Abstract
Stable carbon isotope analysis of biomass and analyses of phospholipid fatty acids (PLFA), glycolipid fatty acids (GLFA), and mycolic acids were used to characterize mixed-substrate utilization by Mycobacterium frederiksbergense LB501T under various substrate regimens. The distinct 13C contents of anthracene and glucose as representatives of typical hydrophobic pollutants and naturally occurring organic compounds, respectively, were monitored during formation into biomass and used to quantify the relative contributions of the two carbon sources to biomass formation. Moreover, the influence of mixed-substrate utilization on PLFA, GLFA, and mycolic acid profiles and cell surface hydrophobicity was investigated. Results revealed that M. frederiksbergense LB501T degrades anthracene and forms biomass from it even in the presence of more readily available dissolved glucose. The relative ratios of straight-chain saturated PLFA to the corresponding unsaturated PLFA and the total fraction of saturated cyclopropyl-branched PLFA of M. frederiksbergense LB501T depended on the carbon source and the various rates of addition of mixed substrates, whereas no such trend was observed with GLFA. Higher proportions of anthracene in the carbon source mixture led to higher cell surface hydrophobicities and more-hydrophobic mycolic acids, which in turn appeared to be valuable indicators for substrate utilization by M. frederiksbergense LB501T. The capability of polycyclic aromatic hydrocarbon (PAH)-degrading bacteria to utilize readily available substrates besides the poorly available PAHs favors the buildup of PAH-degrading biomass. Feeding of supplementary carbon substrates may therefore promote bioremediation, provided that it sustains the pollutant-degrading population rather than other members of the microbial community.
Engineered soil bioremediation aims at stimulating biodegradation by promoting the creation and maintenance of an active, pollutant-degrading microbial community. There is evidence, however, that growth of heterotrophic microbes in terrestrial ecosystems is often restricted by the availability of nutrients or organic carbon (for a review see reference 8). Moreover, practical experience shows—and theoretical considerations confirm—that the bioavailability of extremely hydrophobic contaminants may be too low to build up and maintain the microbial population sizes that would be needed to clean soil in a reasonable time (6). It appears that from a microbe's perspective even a highly contaminated soil may be oligotrophic.
The restricted availability of carbon sources has led to the selection of microorganisms with physiological properties that allow them to survive in inhospitable low-nutrient environments, for instance by adjusting structural and metabolic features (20). Recent observations have indicated that specific bacterial adaptations to the utilization of contaminants of low bioavailability may exist (10, 12, 14, 31). For instance, the utilization of multiple carbon sources was shown to be a suitable bacterial strategy to cope with oligotrophic conditions, and in laboratory experiments bacteria indeed metabolized simultaneously a wide range of organic molecules (20). Addition of supplementary carbon sources may therefore improve bioremediation, provided that it helps to create and sustain pollutant-degrading populations. Information on microbial substrate utilization can be obtained by analytically monitoring the change of the substrate's characteristic content of the minor stable carbon isotope δ-13C into microbial biomass. δ-13C analysis of microbial biomarkers (e.g., cell wall membrane fatty acids) has further been used to link contaminant degradation to the catabolically active microbial community members in situ (24). This requires, however, that (i) the δ-13C content of the contaminant is distinct from that of natural organic carbon; (ii) the extent of enzymatic isotope fractionation, i.e., the differentiation of 13C by bacterial enzymes, is low; and (iii) the influences of substrate usage on the synthesis of fatty acids of taxonomic value are known. An earlier study showed that δ-13C of phospholipid fatty acids (PLFA) and glycolipid fatty acids (GLFA) and specific shifts of PLFA and GLFA patterns of Mycobacterium frederiksbergense LB501T could be used to distinguish between the utilization of anthracene and that of glucose (32).
Mycobacteria are an important group of polycyclic aromatic hydrocarbon (PAH)-degrading bacteria. They possess a complex, highly rigid cell envelope that contains large amounts of C60-C90 fatty acids (mycolic acids) specific for the genus Mycobacterium. Mycolic acids can be extracted from soil and used as markers for the presence of mycobacteria (L. Y. Wick et al., unpublished results). It has been shown that PAH-degrading mycobacteria respond specifically to the growth substrate by modifying the mycolic acid composition of their cell walls (33). PLFA, GLFA, and mycolic acids may therefore be used as diagnostic tools for in situ substrate usage.
Here, we report on mixed-substrate utilization by laboratory-grown M. frederiksbergense LB501T under various substrate regimens, i.e., different molar ratios of anthracene and glucose as representatives of typical hydrophobic pollutants and naturally occurring organic substrates, respectively. The relative contributions of these two carbon sources to biomass formation were quantified, and substrate influences on the composition of cell wall membrane fatty acids (PLFA, GLFA, and mycolic acids) and concomitant cell surface hydrophobicity were described. Our results are important for the development and calibration of new methods that link observed in situ catabolic activity to the identity of the active community members.
MATERIALS AND METHODS
Bacterium and culture conditions.
Strain LB501T (3), an environmental isolate belonging to the recently described species M. frederiksbergense (28, 34), was grown in a minimal medium (30) with anthracene, glucose, or mixtures of both as the carbon sources. Bacterial cultures were inoculated with anthracene-grown inocula to an optical density at 578 nm of ca. 0.025 and were incubated at 25°C on a gyratory shaker at 130 rpm in airtight three-neck 1-liter Erlenmeyer flasks containing 300 ml of medium. Anthracene (>98% [high-pressure liquid chromatography (HPLC)]; Fluka, Buchs, Switzerland) was provided at 2 g liter−1 in the form of crystals with diameters ranging from 0.2 to 0.5 mm. All growth experiments were performed at least in triplicate. Due to its poor solubility of only 3.4 × 10−7 mol liter−1 at 25°C, anthracene was mainly present as crystals throughout the cultivation. The dissolution flux of 2 g of anthracene liter−1 had been determined in separate dissolution experiments in the absence of cells (30) (Table 1, column 1). Control cultures were also grown with anthracene dissolved in dimethyl sulfoxide (DMSO) and continuously added to the culture flask with a syringe pump (74900 series; Cole Parmer, Vernon Hills, Ill.) at a low rate. DMSO at the final concentration accumulating in our cultures had no direct effect on M. frederiksbergense LB501T, as could be seen from identical growth rates during cultivation on glucose in the presence and absence of DMSO. Glucose was provided either at an initial concentration of 2 g liter−1 (excess glucose) or as slowly amended solutions of 0.72, 3.6, or 36 g liter−1 in mineral medium at a flow rate of ca. 0.16 ml h−1, leading to glucose additions of 0.12, 0.58, and 5.8 mg h−1, respectively. The resulting molar glucose supply rates and calculated contributions of anthracene to the total carbon and energy fluxes are given in Table 1 in columns 1 to 4. The relative energy flux rates from anthracene versus glucose in mixed-substrate cultures were deduced according to standard procedures for calculations of Gibbs free enthalpy changes (ΔG°′) (Table 1, column 8). Assuming ammonium as the nitrogen source (1/5 CO2 + 1/20 HCO3− + 1/20 NH4+ + H+ + e− → 1/20 C5H7O2N + 9/20 H2O; ΔG°′pH 7 = 32 kJ/electron equivalent) and oxygen as electron acceptor (1/2 O2 + H+ + e− → 1/2 H2O; ΔG°′pH 7 = −78.2 kJ/electron equivalent), the mineralization of the more reduced anthracene molecule (1/66 C14H10 + 28/66 H2O → CO2 + H+ + e−; ΔG°′pH 7 = 27.6 kJ/electron equivalent) yields more energy than does mineralization of a glucose molecule to CO2 (1/24 C6H12O6 + 1/4 H2O → 1/4 CO2 + H+ + e−; ΔG°′pH 7 = 41.8 kJ/electron equivalent). Accordingly, ΔG°′pH 7 = −72.4 kJ/electron equivalent for anthracene and ΔG°′pH 7 = −28.5 kJ/electron equivalent for glucose utilization can be calculated. This leads to a roughly seven-times-higher molar energy yield for anthracene degradation than for glucose degradation. Cells were harvested at times at which they grew at identical specific rates, which were limited by the low substrate provision (cells growing exponentially on excess glucose excepted). After harvest (about 2 weeks of growth), cells were frozen or analyzed within 4 h. To separate planktonic cells from cells attached to solid anthracene, cultures were filtered through a fritted funnel covered with a paper filter (no. 589/1; Schleicher & Schuell, Dassel, Germany). For the analysis of mycolic acids of attached cells, the filtered crystals were removed from the paper filter and resuspended in 5 ml of 0.9% NaCl solution in a 10-ml test tube. The test tube was exposed to an ultrasonic bath to release attached cells from the crystal surfaces (31), and the resulting suspension was analyzed. The optical density of cell suspensions was determined spectrophotometrically at 578 nm after the samples were carefully shaken.
TABLE 1.
Culture conditions and results of stable carbon isotope, linear growth rate, and contact angle analyses
| C substrate(s) | Molar addition rate (mol h−1)
|
Calculated energy flux (kJ h−1) derived from:
|
Relative linear growth ratea (−) | δ−13C of biomassb (‰) | Calculated contribution of A (%) to:
|
Contribution of solid A to linear growth ratee (%) | Anthracene C in biomassb (%) | Contact angleb (°) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthracene | Glucose | Anthracene | Glucose | C atom flux | Energy flux | ||||||
| Solid anthracene | 2.9 × 10−7 | 0 | 1.4 × 10−3 | 0 | 6.2 ± 1.4 | −23.14 ± 0.52 | 100 | 100 | 100 | 100 | 65 ± 19 |
| Dissolved anthracene | 2.9 × 10−7 | 0 | 1.4 × 10−3 | 0 | 0.7 ± 0.04 | −25.90 ± 0.78 | 100 | 100 | 0 | 100 | NA |
| Excess glucose | 0 | NAc | 0 | NA | NAd | −10.95 ± 0.11 | 0 | 0 | 0 | 0 | 34 ± 6 |
| Limited glucose | 0 | 6.4 × 10−7 | 0 | 4.4 × 10−4 | 1 | −10.83 ± 0.61 | 0 | 0 | 0 | 0 | 40 ± 7 |
| High A-G | 2.9 × 10−7 | 6.4 × 10−7 | 1.4 × 10−3 | 4.4 × 10−4 | 7.8 ± 1.5 | −18.89 ± 2.29 | 50 | 76 | 80 ± 24 | 68 ± 19 | 54 ± 13 |
| Intermediate A-G | 2.9 × 10−7 | 3.2 × 10−6 | 1.4 × 10−3 | 2.2 × 10−3 | 8.4 ± 2.1 | −16.13 ± 5.12 | 17 | 39 | 74 ± 25 | 33 ± 18 | 63±12 |
| Low A-G | 2.9 × 10−7 | 3.2 × 10−5 | 1.4 × 10−3 | 2.2 × 10−2 | 40.3 ± 5 | −11.89 ± 0.88 | 2 | 6 | 15 ± 4 | 8 ± 5 | 34 ± 1 |
n = 5.
n ≥ 3.
NA, not analyzed.
Exponential growth.
Contribution of solid A to linear growth rate = [(relative linear growth rate of culture X/relative linear growth rate for growth on solid anthracene) × 100].
Stable carbon isotope analysis.
The carbon isotope composition of bacterial biomass was determined with a Carlo-Erba CNS analyzer (CE Instruments, Milan, Italy) coupled in continuous flow to an Optima mass spectrometer (Micromass, Manchester, United Kingdom) calibrated with the international carbon isotope standards NBS22. All results are reported in the conventional δ notation with reference to Vienna Peedee belemnite. Analytical reproducibility was ±0.1‰. The percentage of anthracene carbon utilized PCA from substrate mixtures was calculated as
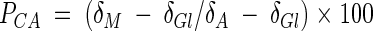 |
(1) |
where δGl, δA, and δM are the δ-13C of the biomass grown on glucose only, anthracene only, and the mixture of both.
Fatty acid analysis.
Total lipids were extracted from whole bacterial cells by a modified Bligh-Dyer method (4) and further fractionated into neutral lipids, glycolipids, and polar lipids by column chromatography on a silica gel as described previously (1). The polar lipids were dried, and the fatty acid methyl esters were generated as described previously (9). The fatty acid methyl esters were separated by gas chromatography (Hewlett Packard HP 5890 series II chromatograph equipped with an HP Ultra 2 capillary column) and identified by using the MIDI program (MIDI, Inc., version 4.0) according to a standardized procedure (32). Fatty acid nomenclature followed standard rules as described elsewhere (19).
Mycolic acid analysis.
The extraction and p-bromophenacyl ester derivatization of mycolic acids followed standard procedures described by Butler and Kilburn (7). As described earlier (33), mycolic acids were analyzed by HPLC with UV detection at 260 nm on an RP-18 column by applying a solvent gradient of CH2Cl2 in MeOH (0 to 13 min, 0 to 10%; 13 to 17 min, 10 to 25%; 17 to 34 min, 25 to 70%; 34 to 41 min, constant 70%). Although HPLC-pure solvents were used, small baseline drifts may thus occur at high sensitivity ranges. Mycolic acid p-bromophenacyl esters eluted between 23 and 36 min.
Characterization of bacterial cell surface hydrophobicity.
Cell surface hydrophobicities were derived from the contact angles θw of water drops on bacterial lawns by using a microscope with a goniometric eye piece (Krüss GmbH, Hamburg, Germany) as described earlier (27).
RESULTS
Growth on individual and mixed substrates.
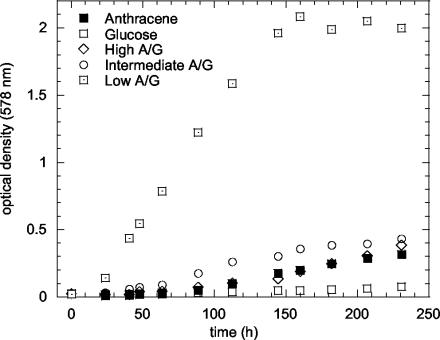
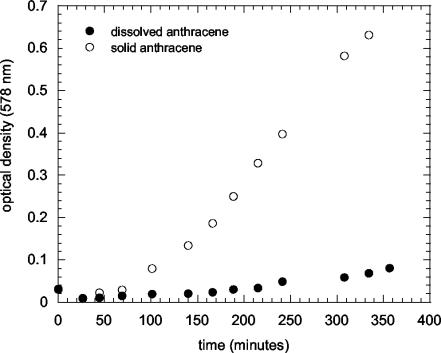
Batch growth curves of M. frederiksbergense LB501T were established with solid anthracene (A) or glucose (G), each provided at a low rate, and three A-G mixtures (low, intermediate, and high A-G; Fig. 1) exhibiting different molar A/G ratios. Pseudolinear growth was observed in all cultures, indicating substrate limitation. Growth rates were calculated by linear regression of the linear growth phases. When expressed relative to the growth rate obtained with limited glucose addition, growth rates were about 6 times higher with solid anthracene only, about 8 times higher for high A-G and intermediate A-G, and 40 times higher for low A-G (Table 1, column 5). These results suggest that degradation of solid anthracene yields more biomass than does degradation of glucose relative to the calculated available energy fluxes (Table 1, column 9). As biofilm formation on anthracene crystals was generally observed, these higher yields can be explained by the fact that energy flux calculations were based on anthracene dissolution rates observed in the absence of bacteria, whereas anthracene-consuming bacteria in biofilms may drive anthracene dissolution more efficiently. To test this hypothesis, we compared growth on solid anthracene with growth on DMSO-dissolved anthracene provided at a rate identical to the abiotically observed dissolution rate of solid anthracene. Figure 2 shows that under these conditions growth with crystalline anthracene was indeed about 10 times faster than growth with DMSO-dissolved anthracene.
FIG. 1.
Representative batch growth curves of M. frederiksbergense LB501T on 2 g of size-fractionated (0.2- to 0.5-mm) solid anthracene liter−1 leading to an anthracene dissolution flux of 9.7 × 10−10 mol ml−1 h−1, dissolved glucose added at a rate of 2.3 × 10−9 mol ml−1 h−1, and additions of 2 g of solid anthracene liter−1 together with dissolved glucose provided at three different rates (Table 1). Growth was determined as optical bacterial density at 578 nm.
FIG. 2.
Representative batch growth curves of M. frederiksbergense LB501T on 2 g of size-fractionated (0.2- to 0.5-mm) solid anthracene liter−1 and with continuous addition of DMSO-dissolved anthracene at an identical substrate carbon flux as that in which 2 g of solid anthracene liter−1 would dissolve under abiotic conditions.
Carbon isotope ratios of biomass.
The δ-13C values of biomass of M. frederiksbergense LB501T grown on the single carbon source were within 1‰ of those of the carbon substrates (δ-13C = −10.70‰ ± 0.04‰ [glucose] and −23.76‰ ± 0.03‰ [anthracene] [Table 1, column 6]) and thus exhibited little isotope fractionation during substrate degradation and biomass formation. An exception was the somewhat 13C-depleted biomass resulting from growth on DMSO-dissolved anthracene. The decreasing proportion of anthracene in the three mixtures with glucose is reflected in the δ-13C content of the biomass. These data together with the observed growth rates reveal simultaneous utilization of both carbon sources at all A/G ratios. δ-13C values were used to calculate the amount of anthracene used for biomass formation during growth on mixed carbon sources according to equation 1. Contributions of anthracene carbon to biomass formation in mixed-substrate cultures were more consistent with anthracene contributions to the total energy flux than with those to the total carbon flux (Table 1, columns 7, 8, and 10).
GLFA and PLFA composition of the cell envelope.
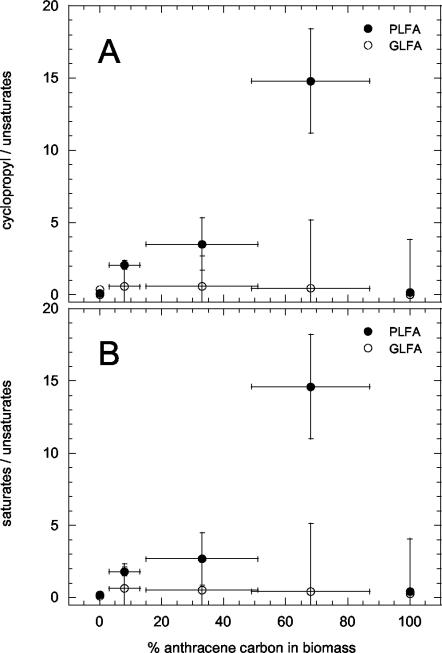
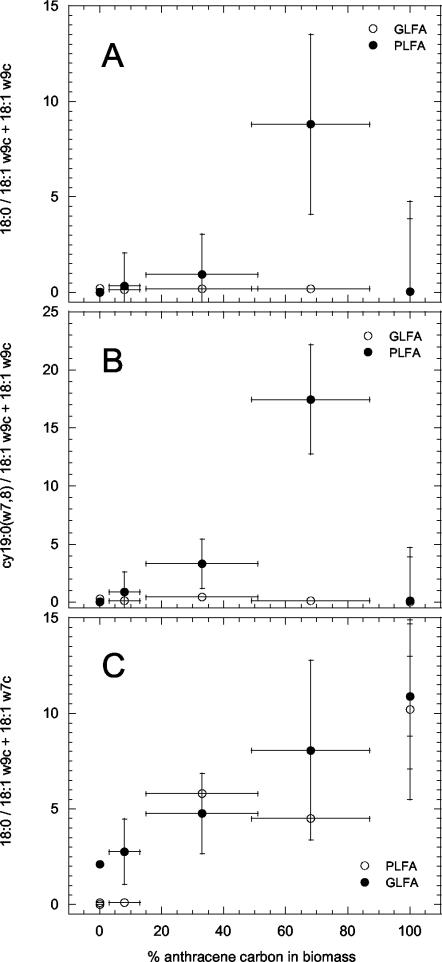
The influence of mixed-substrate utilization on PLFA and GLFA profiles of M. frederiksbergense LB501T was studied because characteristic variations would make them promising markers for substrate utilization. Cells grown on three mixtures of anthracene and glucose were analyzed and compared with those grown on slowly dissolving solid anthracene, excess glucose, or slowly amended glucose (32). Results showed that PLFA and GLFA profiles depended on the kinds of individual growth substrates or substrate mixtures and on the rates at which they became available (Tables 2 and 3). Cells grown on excess glucose were characterized by the lowest relative contents of branched PLFA and GLFA and the highest contents of unsaturated PLFA and GLFA. When grown on slowly provided glucose, the fractions of branched, cyclic, and saturated PLFA and GLFA were drastically increased. Anthracene-grown cells differed from those grown on excess glucose by their lower content of unsaturated PLFA and GLFA and their higher content of branched and saturated PLFA and GLFA. Cells grown on slowly provided glucose and on mixtures of anthracene and glucose were characterized by drastically lower unsaturated PLFA and slightly lower unsaturated GLFA contents. Interestingly the PLFA-GLFA composition of cells grown at low A-G concentrations differed largely from that of cells grown on excess glucose only. The former had considerably higher contents of cyclic PLFA-GLFA and in this aspect resembled cells grown on limited glucose. Changes of saturated and branched fatty acids were less explicit. The ratios of saturated to unsaturated as well as of cyclic to unsaturated PLFA fractions of high A-G, intermediate A-G, and low A-G decreased from 14.6 to 1.8 and from 14.8 to 2.1, respectively (Fig. 3), whereas no such tendencies were found for GLFA. Closer inspection of the most abundant C18 fatty acids reveals a similar trend (Fig. 4A and B), whereas a linear relationship between the anthracene incorporated in the biomass and the ratio of the 18:1ω9c to 18:1ω7c fatty acids was observed for both GLFA and PLFA (Fig. 4C). Anthracene-grown cells exhibited an up to 10-fold-increased 18:1ω9c/18:1ω7c fatty acid ratio.
TABLE 2.
Relative abundance of PLFA of M. frederiksbergense LB501T grown in minimal medium containing anthracene or glucose as sole carbon source or mixtures of both
| PLFA | Fatty acid content (%) for substrate:
|
|||||
|---|---|---|---|---|---|---|
| Anthracenea | Excess glucosea | Limited glucosea | High A-G | Intermediate A-G | Low A-G | |
| Saturated straight chain | ||||||
| 14:0 | 0.6 ± 0.6 | 1.1b | 1.2b | 0.4 ± 0.1 | 0.6 ± 0.4 | |
| 15:0 | 1.3 ± 0.7 | 1.0 ± 0.1 | ||||
| 16:0 | 15.5 ± 3.5 | 12.0 ± 0.6 | 14.2 ± 3.3 | 23.2 ± 8.1 | 21.2 ± 4.1 | 30.9 ± 3.9 |
| 17:0 | 0.4 ± 0.2 | 1.0 ± 0.6 | 0.5 ± 0.3 | 0.5 ± 0.1 | ||
| 18:0 | 1.9 ± 0.7 | 0.7 ± 0.0 | 5.8 ± 1.5 | 8.8 ± 3.5 | 7.7 ± 5.2 | 5.2 ± 4.2 |
| 20:0 | 4.1 ± 0.9 | 1.1 ± 0.3 | 1.0 ± 0.1 | 2.3 ± 2.9 | ||
| Sum | 22.1 ± 3.7 | 12.7 ± 0.6 | 22.6 ± 3.6 | 36.5 ± 8.9 | 32.1 ± 7.2 | 38.2 ± 5.7 |
| Saturated methyl branched | ||||||
| a17:0 | 0.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.2 | 0.9 ± 0.1 | 0.7 ± 0.6 | |
| i18:0 | 0.5 ± 0.1 | 0.8 ± 0.7 | 1.9 ± 0.7 | 1.3 ± 0.9 | 0.9 ± 1.0 | |
| 10-Me-17:0 | 0.5 ± 0.0 | |||||
| 10-Me-18:0 | 16.9 ± 12.1 | 4.3 ± 0.2 | 4.8 ± 2 | 16.7 ± 10.3 | 14.0 ± 5.0 | 2.6 ± 2.6 |
| Sum | 17.6 ± 12.1 | 4.8 ± 0.2 | 6.8 ± 2.1 | 19.9 ± 10.3 | 16.6 ± 5.1 | 4.9 ± 2.9 |
| Saturated cyclopropyl branched | ||||||
| cy17:0(ω7,8) | 3.8 ± 0.5 | 3.4 ± 0.6 | 10.9 ± 1.4 | 17.8 ± 9.4 | 14.9 ± 0.2 | 29.9 ± 4.9 |
| cy19:0(ω7,8) | 4.2 ± 0.2 | 3.9 ± 0.5 | 55.2 ± 5.9 | 19.2 ± 9.7 | 27.2 ± 8.6 | 13.6 ± 10.5 |
| Sum | 8.0 ± 0.5 | 7.3 ± 0.8 | 66.1 ± 6.1 | 37.0 ± 13.5 | 42.1 ± 8.6 | 43.5 ± 11.6 |
| Unsaturated | ||||||
| 16:1ω7c | 7.5 ± 3.2 | 3.0 ± 0.3 | 1.7b | 4.0b | ||
| 16:1ω9c | 0.5 ± 0.1 | 0 | ||||
| 18:1ω5c | 0.7 ± 0.0 | |||||
| 18:1ω7c | 3.2 ± 1.4 | 54.8 ± 0.1 | 0.2b | 1.2b | 14.1b | |
| 18:1ω9c | 32.7 ± 4.4 | 4.6 ± 0.0 | 0.9b | 7b | 1.3b | |
| a17:1 | 1.5 | 1.4 ± 0.8 | 1.4 ± 0.3 | 1.3 | ||
| a18:1 | 2.2 ± 0.2 | |||||
| 11-Me-18:1ω7c | 4.3 ± 4.0 | 2.7 ± 0.2 | 0.6b | |||
| Sum | 50.4 ± 6.9 | 65.8 ± 0.4 | 1.5b | 2.5b | 11.9 ± 0.3 | 20.7b |
| Hydroxy substituted | ||||||
| 2-OH-14:0 | 0.3 ± 0.0 | 4.5 ± 1.5 | ||||
| 16:0 N alcohol | 1.8 ± 1.2 | |||||
| 16:0 2-OH | 1.0 ± 0.3 | |||||
| 18:1 2-OH | 0.6 ± 0.2 | 1.2 ± 0.4 | 0.7 ± 0.3 | 0.6b | ||
| Sum | 2.1 ± 1.2 | 5.5 ± 1.5 | 0.6 ± 0.2 | 1.2 ± 0.4 | 0.7 ± 0.3 | 0.6b |
| No. of replicates | 3 | 3 | 3 | 2 | 2 | 2 |
Data taken partly from reference 32.
Found in only one of the replicates tested.
TABLE 3.
Relative abundance of GLFA of M. frederiksbergense LB501T grown in minimal medium containing anthracene or glucose as sole carbon source or mixtures of both
| GLFA | Fatty acid content (%) for substrate:
|
|||||
|---|---|---|---|---|---|---|
| Anthracenea | Excess glucosea | Limited glucosea | High A-G | Intermediate A-G | Low A-G | |
| Saturated straight chain | ||||||
| 14:0 | 1.3 ± 0.3 | 0.9 ± 0.0 | 2.2 ± 1.1 | 1.6 ± 0.3 | 1.3 ± 0.5 | 1.4 ± 0.8 |
| 15:0 | 0.8 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.1 | ||
| 16:0 | 11.9 ± 3.9 | 4.4 ± 0.4 | 14.5 ± 4.5 | 8.8 ± 2.8 | 11.3 ± 1.3 | 18.4 ± 2.6 |
| 17:0 | 0.6 ± 0.1 | 0.5 ± 0.3 | 0.4 ± 0.0 | 0.4 ± 0.1 | ||
| 18:0 | 3.4 ± 1.4 | 2.7 ± 0.2 | 7.8 ± 3.2 | 7.6 ± 1.0 | 6.3 ± 2.6 | 5.2 ± 1.4 |
| 20:0 | 1.8 ± 0.5 | 0.4b | 0.5 ± 0.1 | 0.5 ± 0.3 | 0.4 ± 0.1 | |
| Sum | 18.4 ± 4.2 | 8 ± 0.5 | 26.3 ± 5.6 | 19.5 ± 3 | 20.3 ± 3 | 26.3 ± 3.1 |
| Saturated methyl branched | ||||||
| i15:0 | 0.4b | 0.4 ± 0.2 | 0.2 ± 0.0 | 0.2 ± 0.0 | ||
| a15:0 | 0.7 ± 0.4 | 3.1 ± 1.8 | 1.0 ± 0.5 | 1.1 ± 1.2 | 0.8 ± 0.4 | |
| i16:0 | 0.7 ± 0.2 | 0.7 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.5 | ||
| i17:0 | 0.4 ± 0.1 | 0.4 ± 0.4 | 0.6 ± 0.6 | 0.4 ± 0.2 | ||
| a17:0 | 0.7 ± 0.4 | 0.7 ± 0.1 | 2.0 ± 1.4 | 1.0 ± 0.5 | 1.8 ± 1.9 | 1.1 ± 0.4 |
| i18:0 | 0.6 ± 0.4 | 0.6 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.6 | 0.5 ± 0.2 | |
| a18:0 | 3.7 ± 0.2 | 3.0 ± 2.2 | 3.4 ± 1.2 | 3.4 ± 1.7 | 2.3 ± 0.4 | |
| 10-Me-18:0 | 4.0 ± 2.1 | 0.6 ± 0.2 | 2.8 ± 1.2 | 1.7 ± 1.5 | 6.3 ± 8.1 | 1.4 ± 0.9 |
| Sum | 9.7 ± 2.3 | 1.9 ± 0.2 | 12.4 ± 3.4 | 9.6 ± 2.1 | 14.9 ± 8.6 | 7.4 ± 1.3 |
| Saturated cyclopropyl branched | ||||||
| cy17:0(ω7,8) | 5.1 ± 6.3 | 15.3 ± 19.4 | 8.9 ± 4.5 | 19.5 ± 3.5 | ||
| cy19:0(ω7,8) | 0.7 ± 0.2 | 0.3b | 9.2 ± 10.2 | 5.2 ± 4.9 | 13.6 ± 10.1 | 4.1 ± 1.0 |
| Sum | 0.7 ± 0.2 | 0.3b | 14.3 ± 12 | 20.5 ± 20 | 22.5 ± 11 | 23.6 ± 3.6 |
| Unsaturated | ||||||
| 16:1ω7c | 6.4 ± 2.3 | 3.4 ± 0.2 | 3.2 ± 0.4 | 2.4 ± 0.7 | 3.1 ± 1.0 | 4.1 ± 0.6 |
| 16:1ω9c | 0.8 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.4 ± 0.2 | ||
| 17:1ω6 | 2.1 ± 0.2 | |||||
| 17:1ω7c | 4.2 ± 3.0 | 3.3 ± 2.1 | 1.9b | |||
| 17:1ω8c | 0.7 ± 0.3 | 0.8 ± 0.0 | 0.5 ± 0.2 | 0.7 ± 0.0 | ||
| 18:1ω7c | 4.5 ± 1.5 | 22.9 ± 6.7 | 4.1 ± 1.5 | 4.3 ± 2.1 | 5.5 ± 1.9 | 9.2 ± 5.4 |
| 18:1ω9c | 49.0 ± 4.2 | 47.9 ± 7.1 | 29.9 ± 20.8 | 34.7 ± 10.8 | 26.2 ± 7.4 | 25.4 ± 5.0 |
| a17:1 | 1.3b | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.6b | ||
| a18:1 | 3.6 ± 0.1 | 4.2 ± 0.6 | ||||
| 11-Me-18:1ω7c | 1.2 ± 0.9 | 1.0 ± 0.4 | 2.1 ± 2.8 | 0.7 ± 0.5 | 1.8 ± 1.7 | 0.8b |
| Sum | 71.8 ± 5.9 | 83.0 ± 10.1 | 41.3 ± 21 | 45.7 ± 11.0 | 38 ± 7.9 | 40.8 ± 7.4 |
| Hydroxy substituted | ||||||
| 16:0 N alcohol | 0.6 ± 0.3 | 2.7 ± 0.6 | ||||
| Sum | 0.6 ± 0.3 | 2.7 ± 0.6 | ||||
| No. of replicates | 4 | 3 | 3 | 2 | 2 | 2 |
Data taken partly from reference 32.
Detected in only one of the replicates tested.
FIG. 3.
Ratios of the cyclopropyl-branched and the unsaturated fatty acids (A) and the ratios of saturated straight-chain and unsaturated fatty acids (B) as a function of the percentage of anthracene carbon in biomass of M. frederiksbergense LB501T grown on solid anthracene, excess glucose, or various mixtures of anthracene and glucose.
FIG. 4.
Ratios of the 18:0 fatty acids and the sum of 18:1ω7c + 18:1ω9c (A), cy19:0(ω7,8) and the sum of 18:1ω7c + 18:1ω9c (B), and 18:1ω9c and 18:1ω7c (C) as a function of the percentage of anthracene carbon in biomass of M. frederiksbergense LB501T grown on solid anthracene, excess glucose, or various mixtures of anthracene and glucose.
Mycolic acid composition.
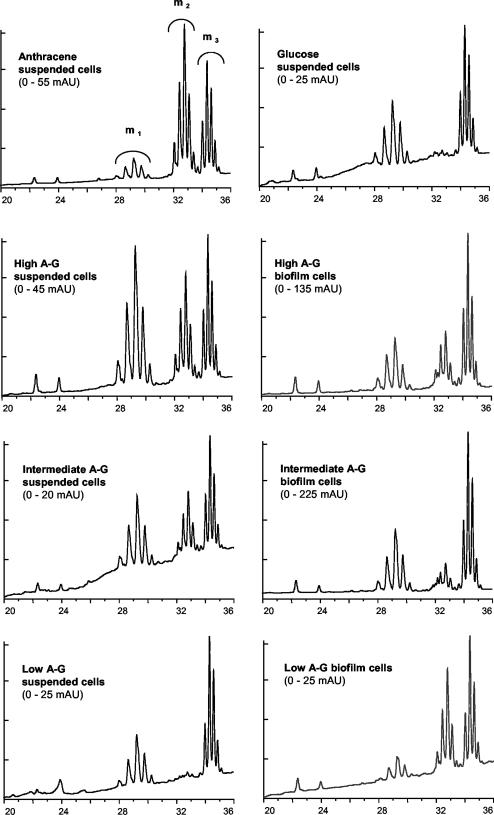
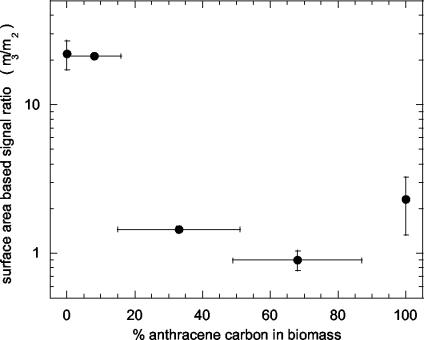
Representative HPLC chromatograms of derivatized mycolic acids of M. frederiksbergense LB501T show a clear difference between mycolic acid profiles of cells grown on anthracene and those of cells grown on glucose (Fig. 5). Anthracene-grown cells exhibited three signal clusters (referred to as multiplets m1, m2, and m3) eluting at 28 to 30.5, 31 to 34, and 34 to 35 min, respectively. In contrast, multiplet m1 was much more pronounced in glucose-utilizing cells, whereas multiplet m2 was nearly absent. When anthracene crystals were present, cultures of M. frederiksbergense LB501T split up into a fraction forming a biofilm on the surfaces of anthracene crystals and a suspended fraction. Suspended and biofilm cells growing on high A-G and intermediate A-G showed all three signal multiplets, including a pronounced multiplet m2. This is in accordance with the results obtained by stable isotope analysis and growth curves of the same cultures. Suspended cells utilizing low A-G mixtures had a similar mycolic acid pattern as did glucose-utilizing cells. In contrast, mycolic acid profiles of biofilm cells from the same culture resembled those of bacteria utilizing anthracene. The ratio of the surface area-based multiplet/signal ratios (m3/m2) of derivatized mycolic acids of suspended M. frederiksbergense LB501T showed a clear relationship with the percentage of anthracene carbon in the biomass of the respective cultures (Fig. 6).
FIG. 5.
HPLC chromatograms depicting mycolic acid profiles of M. frederiksbergense LB501T grown on either solid anthracene, excess glucose, or various mixtures of anthracene and glucose as described in Table 1. A distinction was made between bacteria present in suspension and those in biofilms adhering to anthracene crystals. Signal multiplets of mycolic acids eluting after 28 to 30.5, 31 to 34, and 34 to 35 min are designated as m1, m2, and m3, respectively. Values in parentheses represent the ranges of the y axis in milliabsorption units (mAU) at 260 nm. The x axis shows time in minutes.
FIG. 6.
Surface area-based multiplet-signal ratios (m3/m2) of derivatized mycolic acids of suspended M. frederiksbergense LB501T cells as a function of the percentage of anthracene carbon in biomass calculated from carbon-specific stable isotope measurements (n ≥ 3).
Cell surface hydrophobicity after growth on single and mixed substrates.
Cell surface hydrophobicity, as analyzed by contact angle measurements, reflected the different mycolic acid profiles of the cells from the corresponding cultures. Cells growing in suspension solely on anthracene were more hydrophobic and exhibited a water contact angle (θ) of 85 ± 4, whereas glucose-grown cells were more hydrophilic (θ = 34° ± 6°). Contact angles of cells growing in suspension on mixed substrates were 54° ± 13° (high A-G), 63° ± 12° (intermediate A-G), and 34° ± 1° (low A-G). Despite some scatter of the results obtained with high A-G and intermediate A-G in the triplicate cultures, there appears to be a correlation between contact angles of suspended M. frederiksbergense LB501T and the percentage of anthracene carbon utilized for biomass formation in the respective cultures.
DISCUSSION
Mixed-substrate utilization.
We showed that M. frederiksbergense LB501T degrades solid anthracene in the presence of similar and considerably higher concentrations of dissolved glucose and uses both carbon sources simultaneously for growth. Growth data, stable isotope compositions of biomass, and variations of PLFA-GLFA and mycolic acid profiles as well as concomitant cell surface characteristics led to this conclusion. Our attempt to feed dissolved glucose at controlled rates relative to anthracene dissolution rates revealed that biofilms apparently promoted the dissolution of anthracene beyond the rates observed in the absence of bacteria. This is in accordance with earlier theoretical considerations and observations both suggesting that bacterial biofilms located in the immediate vicinity of solid or sorbed substrates can create very high dissolution fluxes because they drive dissolution from inside the diffusion layer surrounding the substrate source (11, 15). In contrast, dissolution of crystals in bacterium-free aqueous medium is driven by the reduced bulk concentration of the solute through diffusion layers of up to 100 μm in width, depending on the crystal size and shaking velocity. According to Fick's first law of diffusion, the slope of the concentration gradient as the driving force for substrate dissolution is inversely proportional to the distance between the source (the anthracene crystal) and the sink (the biofilm versus the bulk aqueous solution). Growth on anthracene crystals was 10 times faster than on dissolved anthracene provided at nominally equal flux (Fig. 2) and about 6 times faster than on dissolved glucose delivered at the same nominal energy flux rate (Fig. 1). Considering the various assumptions (described in Materials and Methods) made about the energy yield of anthracene versus glucose, this discrepancy of 40% is relatively small and confirms the positive effect of a colonization of the substrate source. The relative contributions of anthracene carbon to the biomass in mixed-substrate cultures were in accordance with calculations of relative energy fluxes from both carbon sources.
PLFA and GLFA as markers for substrate utilization and pollutant-degrading Mycobacterium populations.
A recent study reported on variations of PLFA and GLFA profiles of M. frederiksbergense LB501T that were controlled by both the identity of growth substrates and their bioavailability (32). Anthracene-grown cells showed clear indications of an adaptation to the utilization of this hydrophobic compound. In contrast, cells grown on molar glucose fluxes, which matched the dissolution flux of solid anthracene in the absence of bacteria, showed signs of starvation stress such as a drastically enhanced fraction of cyclic fatty acids (32). Substrate influences on the composition of bacterial membranes need to be known because they may affect the value of PLFA and GLFA as taxonomic markers (13, 21, 23, 32).
Information on substrate influences is also important when δ-13C values of carbon sources are used for an identification of the microbial populations assimilating these substrates, based on comparisons with δ-13C-specific environmental biomarker PLFA-GLFA (e.g., see reference 5). We were therefore interested in how the simultaneous utilization of more than one substrate, such as expected in oligotrophic environments, would influence PLFA and GLFA patterns. Results of PLFA analyses revealed that the relative ratios of straight-chain saturated to the corresponding unsaturated fatty acids and the total fraction of saturated cyclopropyl-branched fatty acid profiles of M. frederiksbergense LB501T depend on the carbon sources and the various rates of addition of mixed substrates (Fig. 3). In cultures growing on mixed substrates a continuous increase of the relative ratios of straight-chain saturated to the corresponding unsaturated fatty acids and the saturated cyclopropyl-branched to the unsaturated fatty acids was observed with increasing anthracene carbon fractions in the biomass. No such trend was observed with GLFA or with cultures growing on single carbon sources such as anthracene or excessively available glucose. Many studies have shown that bacteria can change their membrane fluidity by modifying their membrane-bound PLFA and GLFA profiles. Typical changes include increased ratios of saturated to unsaturated fatty acids or increased fractions of cyclopropyl fatty acids (29). They were found in response to environmental stress conditions such as chemical contamination (23), drought (18), temperature changes (16), starvation (13), and membrane-active substances (25). Based on this reasoning, cells grown on limited glucose and on A-G mixtures, particularly those on intermediate A-G, would exhibit signs of stress, whereas cells growing on solid anthracene or excess glucose would not exhibit such signs (Fig. 3 and 4).
Our expectation of a stepwise transition from the anthracene pattern via the various mixtures to the excess glucose pattern was indeed partly fulfilled, e.g., for the ratios of the most abundant unsaturated PLFA and GLFA, 18:1ω9c and 18:1ω7c (Tables 2 and 3). Figure 4C shows the dependence of this ratio on the content of anthracene carbon in the biomass. A further important finding was the presence of the mycobacterium-specific PLFA 10-Me-18:0 (tuberculostearic acid) in all cultures in proportions between 3 and 17% (Table 2). Despite some scatter in the data and a probable trend toward higher relative fractions of this PLFA in cells predominantly grown on anthracene, it appears that the presence of this PLFA in soil extracts can be used to identify utilizing mycobacteria regardless of their actual growth substrate(s). It was shown before that the δ-13C labels of PLFA-GLFA reflect those of the growth-supporting carbon source (32). Together with the new information about their relative quantities in the biomass of mixed-substrate-utilizing populations, this may help to link in situ substrate utilization to Mycobacterium spp. populations.
Mycolic acids as markers for substrate utilization and pollutant-degrading Mycobacterium populations.
Long-chain (C60 to C90) mycolic acids are restricted to the genus Mycobacterium, which makes them a powerful criterion for the in situ identification of this genus. In a recent study (31, 33) it was shown that the average chain length of mycolic acids of several Mycobacterium spp. and their concomitant cell surface hydrophobicities were positively correlated with the aqueous solubility of their growth substrates. Here we showed that this relationship also exists with the average hydrophobicities of mixtures of the hydrophobic substrate anthracene and the hydrophilic substrate glucose. Higher proportions of glucose in the substrate mixture led to earlier-eluting mycolic acids and lower cell surface hydrophobicities. Even more importantly, the presence of distinct length classes of mycolic acids, visible as peak multiplets in HPLC chromatograms, provided information about the extents of the utilization of one or the other substrate in the provided mixture. In our setup it seems that a pronounced multiplet m1 is linked to glucose utilization whereas multiplet m2 follows anthracene utilization (33). Suspended and biofilm cells growing on high A-G and intermediate A-G showed all three signal multiplets, including a pronounced multiplet m2, indicating simultaneous utilization of glucose and anthracene, whereas suspended cells utilizing low-A-G mixtures had a similar mycolic acid pattern as did glucose-utilizing cells. Mycolic acid profiles of biofilm cells from the same culture, however, resembled those of bacteria utilizing anthracene. This indicates an interesting split-up of the culture in glucose-utilizing suspended cells and anthracene-utilizing biofilm cells. Future work will thus have to further calibrate these relationships by relating the relative quantities of structurally well-characterized mycolic acids and their length classes to their δ-13C labels.
Implications for bioremediation.
The finding that M. frederiksbergense LB501T degrades and grows on solid anthracene in the presence of much more bioavailable dissolved glucose is of special interest, as PAH degraders isolated from PAH-contaminated soils are often mycobacteria, indicating that this genus might play a major role in the biodegradation of PAHs in contaminated soils (3, 17). Mixed-carbon-source utilization is of high relevance for PAH bioremediation, as degradation of poorly bioavailable carbon sources alone is unlikely to bring about the metabolically active biomass needed to remedy contaminated soils (6). In ecological terms, the carrying capacity of a contaminated environment for a pollutant-degrading microbial population may rise when it provides additional growth-supporting carbon sources. The capability of PAH-degrading bacteria to simultaneously utilize readily available carbon sources may therefore be used to decouple the formation of actively pollutant-degrading biomass from the pollutant degradation itself. An important consequence of mixed-substrate maintenance of bacterial population is reduced environmental threshold concentrations of each of the individual substrates (22). For instance, it has been observed that many natural and xenobiotic carbon sources can be utilized simultaneously when present at concentrations of nanograms per liter or lower (2, 22, 26). Simultaneous utilization of the potentially available carbon sources at concentrations lower than those observed during single-substrate growth may thus be a crucial factor for the efficient and fast growth of microorganisms in the environment (22). Addition of extra carbon sources may therefore promote bioremediation, provided that it sustains the pollutant-degrading population rather than promoting degradation by other members of the microbial community.
Acknowledgments
Financial support of this study by EC Biotech programs (contracts BIO4-CT97-2015 and QLK3-CT-1999-00326) and the Swiss Federal Office for Education and Science (contracts 96.0404 and 99.0366) is greatly acknowledged.
REFERENCES
- 1.Abraham, W.-R., H. Meyer, S. Lindholst, M. Vancanneyt, and J. Smit. 1997. Phospho- and sulfolipids as biomarkers of Caulobacter sensu lato, Brevundimonas and Hyphomonas. Syst. Appl. Microbiol. 20:522-539. [Google Scholar]
- 2.Alexander, M. 1999. Biodegradation and bioremediation, 2nd ed. Academic Press, San Diego, Calif.
- 3.Bastiaens, L., D. Springael, P. Wattiau, H. Harms, R. de Wachter, H. Verachtert, and L. Diels. 2000. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microbiol. 66:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bligh, E. G., and W. M. Dyer. 1959. A rapid method of lipid extraction and purification. Can. J. Biochem. Physiol. 35:911-917. [DOI] [PubMed] [Google Scholar]
- 5.Boschker, H. T. S., and J. J. Middelburg. 2002. Stable isotopes and biomarkers in microbial ecology. FEMS Microbiol. Ecol. 40:85-95. [DOI] [PubMed] [Google Scholar]
- 6.Bosma, T. N. P., P. J. M. Middeldorp, G. Schraa, and A. J. B. Zehnder. 1997. Mass transfer limitation of biotransformation: quantifying bioavailability. Environ. Sci. Technol. 31:248-252. [Google Scholar]
- 7.Butler, W. R., and J. O. Kilburn. 1988. Identification of major slowly growing pathogenic mycobacteria and Mycobacterium gordonae by high-performance liquid chromatography of their mycolic acids. J. Clin. Microbiol. 26:50-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egli, T. 1995. The ecological and physiological significance of the growth of heterotrophic microorganisms with mixtures of substrates. Adv. Microb. Ecol. 14:305-386. [Google Scholar]
- 9.Fredrickson, H. L., T. E. Cappenberg, and J. W. D. Leeuw. 1986. Polar lipid ester-linked fatty acid composition of Lake Vechten Seston: an ecological application of lipid analysis. FEMS Microbiol. Ecol. 38:381-396. [Google Scholar]
- 10.Friedrich, M., R. J. Grosser, A. Kern, W. P. Inskeep, and D. M. Ward. 2000. Effect of model sorptive phases on phenanthrene degradation: molecular analysis of enrichments and isolates suggests selection based on bioavailability. Appl. Environ. Microbiol. 66:2703-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia, J. M., L. Y. Wick, and H. Harms. 2000. Influence of the nonionic surfactant Brij 35 on the bioavailability of solid and sorbed dibenzofuran. Environ. Sci. Technol. 66:2033-2039. [DOI] [PubMed] [Google Scholar]
- 12.Grosser, R. J., M. Friedrich, D. M. Ward, and W. P. Inskeep. 2000. Influence of different chemical treatments on transport of Alcaligenes paradoxus in porous media. Appl. Environ. Microbiol. 61:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guckert, J. B., M. A. Hood, and D. C. White. 1986. Phospholipid ester-linked fatty acid profile change during nutrient deprivation of Vibrio cholerae: increases in trans/cis ratio and proportions of cyclopropyl fatty acids. Appl. Environ. Microbiol. 52:794-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerin, W. F., and S. A. Boyd. 1992. Differential bioavailability of soil-sorbed naphthalene to two bacterial species. Appl. Environ. Microbiol. 58:1142-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms, H., and A. J. B. Zehnder. 1995. Bioavailability of sorbed 3-chlorodibenzofuran. Appl. Environ. Microbiol. 61:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahnke, L. L. 1992. The effects of growth temperature on the methyl sterol and phospholipid fatty acid composition of Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 93:209-212. [DOI] [PubMed] [Google Scholar]
- 17.Kanaly, R. A., and S. Harayama. 2000. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons. J. Bacteriol. 182:2059-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieft, T. L., D. B. Ringelberg, and D. C. White. 1994. Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium. Appl. Environ. Microbiol. 60:3292-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleikemper, J., O. Pelz, M. H. Schroth, and J. Zeyer. 2002. Sulfate-reducing bacterial community response to carbon source amendments in contaminated aquifer microcosms. FEMS Microbiol. Ecol. 42:109-118. [DOI] [PubMed] [Google Scholar]
- 20.Kovárová-Kovar, K., and T. Egli. 1998. Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol. Mol. Biol. Rev. 62:646-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laura, D., G. DeSocio, R. Frassanito, and D. Rotilo. 1996. Effects of atrazine on Ochrobactrum anthropi membrane fatty acids. Appl. Environ. Microbiol. 62:2644-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lendenmann, U., M. Snozzi, and T. Egli. 1996. Kinetics of the simultaneous utilization of sugar mixtures by Escherichia coli in continuous culture. Appl. Environ. Microbiol. 62:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacNaughton, S. J., J. R. Stephen, A. D. Venosa, G. A. Davis, Y. Y. Chang, and D. C. White. 1999. Microbial population changes during bioremediation of an experimental oil spill. Appl. Environ. Microbiol. 65:3566-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelz, O., C. Hesse, M. Tesar, R. B. Coffin, and W. R. Abraham. 1997. Development of methods to measure carbon isotope ratios of bacterial biomarkers in the environment. Isotop. Environ. Health Stud. 33:131-144. [Google Scholar]
- 25.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1995. Mechanism of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subba-Rao, R. V., H. E. Rubin, and M. Alexander. 1982. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl. Environ. Microbiol. 43:1139-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wattiau, P., D. Springael, S. N. Agathos, and S. Wuertz. 2002. Use of the pAL5000 replicon in PAH-degrading mycobacteria: application for strain labelling and promoter probing. Appl. Microbiol. Biotechnol. 59:700-705. [DOI] [PubMed] [Google Scholar]
- 29.White, D. C., H. C. Pinkart, and D. B. Ringelberg. 1997. Biomass measurements: biochemical approaches, p. 91-101. In C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Walter (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 30.Wick, L. Y., T. Colangelo, and H. Harms. 2001. Kinetics of mass transfer-limited bacterial growth on solid PAHs. Environ. Sci. Technol. 35:354-361. [DOI] [PubMed] [Google Scholar]
- 31.Wick, L. Y., A. Ruiz de Munain, D. Springael, and H. Harms. 2002. Responses of Mycobacterium sp. LB501T to the low bioavailability of solid anthracene. Appl. Microbiol. Biotechnol. 58:378-385. [DOI] [PubMed] [Google Scholar]
- 32.Wick, L. Y., O. Pelz, S. M. Bernasconi, N. Andersen, and H. Harms. 2003. Influence of the growth substrate on ester-linked phospho- and glycolipid fatty acids of Mycobacterium sp. LB501T. Environ. Microbiol. 5:672-680. [DOI] [PubMed] [Google Scholar]
- 33.Wick, L. Y., P. Wattiau, and H. Harms. 2002. Influence of the growth substrate on the mycolic acid profiles of mycobacteria. Environ. Microbiol. 4:612-616. [DOI] [PubMed] [Google Scholar]
- 34.Willumsen, P., U. Karlson, E. Stackebrandt, and R. M. Kroppenstedt. 2001. Mycobacterium frederiksbergense sp. nov., a novel polycyclic aromatic hydrocarbon-degrading Mycobacterium species. Int. J. Syst. Evol. Microbiol. 51:1715-1722. [DOI] [PubMed] [Google Scholar]