grown on Luria-Bertani agar plates containing 50 μg of ampicillin/ml. Distinct single colonies were picked and grown in Luria-Bertani broth containing 50 μg of ampicillin/ml. Plasmids were purified with the Wizard Plus Minipreps DNA purification system (Promega Corp., Madison, Wis.). The sample was sequenced by using M13 reverse primers at the BWH DNA Core Sequencing Facility (Boston, Mass.) and confirmed by the VCU Massey Cancer Center Nucleic Acids Research Facility (Richmond, Va.).
RESULTS
Nineteen samples collected from domestic sources were labeled and transported to the laboratory (Table 1). Samples were dispensed into tissue culture flasks or placed on nonnutrient agar E. coli plates. Samples were observed daily for the presence of trophozoites or cysts. At 44°C, thermotolerant amoebae were observed, but many were encysted after 72 h. Eleven samples, numbered 2, 3, 4, 5, 11, 12, 13, 14, 15, 16, and 17, maintained for 4 days in Page's amoeba saline at 37°C, were tested by nested PCR. Of the 11 samples initially subjected to PCR, 4 were positive and 7 were negative for N. fowleri (Fig. 1). The PCR product from sample 4, which was PCR positive for N. fowleri, was cloned and sequenced to confirm the identity of the product. Sequencing of the PCR product confirmed the amplification of the Mp2Cl5 gene to 99% identity.
TABLE 1.
PCR results for water and swipe samples collected from the homes of two children who died from PAM and the home of an adjacent neighbora
| Sample source | Assigned sample no.b | PCRd | No. of positive assays/total no. of assayse |
|---|---|---|---|
| Kitchen sink trap swipe | 1 | − | 0/2 |
| Master bathroom sink trap swipe | 2 | + | 3/5 |
| Master bathroom sink trap swipe | 3 | + | 5/5 |
| Guest bathroom sink trap swipe | 4 | + | 3/5 |
| Guest bathroom sink trap swipe | 5 | + | 1/3 |
| Guest bathroom residual sink water | 6 | + | 4/5 |
| Garbage disposal swipe | 7 | − | 0/3 |
| Bathroom sink trap swipe | 11 | + | 2/6 |
| Bathroom sink residual water | 12 | + | 3/6 |
| Double-sink (bedroom) trap swipe | 13 | + | 2/6 |
| Double-sink (bedroom) residual water | 14 | + | 2/6 |
| Double-sink (bedroom) swipe | 15 | + | 4/6 |
| Double-sink (bedroom) residual water | 16 | + | 7/7 |
| Kitchen sink swipe | 17 | + | 3/5 |
| Kitchen sink R.O. water | 18 | + | 2/3 |
| Kitchen R.O. water | 19 | + | 1/4 |
| Guest bathroom sink | 20 | + | 1/5 |
| Soil exposed to continuous water leak | 23 | + | 2/4 |
| Wynd II filterc | 24 | + | 5/5 |
Samples were collected from domestic sites and placed in Page's amoeba saline.
Domestic samples: patient 1, samples 1 to 7; patient 2, samples 13 to 20; 23; neighbor's home, samples 11 and 12.
Micro-Wynd II filter water collected from both bathtubs and passed through a 1-μm-pore-size polypropylene filter.
A nested-PCR assay described in Materials and Methods was performed with each sample.
Samples were tested multiple times during a 3-month period after culture at 37°C in ATCC medium 802 alternating with Page's amoeba saline.
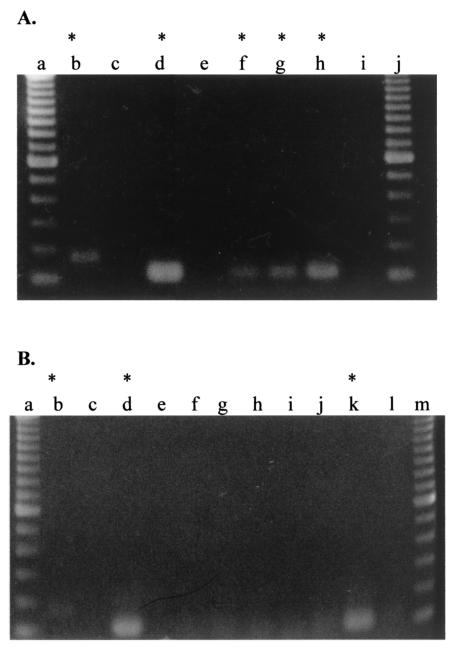
FIG. 1.
Nested-PCR assay of domestic water samples after culture at 37°C for 4 days. Samples were maintained in tissue culture flasks for 4 days at 37°C. Cultures were harvested with a cell scraper and centrifuged to obtain a pellet and supernatant. The pellet was used for nested PCR, and the supernatant was discarded. The first and second PCR-positive and -negative controls were examined on the gel since this was the first PCR assay performed with domestic water samples. (A) Nested-PCR products were demonstrated on a 4% NuSieve gel. Lanes: a and j, 100-bp ladder; b, positive control for first PCR; c, negative control for first PCR; d, positive control for second PCR; e, negative control for second PCR; f, sample 2; g, sample 3; h, sample 4; and i, sample 5. (B) Nested PCR products were demonstrated on a 1.5% agarose gel. Lanes: a and m, 100-bp ladder; b, positive control for first PCR; c, negative control for first PCR; d, positive control for second PCR; e, negative control for second PCR; f, sample 11; g, sample 12; h, sample 13; i, sample 14; j, sample 15; k, sample 16; l, sample 17. Asterisks indicate samples that were positive for N. fowleri by PCR.
Samples 3, 4, and 16, which were PCR positive, were subjected to a test for flagellates because of the number of amoebae seen by visual observation after 4 days of culture at 37°C. Transformation of amoebae to flagellates was observed 30 min after the cultures were switched from ATCC medium 802 to water and were maintained in a shaker incubator. Flagellates were identified in cultures by use of an inverted light microscope (29). The PCR assay was repeated with samples collected after 1 week in culture in liquid medium to determine whether culturing in liquid medium at 37°C would improve detection of N. fowleri, since only 10 ml of each original sample was analyzed. Figure 2A demonstrates that culturing the samples for 1 week in liquid medium at 37°C resulted in additional positive cultures. Samples 11, 12, 13, and 14, which were initially negative by PCR, were positive after culture in ATCC medium 802. To confirm the results of the previous PCR assays, samples maintained in liquid medium at 37°C, which were originally positive, were retested within 1 month of receipt. Samples were cultured continuously, with growth alternated between ATCC medium 802 and Page's amoeba saline. All samples remained positive by PCR on repeat assay with the exception of sample 20 (Fig. 2B). Sample 20 was positive by PCR only one out of five times tested. This sample was negative by Western immunoblot analysis using polyclonal anti-N. fowleri antiserum (Fig. 3A). The Micro Wynd filter was used for samples in which water from bathtubs where both fatal cases occurred tested positive by the PCR assay (Fig. 2B). Samples testing positive for N. fowleri by PCR are given in Table 1. All samples tested by PCR were kept at 37°C in tissue culture flasks. Samples 3, 16, and 24 were positive by PCR for at least five assays. Two samples, 1 and 7, were negative for N. fowleri by PCR throughout the 3-month testing period. Sample 1 was a swipe sample from the kitchen sink trap, and sample number 7 was obtained from the garbage disposal. Both negative samples were swipe samples, but residual water was not obtained from these areas.
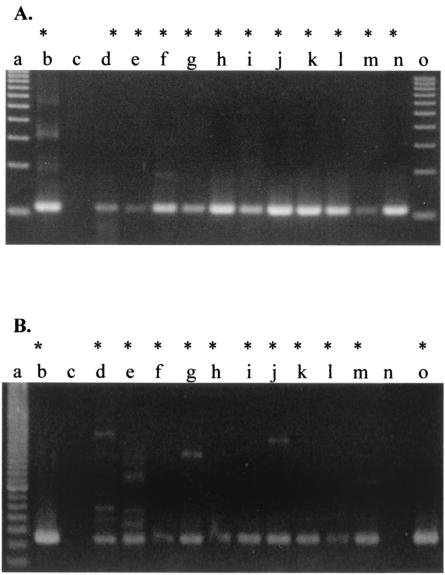
FIG. 2.
Samples cultured for 1 week (A) and 1 month (B) at 37°C were analyzed by PCR. The samples in tissue culture flasks were harvested with a sterile cell scraper and centrifuged to obtain a supernatant and pellet. The pellet was used for nested PCR. PCR products were demonstrated on a 4% NuSieve gel. (A) Lanes: a and o, 100-bp ladder; b, positive control; c, negative control; d, sample 11; e, sample 12; f, sample 13; g, sample 14; h, sample 15; i, sample 16; j, sample 17; k, sample 18; l, sample 19; m, sample 20; n, sample 23. (B) Samples for PCR were prepared after 1 month in continuous culture. Lanes: a, 20-bp ladder; b, positive control; c, negative control; d, sample 2; e, sample 3; f, sample 4; g, sample 6; h, sample 11; i, sample 12; j, sample 14; k, sample 15; l, sample 16; m, sample 17; n sample 20; o, sample Wynd II filter. All domestic samples tested after 1 month were positive, with the exception of sample 20. Asterisks indicate samples that were positive by PCR analysis.
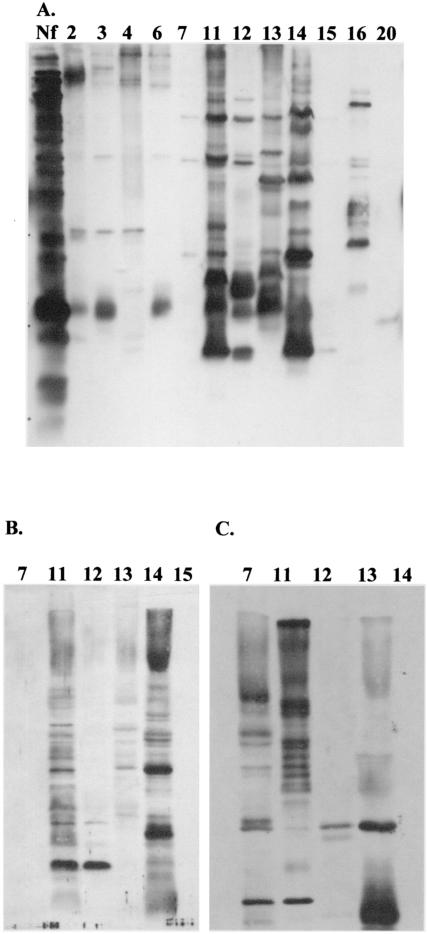
FIG. 3.
Western immunoblot analysis of domestic water samples by using polyclonal anti-N. fowleri and anti-Acanthamoeba antibodies and monoclonal antibody 5D12 for N. fowleri. Samples harvested at 1 month of culture at 37°C were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and separated proteins were transferred to nitrocellulose membranes. The membranes were incubated in the primary antibodies rabbit polyclonal anti-N. fowleri (A), monoclonal 5D12 anti-N. fowleri (B), and rabbit polyclonal anti-Acanthamoeba (C). Secondary antibodies were goat anti-rabbit or rabbit anti-mouse antibodies. The blots were developed by using enhanced chemiluminescence. Lane Nf, a whole-cell lysate of N. fowleri (ATCC 30894) used as a control. Other lanes are labeled with the sample numbers identified in Table 1.
Samples maintained at 37°C for 3 months in liquid culture were harvested for Western immunoblot analysis. Select samples were reacted with anti-N. fowleri antibody or anti-Acanthamoeba antibody. Using polyclonal anti-N. fowleri or anti-Acanthamoeba antisera, both N. fowleri and Acanthamoeba were detected (Fig. 3A and C). Four of five samples analyzed by Western immunoblot demonstrated reactivity with monoclonal antibody 5D12, which is specific for N. fowleri (34, 38) (Fig. 3B). The organisms in sample 7, which was negative for N. fowleri by PCR, were also below the detection limit for N. fowleri by Western immunoblot analysis using polyclonal or monoclonal antibodies to N. fowleri (Fig. 3A and B). However, sample 7 was positive for Acanthamoeba when anti-Acanthamoeba antiserum was used (Fig. 3C). Visual observation by light microscopy confirmed that cultures contained limax amoebae (Naegleria spp.) and Acanthamoeba (Fig. 4).
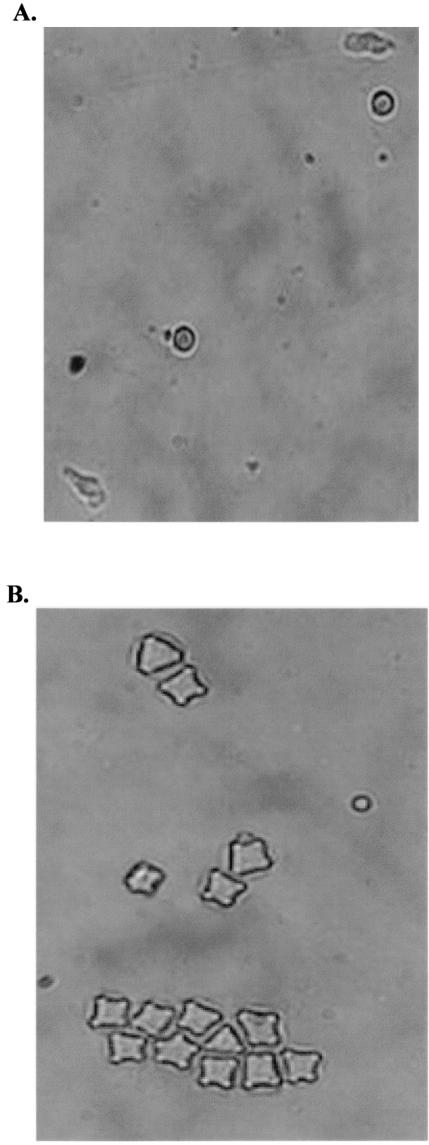
FIG. 4.
Cultures of domestic samples were observed daily for the presence of trophozoites, cysts, or flagellates. (A) Limax-type amoebae were observed in sample 16, which was positive for N. fowleri by PCR. (B) Acanthamoeba cysts were observed in sample 7, which was negative for N. fowleri by PCR. Magnification, ×400.
DISCUSSION
In the present study, a nested-PCR assay was used to identify N. fowleri in samples collected from domestic sites in Arizona where two cases of PAM occurred in previously healthy children. We have shown previously that the nested-PCR assay can detect as few as five amoebae in 50 ml of water (35). In the present study, samples were collected in approximately 250-ml volumes but only 10 ml of each sample was tested initially by PCR. Analysis of these 10-ml samples by PCR indicated the presence of N. fowleri in 4 of 11 samples after 4 days of culture at 37°C. Culture of the samples for 1 week followed by nested PCR increased the frequency of detection of N. fowleri such that 11 of 11 samples were positive. Samples were cultured prior to PCR to eliminate the possibility of the presence of inhibitors of Taq polymerase in the environmental samples, which would result in false negatives. Inhibitors of PCRs have been described by others for water and soil samples (32, 37, 41). Three domestic samples, labeled 3, 4, and 16, containing thermotolerant amoebae which were positive by PCR after 4 days of culture, were selected for a test for flagellates. Flagellates were detected in all three samples, indicating that a thermotolerant amoeboflagellate was present. Transformation of amoebae into flagellates is a distinctive feature of Naegleria (29). Select samples were then examined by Western immunoblot analysis using polyclonal antiserum elicited against N. fowleri. Samples analyzed by Western immunoblotting confirmed the presence of Naegleria amoebae in the samples. However, the immunological technique using polyclonal antiserum is genus specific and cannot distinguish the species N. fowleri from N. lovaniensis. Sufficient amounts of samples from five cultures were tested for N. fowleri by using a monoclonal antibody (5D12) specific to the amoeba, which indicated that N. fowleri was present (34, 38). The samples were also tested for the organisms of the genus Acanthamoeba by Western immunoblot analysis, since visual assays using light microscopy indicated that the cultures also contained Acanthamoeba cysts, which are readily recognized using morphological criteria (27-29, 33). Acanthamoeba was also detected by immunoblot analysis in several domestic samples, an additional human health hazard since Acanthamoeba spp. have been associated with amoebic keratitis and granulomatous amoebic encephalitis (27-29). Animal pathogenicity tests have been used to distinguish pathogenic from nonpathogenic Naegleria spp. Although Naegleria australiensis is pathogenic for mice, it has not been isolated from a human case of PAM (30). However, animal pathogenicity tests do not distinguish N. fowleri from N. australiensis. We have employed the nested-PCR technique previously to distinguish N. fowleri from other Naegleria species as well as from amoebae of the genus Acanthamoeba (35). The specificity of the product generated by nested PCR was confirmed by cloning and sequencing the PCR product. Thus, the nested-PCR assay not only eliminates the need to do animal pathogenicity testing but also constitutes a highly sensitive tool for discriminating N. fowleri from other Naegleria species as well as from amoebae of the genus Acanthamoeba and other free-living amoebae commonly found in the environment (35; Maclean et al., submitted). Differentiation of N. fowleri from pathogenic species of Acanthamoeba is important because the courses of the diseases and the treatment regimens are different (27-30).
Nineteen sites in the households associated with PAM victims and a neighboring home were sampled. Seventeen of these sites were shown to be positive for N. fowleri by PCR. Of particular interest is that N. fowleri was detected in residual water from the sink pipes in both homes as well as from the Micro-Wynd filter, which was used to filter bathtub water from the homes. These results are consistent with the source of infection for these two children being the domestic water source, because neither child had a history of swimming in a natural freshwater lake or pond prior to the onset of symptoms of PAM. However, both victims routinely played in the bathtub.
Infection with N. fowleri has been acquired through modes other than conventional swimming or diving in ponds and lakes. Sniffing water into the nasal passages as a religious ritual prior to prayer (24), total immersion in bathwater (1-3, 31), playing in a warm muddy puddle after rain (4), and immersion of the head in a trough of water on a school playground (16) have been described as sources of infection with these amoebae. An 8-month-old infant was thought to have acquired an infection with N. fowleri during a full-submersion baptism ceremony in a natural body of water (5).
The occurrence of N. fowleri in the domestic water supply has been reported previously (1-3). In South Australia, household water delivered via overland pipelines during a prolonged period of hot weather was attributed as the source of PAM in children in backyard wading pools or in bathtubs. N. fowleri was recovered from a sample of tap water taken from a home where a fatal case of PAM occurred. In two Australian cases, houses had remained unoccupied for considerable periods of time during warm weather (3, 8, 31). It has been suggested that under such climatic conditions, N. fowleri can multiply to significant numbers in warm stagnant sections of domestic water supplies (3, 8, 31). In this context, a number of studies have shown that, out of several physical and chemical characteristics of water, elevated temperature has been one of the most important factors accounting for the increased incidence and higher levels of N. fowleri (16-18). The cases studied in the present report emphasize that PAM should be considered in the differential diagnosis of unexplained meningoencephalitis since not all cases of this disease are associated with freshwater sports. The nested-PCR assay provides a rapid, sensitive, and specific method to determine the presence of N. fowleri in environmental and recreational sources.
Acknowledgments
This work was supported in part by a grant from the Foundation of Immunotoxicology, Richmond, Va.
The monoclonal antibody was provided by Electricite de France, Paris.
REFERENCES
- 1.Anderson, K., and A. Jamieson. 1972. Primary amoebic meningoencephalitis. Lancet i:902-903. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K., and A. Jamieson. 1972. Primary amoebic meningoencephalitis. Lancet ii:379. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K., A. Jamieson, J. B. Jadin, and E. Willaert. 1973. Primary amoebic meningoencephalitis. Lancet ii:672-673. [DOI] [PubMed] [Google Scholar]
- 4.Apley, J., S. K. Clarke, A. P. Roome, S. A. Sandry, G. Saygi, B. Silk, and D. C. Warhurst. 1970. Primary amoebic meningoencephalitis in Britain. Br. Med. J. 1:596-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett, N. D., A. M. Kaplan, R. J. Hopkin, M. A. Saubolle, and M. F. Rudinsky. 1996. Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr. Neurol. 15:230-234. [DOI] [PubMed] [Google Scholar]
- 6.Brown, T. J., R. T. Cursons, E. A. Keys, M. Marks, and M. Miles. 1983. The occurrence and distribution of pathogenic free-living amoebae in thermal areas of the North Island of New Zealand. N. Z. J. Mar. Freshw. Res. 17:59-69. [Google Scholar]
- 7.Callicott, J. H. 1968. Amebic meningoencephalitis due to free-living amoebas of the Hartmanella (Acanthamoeba)-Naegleria group. Am. J. Clin. Pathol. 49:84-91. [DOI] [PubMed] [Google Scholar]
- 8.Carter, R. F. 1972. Primary amoebic meningo-encephalitis: an appraisal of present knowledge. Trans. R. Soc. Trop. Med. Hyg. 66:193-213. [DOI] [PubMed] [Google Scholar]
- 9.Cerva, L., K. Novak, and C. G. Culbertson. 1968. An outbreak of amoebic meningoencephalitis. Am. J. Epidemiol. 88:436-444. [DOI] [PubMed] [Google Scholar]
- 10.Cooter, R. 2002. The history of the discovery of primary amoebic meningoencephalitis. Aust. Fam. Physician 31:399-400. [PubMed] [Google Scholar]
- 11.Cursons, R. T., T. J. Brown, B. J. Bruns, and D. E. Taylor. 1976. Primary amoebic meningoencephalitis contracted in a thermal pool of the Waikato River-Taupo: a case report. N. Z. Med. J. 84:479-481. [PubMed] [Google Scholar]
- 12.Darby, C. P., S. E. Conradi, T. W. Holbrook, and C. Chatellier. 1979. Primary amebic meningoencephalitis. Am. J. Dis. Child. 133:1025-1027. [DOI] [PubMed] [Google Scholar]
- 13.De Jonckheere, J., P. Van Dijck, and H. Van de Voorde. 1975. The effect of thermal pollution on the distribution of Naegleria fowleri. J. Hyg. 75:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeNapoli, T. S., J. Y. Rutman, J. R. Robinson, and M. M. Rhodes. 1996. Primary amoebic meningoencephalitis after swimming in the Rio Grande. Tex. Med. 92:59-63. [PubMed] [Google Scholar]
- 15.Dingley, D. 1996. Commentary: safe water practices can lower risk of contracting primary amoebic meningoencephalitis. Tex. Med. 92:28-29. [PubMed] [Google Scholar]
- 16.Dorsch, M. M., A. S. Cameron, and B. S. Robinson. 1983. The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia. Trans. R. Soc. Trop. Med. Hyg. 77:372-377. [DOI] [PubMed] [Google Scholar]
- 17.Griffin, J. L. 1972. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science 178:869-870. [DOI] [PubMed] [Google Scholar]
- 18.Griffin, J. L. 1983. The pathogenic amoeboflagellate Naegleria fowleri: environmental isolations competitors, ecologic interactions, and the flagellate-empty habitat hypothesis. J. Protozool. 30:403-409. [DOI] [PubMed] [Google Scholar]
- 19.Hecht, R. H., A. H. Cohen, J. Stoner, and C. Irwin. 1972. Primary amebic meningoencephalitis in California. Calif. Med. 117:69-73. [PMC free article] [PubMed] [Google Scholar]
- 20.Kadlec, V., L. Cerva, and J. Skvarova. 1978. Virulent Naegleria fowleri in an indoor swimming pool. Science 201:1025. [DOI] [PubMed] [Google Scholar]
- 21.Kasprzak, W., T. Mazur, and L. Cerva. 1982. Naegleria fowleri in thermally polluted waters. Folia Parasitol. 29:211-218. [PubMed] [Google Scholar]
- 22.Kyle, D. E., and G. P. Noblet. 1985. Vertical distribution of potentially pathogenic free-living amoebae in fresh water lakes. J. Protozool. 32:99-105. [DOI] [PubMed] [Google Scholar]
- 23.Lares-Villa, F., J. F. De Jonckheere, H. De Moura, A. Rechi-Iruretagoyena, E. Ferreira-Guerrero, G. Fernandez-Quintanilla, C. Ruiz-Matus, and G. S. Visvesvara. 1993. Five cases of primary amebic meningoencephalitis in Mexicali, Mexico: study of the isolates. J. Clin. Microbiol. 31:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawande, R. V., J. T. Macfarlane, W. R. Weir, and C. Awunor-Renner. 1980. A case of primary amebic meningoencephalitis in a Nigerian farmer. Am. J. Trop. Med. Hyg. 29:21-25. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2002. Surveillance for waterborne-disease outbreaks—United States, 1999-2000. Morb. Mortal. Wkly. Rep. Surveill. Summ. 51:1-47. [PubMed] [Google Scholar]
- 26.Marciano-Cabral, F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marciano-Cabral, F., R. Puffenbarger, and G. A. Cabral. 2000. The increasing importance of Acanthamoeba infections. J. Eukaryot. Microbiol. 47:29-36. [DOI] [PubMed] [Google Scholar]
- 28.Marciano-Cabral, F., and G. Cabral. 2003. The importance of Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, A. J. 1985. Free-living amoebas: natural history, prevention, diagnosis, pathology and treatment of disease. CRC Press, Boca Raton, Fla.
- 30.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living amphizoic and opportunistic amoebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, G., G. Cullity, I. Walpole, J. O'Connor, and P. Masters. 1982. Primary amoebic meningoencephalitis in Western Australia. Med. J. Aust. 1:352-357. [DOI] [PubMed] [Google Scholar]
- 32.Orlandi, P. A., and K. A. Lampel. 2000. Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J. Clin. Microbiol. 38:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page, F. C. 1988. A new key to freshwater and soil gymnamoeba with instructions for culture. Freshwater Biological Association, Ambleside, United Kingdom.
- 34.Reveiller, F. L., F. Marciano-Cabral, P. Pernin, P. A. Cabanes, and S. Legastelois. 2000. Species specificity of a monoclonal antibody produced to Naegleria fowleri and partial characterization of its antigenic determinants. Parasitol. Res. 86:634-641. [DOI] [PubMed] [Google Scholar]
- 35.Reveiller, F. L., P. A. Cabanes, and F. Marciano-Cabral. 2002. Development of a nested PCR assay to detect the pathogenic free-living amoeba Naegleria fowleri. Parasitol. Res. 88:443-450. [DOI] [PubMed] [Google Scholar]
- 36.Rodriquez-Zaragoza, S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 37.Sluter, S. D., S. Tzipori, and G. Widmer. 1997. Parameters affecting polymerase chain reaction detection of waterborne Cryptosporidium parvum oocysts. Appl. Microbiol. Biotechnol. 48:325-330. [DOI] [PubMed] [Google Scholar]
- 38.Sparagano, O., E. Drouet, R. Brebant, E. Manet, G.-A. Denoyel, and P. Pernin. 1993. Use of mononclonal antibodies to distinguish pathogenic Naegleria fowleri (cysts, trophozoites, or flagellate forms) from other Naegleria species. J. Clin. Microbiol. 31:2758-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens, A. R., J. De Jonckheere, and E. Willaert. 1980. Naegleria lovaniensis new species: isolation and identification of six thermophilic strains of a new species found in association with Naegleria fowleri. Int. J. Parasitol. 10:51-64. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, J. P., K. A. Hendricks, and D. D. Dingley. 1996. Amebic meningoencephalitis. Infect. Med. 13:1021-1024. [Google Scholar]
- 41.Tebbe, C. C., and W. Vahjen. 1993. Interference of humic acids and DNA extracted directly from soil in detection and transformation of recombinant DNA from bacteria and a yeast. Appl. Environ. Microbiol. 59:2657-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyndall, R. L., K. S. Ironside, P. L. Metler, E. L. Tan, T. C. Hazen, and C. B. Fliermans. 1989. Effect of thermal additions on the density and distribution of thermophilic amoebae and pathogenic Naegleria fowleri in a newly created cooling lake. Appl. Environ. Microbiol. 55:722-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Driessche, E., J. Vandepitte, P. J. Van Dijck, J. De Jonckheere, and H. Van de Voorde. 1973. Primary amoebic meningoencephalitis after swimming in stream water. Lancet ii:971-972. [DOI] [PubMed] [Google Scholar]
- 44.Warhurst, D. C. 1985. Pathogenic free-living amoebae. Parasitol. Today 1:24-28. [DOI] [PubMed] [Google Scholar]
- 45.Wellings, F. M., P. T. Amuso, S. L. Chang, and A. L. Lewis. 1977. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl. Environ. Microbiol. 34:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellings, F. M., P. T. Amuso, A. L. Lewis, M. J. Farmelo, D. J. Moody, and C. L. Osikowicz. 1979. Pathogenic Naegleria fowleri: distribution in nature. EPA-600/1-79-018. U.S. Environmental Protection Agency, Washington, D.C.