Abstract
The range of sulfur compounds in fuel oil and the substrate range and preference of the biocatalytic system determine the maximum extent to which sulfur can be removed by biodesulfurization. We show that the biodesulfurization apparatus in Rhodococcus sp. strain ECRD-1 is able to attack all isomers of dibenzothiophene including those with at least four pendant carbons, with a slight preference for those substituted in the α-position. With somewhat less avidity, this apparatus is also able to attack substituted benzothiophenes with between two and seven pendant carbons. Some compounds containing sulfidic sulfur are also susceptible to desulfurization, although we have not yet been able to determine their molecular identities.
Oil refineries remove most of the sulfur initially present in diesel fuel distillate fractions by hydrodesulfurization, a process in which sulfur-containing species react with high-pressure hydrogen, in the presence of a catalyst, to produce H2S and desulfurized products (17). The diverse sulfur-containing hydrocarbons present in these oils react differentially in this process, and sterically hindered dibenzothiophenes are among the most resistant (10). Biodesulfurization, a process in which sulfur is removed by an enzymatic process that yields sulfate, may provide an alternative or perhaps complementary technology for attaining low-sulfur fuels, particularly in light of the ever more stringent limits for residual sulfur content in these fuels (4). Despite considerable progress over the last decade (7, 12, 13), there are still many questions about the biodesulfurization process. Most microbiological work has focused on model compounds, particularly dibenzothiophene and occasionally its alkylated forms, but rarely in real refinery feeds. Folsom et al. (5) have reported the biodesulfurization of a previously hydrodesulfurized middle distillate cut (boiling range, 149 to 428°C) that contained 1,850 ppm total sulfur prior to the bioprocess. X-ray absorption spectroscopy indicated that >95% of this sulfur was thiophenic. Biodesulfurization with Rhodococcus erythropolis I-19 reduced the sulfur level to 615 ppm, and the residual sulfur consisted of some 75% thiophenic, 11% sulfidic, and 14% partially oxidized and oxygenated species. Folsom et al. reported the identification of the thiophenic forms by using gas chromatography with mass spectrometry and concluded that the overall rate of biodesulfurization was affected and limited by the concentration and distribution of the alkylated dibenzothiophenes.
We too have studied the biodesulfurization of a hydrodesulfurized refinery oil fraction, in our case light catalytic-cycle oil (LCCO) with a middle distillate boiling range of 175 to 350°C (9). Biodesulfurization by Rhodococcus sp. strain ECRD-1 reduced the sulfur content of the oil from 669 to 56 ppm sulfur. X-ray absorption spectroscopy indicated that all the sulfur-containing species in the initial oil were thiophenic; after desulfurization, some 62% of the residual sulfur was thiophenic while 37% was more oxidized, with the latter essentially equally divided between sulfones and sultines. In addition, we have studied biodesulfurization of a straight-run middle distillate fraction of Oregon Basin crude oil that had not been hydrotreated (OB oil) (9). In these experiments, the sulfur content was reduced from 20,000 to 14,000 ppm and 60% of the remaining sulfur was present as oxidized and oxygenated species.
In this study, we examined the apparent substrate specificity of the biodesulfurization process in these refinery feeds (8, 9, 11). The genes encoding dibenzothiophene desulfurization have been extensively characterized, and they have been named sox (sulfur oxidation) by Denome et al. (2) and dsz (desulfurization) by Piddington et al. (16). Both groups identified three genes, forming a single operon, that were necessary and sufficient to confer the desulfurization activity to recipient Rhodococcus and Escherichia coli strains. Moreover, the desulfurization ability of Rhodococcus sp. strain ECRD-1, the prototype desulfurization strain IGTS8, and other desulfurization strains appears to be an exclusive property of a 4-kb gene locus on a large plasmid (1), the loss of which results in the loss of the desulfurization phenotype. Taken together, these results indicate that the only desulfurization system operating in Rhodococcus sp. strain ECRD-1 is the dsz or sox system. We therefore attribute both the induced and constitutive low-level desulfurization activities in this strain to this system and use the latter to reveal the relative substrate preferences of the system when the middle distillate fraction of OB oil is undergoing desulfurization.
MATERIALS AND METHODS
Rhodococcus sp. strain ECRD-1 was grown as previously described (11), with the refinery oils (untreated OB oil and hydrotreated LCCO, both middle distillate oils) as the sole sources of sulfur except in some experiments with the OB oil in which sulfate (0.2 g of MgSO4 · 0.7H2O liter−1) was added. In the presence of sulfate, desulfurization activity is not induced and only constitutive levels of the activity are present (8, 9, 11). Oils were added at 1 ml per liter of culture. To preserve oil hydrocarbons, sodium acetate (10 g of sodium acetate per liter) and 10 ml of decane per liter were added as carbon sources; the organisms consumed this in preference to the hydrocarbons in the oils (9). After 7 days, oil was extracted from the cultures by using methylene chloride as previously described (9).
Gas chromatography coupled with mass spectrometry was carried out as previously described (3), both in the total-ion mode and with selected ion monitoring. Benzothiophene, dibenzothiophene, 4,6-dimethylbenzothiophenes, and 4,6-diethyldibenzothiophenes were identified using authentic standards (9), and other alkylated dibenzothiophenes were identified based on the assignments of Mössner and Wise (14). The desulfurization pathway has been shown to proceed primarily through the sulfone and sultine (the sulfinic acid lactone) (6) and to a lesser extent the sultone (the sulfonic acid lactone) forms (15) to yield hydroxylated forms. The respective hydroxybiphenyls, sulfones, and sultines were searched for, without success, by extrapolating the molecular masses and spectra from those of authentic standards of 2-phenylphenol, dibenzothiophene sulfone and sultine, respectively (7).
RESULTS
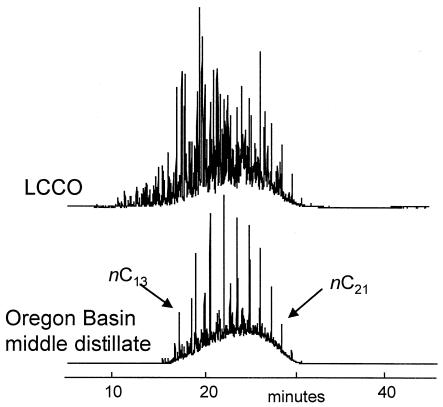
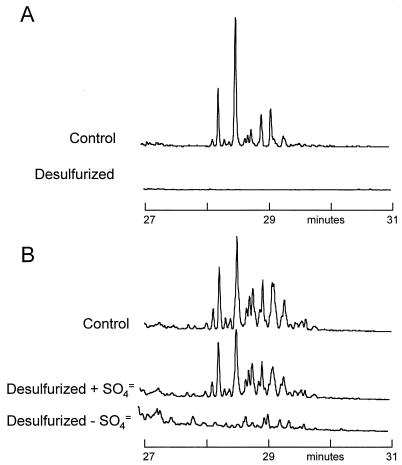
Figure 1 shows the total-ion chromatograms of the two oils before biodesulfurization. These chromatograms remain essentially unchanged after biodesulfurization, indicating that no significant hydrocarbon degradation occurred (data not shown). In order to study the loss of individual sulfur heterocycles in the biodesulfurization process, it was necessary to identify internal conserved species within the oil. Ideally such species should not be affected during the desulfurization process and have chromatographic properties similar to those of the compounds under study. As shown in Fig. 2, phenanthrene and its alkylated forms served as appropriate internal species for this study; there was no evidence of any degradation during desulfurization, the phenanthrenes were present in appropriately high concentrations, and the phenanthrenes had chromatographic properties similar to those of the dibenzothiophenes and substituted benzothiophenes that are the principal sulfur-containing species in these oils. All subsequent figures have been normalized to the amount of phenanthrene or the respective alkylated forms in the oils.
FIG. 1.
Total-ion gas chromatography-mass spectrometry chromatograms of the two oils used, an LCCO and a middle distillate cut of OB crude oil.
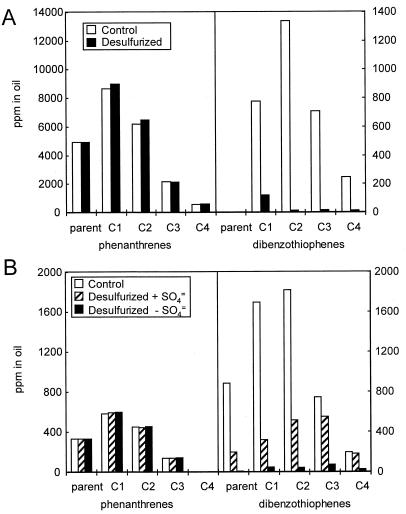
FIG. 2.
Concentrations of phenanthrenes and dibenzothiophenes in LCCO (A) and a mid-distillate cut of OB oil (B) before and after biodesulfurization. The Cn designations on the abscissa indicate the alkylated compounds with the indicated number (n) of pendant carbons; e.g., C1, all the methyl isomers; C2, all the dimethyl and ethyl isomers, etc.
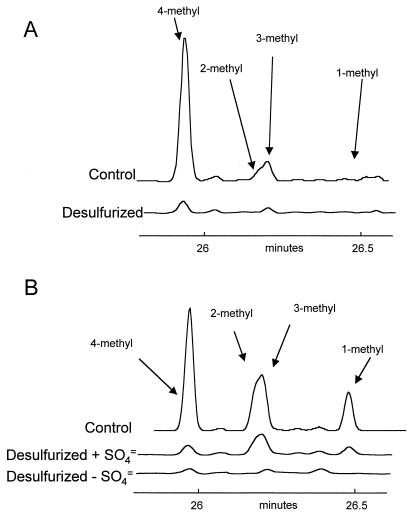
The LCCO contains essentially no dibenzothiophene but has significant amounts of alkylated dibenzothiophenes, up to those with at least four pendant methyl groups (C4). The mass per charge (m/z) for alkylated species increases by 14 for each additional carbon, regardless of whether it is an independent methyl substituent or an additional carbon in a side chain (e.g., ethyl to propyl). Thus, it is difficult to distinguish, for example, dimethyl- and ethyl-substituted compounds, although it is straightforward to distinguish these from diethyl-substituted forms. Figure 3 shows the methyl dibenzothiophenes (C1 dibenzothiophenes), which were identified by using the parameters described by Mössner and Wise (14). The distribution of isomers in the initial oils is a reflection both of the intrinsic history of the oils and their prior processing. The LCCO had been extensively hydrodesulfurized during its production, while the middle distillate OB oil cut had merely been distilled. The total amounts of methyldibenzothiophenes (Fig. 2) reflect this, namely, 800 ppm in the LCCO [it is important to note that this and other references to the amount of a compound(s) in the oils refers to the concentration of the compound(s), not of the associated sulfur] and 1,700 ppm in the OB oil cut. Furthermore, the relative abundance of the isomers is consistent with the expectation that dibenzothiophenes substituted α to the sulfur (positions 4 and 6) are the most resistant to hydrodesulfurization, since a comparison of the relative abundances of 4-methyldibenzothiophene in the two oils suggests that the other isomers have been preferentially removed from the LCCO. Interestingly, it is clear from the OB oil treated in the presence of sulfate that the 4-methyl derivative is the most readily removed by biodesulfurization and that the 3-methyl derivative is the least susceptible to removal (Fig. 3B). Nevertheless, traces of 4-methyldibenzothiophene and to a lesser degree 3-methyldibenzothiophene appear to remain in both oils after extensive biodesulfurization. This suggests that the concentration of these compounds becomes rate limiting for the initial enzyme of the biodesulfurization pathway at the levels achieved during biodesulfurization. In contrast, 1-methyl dibenzothiophene is removed to below detection levels, which we estimate to be about 2 ppm in the oil.
FIG. 3.
Methyl dibenzothiophenes (m/z = 198) in LCCO (A) and a mid-distillate cut of OB oil (B) before and after desulfurization.
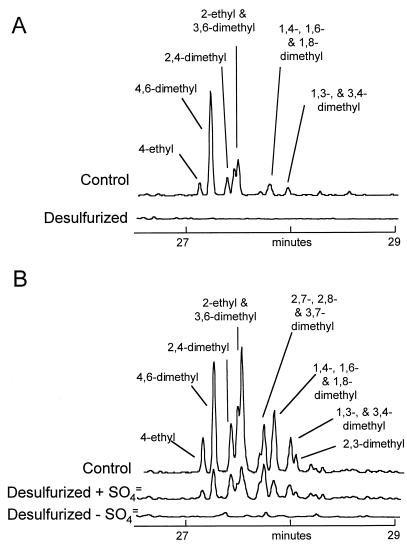
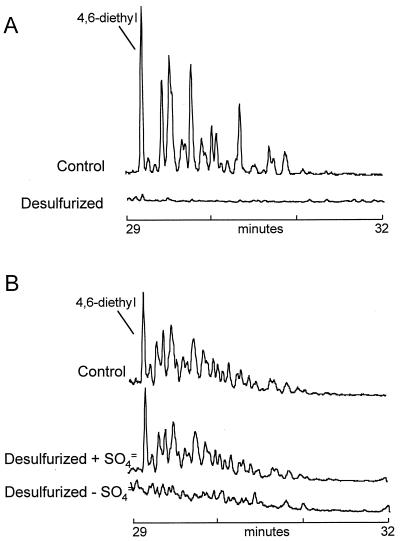
Figures 4, 5, and 6 show similar data for the C2 (dimethyl and ethyl), C3 (trimethyl, methyl-ethyl, and propyl), and C4 (tetramethyl, diethyl, methyl-propyl, and butyl) dibenzothiophenes. Again, the initial distribution of the isomers was consistent with the preferential removal by hydrotreatment of non-α substituted compounds from the LCCO, with 4,6-dimethyl and 4,6-diethyl substituents being particularly conserved. In contrast, the biodesulfurization of these compounds, in the fully induced system, was essentially complete, although the biodesulfurization by the constitutive system decreased with increasing alkylation. Nevertheless, the use of the constitutive system allowed us to see a clear isomer preference for the dimethyl dibenzothiophenes. This is shown graphically in Fig. 7. For example, 4-ethyldibenzothiophene and 4,6-dimethyldibenzothiophenes were desulfurized more extensively than was 2,4-dimethyldibenzothiophene. Interestingly, 4-methyldibenzothiophene seemed to have been removed more extensively, at least on a percentage basis, than its unalkylated parent, and indeed the aggregate methyl dibenzothiophenes were removed in preference to the parent.
FIG. 4.
Dimethyl and ethyl dibenzothiophenes (m/z = 212) in LCCO (A) and a mid-distillate cut of OB oil (B) before and after desulfurization.
FIG. 5.
Trimethyl, ethyl-methyl, and propyl dibenzothiophenes (m/z = 226) in LCCO (A) and a mid-distillate cut of OB oil (B) before and after desulfurization.
FIG. 6.
Tetramethyl, diethyl, propyl-methyl, and butyl dibenzothiophenes (m/z = 240) in LCCO (A) and a mid-distillate cut of OB oil (B) before and after desulfurization.
FIG. 7.
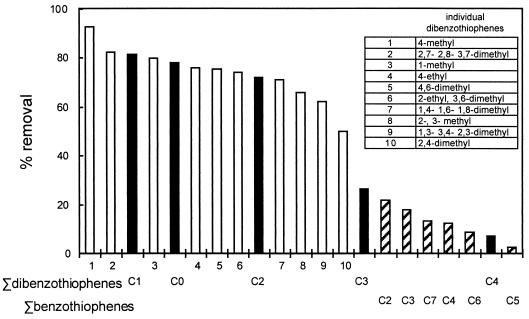
Relative removal of dibenzothiophenes and benzothiophenes from the middle-distillate cut of OB oil in the presence of sulfate with the desulfurization system operating at only constitutive levels. Compounds are ranked in order of their relative depletion. Bars with solid and hatched lines represent the sum of the results for alkylated compounds with n pendant carbons, with C0 indicating the unalkylated parent; open bars represent individual dibenzothiophenes.
Figure 7 also includes the alkylated benzothiophenes (C2 benzothiophenes [m/z = 162 etc.). The distillation cuts under study here do not contain any unalkylated benzothiophene or methyl], C3 benzothiophenes [m/z = 176], benzothiophene, but there are significant amounts of C2 to C7 benzothiophenes in the unprocessed middle distillate cut of the OB oil. They were essentially undetectable in the LCCO, presumably because they were removed during hydrodesulfurization processing. Figure 7 shows that the substituted benzothiophenes are not as effectively desulfurized by the constitutive system as the dibenzothiophenes.
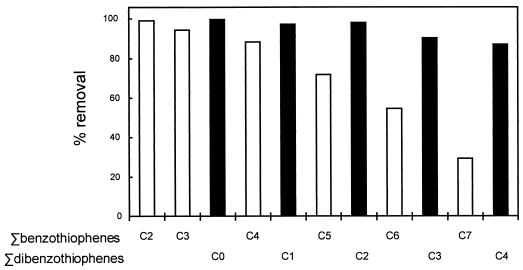
Figure 8 shows the maximum extent of desulfurization achieved for the alkylated benzothiophenes and dibenzothiophenes in the middle distillate cut of OB oil when treated by the fully induced biodesulfurization system. Alkylated dibenzothiophenes with up to four pendant carbons are all extensively depleted, with slightly greater removal of C0 to C2 isomers than of C3 to C4 isomers. The desulfurization of the alkylbenzothiophenes also exhibited the preferential removal of some isomers (cf. Fig. 3 and 4 and data not shown) but with a much more pronounced preference for the less substituted forms.
FIG. 8.
Relative removal of alkylated dibenzothiophenes and benzothiophenes from the middle-distillate cut of OB oil with the desulfurization system fully induced. The bars represent the sum of the results for alkylated compounds with n pendant carbons, with C0 indicating the unalkylated parent; they are ranked in order of their approximate elution from a gas chromatography column (i.e., in order of their approximate boiling points).
DISCUSSION
Biodesulfurization offers the potential of removing sulfur from distillate fuels, especially in the diesel range, to very low levels. Whether the process will be able to reliably meet the recently adopted U.S. Environmental Protection Agency mandate (4) of 15 ppm sulfur in highway diesel fuel, to be phased in starting in 2006, remains to be seen. Clearly it is important to understand the limitations of the current microbial strains, at the molecular level, if such targets are to be reliably achieved by biodesulfurization.
Rhodococcus sp. strain ECRD-1 was initially selected for its ability to remove the sulfur from 4,6-diethyldibenzothiophene, so it is not surprising that it was effective at completely removing this compound from both oils (Fig. 6). Indeed, there is no evidence that any dibenzothiophene isomer with up to four carbon substituents is particularly resistant to desulfurization, even though there is a slight preference for compounds substituted in the α-position (C4 and/or C6). We were unable to rigorously detect dibenzothiophenes with more than four substituent carbons, but the order of elution of the C4 substituted forms from the gas chromatography column relative to that of the whole oil suggests that the oils used here may have contained some C5 and C6 substituted forms. These compounds may be partially responsible for the substantial amount of sulfur still present in the biodesulfurized middle distillate cut of the OB oil. As discussed below, there are other candidates as well.
The desulfurization system of Rhodococcus sp. strain ECRD-1 is clearly less active against alkylated benzothiophenes than against alkylated dibenzothiophenes, but even in this case there is no evidence that any particular isomer is completely resistant; on the contrary, they all seem to serve as rather poor substrates, and this phenomenon becomes more apparent with increasing alkylation. We were unable to rigorously detect benzothiophenes with more than seven substituent carbons, but the distillation properties of the C7 substituted forms suggest that the oils used here may have contained benzothiophenes with more than 15 pendant carbons. Small benzothiophenes are readily removed during hydrodesulfurization (10, 17), and they were absent from the LCCO, but these larger benzothiophenes may be important contributors to the residual sulfur in the unprocessed mid-distillate cut.
X-ray absorption spectroscopy indicated that all the sulfur in the LCCO was originally thiophenic (i.e., associated with aromatic rings), not sulfidic (i.e., with alkyl ligands) (9), presumably because any sulfidic sulfur had been removed in the hydrotreatment that this oil had received prior to biodesulfurization. After the biodesulfurization, more than 92% of the sulfur had been removed and approximately 37% of the residuum was present as more oxidized forms of sulfur that could be modeled reasonably well as sulfones and sultines. This implies that the first enzyme(s) in the desulfurization process has a broader specificity, or at least a higher activity, than subsequent ones. We attempted to identify the partially oxidized forms, looking for alkylated forms of dibenzothiophene-sulfone and -sultine, but without success. We were also unable to detect the predicted hydroxylated end products of the biodesulfurization process because the oil contained many hydrocarbons that had the same molecular mass per charge as the expected products. Thus, the sensitivity to additional compounds introduced by biodesulfurization was too low to allow us to detect the compounds even if there had been a quantitative conversion.
In contrast, the sulfur in the nonhydrotreated mid-distillate cut from OB oil was approximately 50% sulfidic and 50% thiophenic (9). Thirty percent of the initial sulfur was removed in the biodesulfurization, and approximately equal amounts of the two forms were removed. Approximately half of the residual sulfur was more oxidized than the original forms, but it is not clear whether these came from one or both of the precursor forms. As with the LCCO, we attempted to identify the partially oxidized forms by looking for alkylated forms of benzothiophene-sulfone and -sultine and dibenzothiophene-sulfone and -sultine, but without success. Again we were unable to detect fully desulfurized products because a high background from hydrocarbons overwhelmed the sensitivity of our experiments to such products.
In summary, we have shown that the biodesulfurization apparatus in Rhodococcus sp. strain ECRD-1 is able to attack all isomers of dibenzothiophene up to those with at least 4 pendant carbons, with a slight preference for those substituted in the α-position. With somewhat less avidity, ECRD-1 is also able to attack substituted benzothiophenes with between 2 and 7 pendant carbons. The organism is also able to attack at least some compounds containing sulfidic sulfur, although we have not been able to identify their molecular identities. It is important to note, however, that the experiments reported here describe the substrate preference of the initial enzymes of the desulfurization pathway. X-ray absorption spectroscopy (8, 9) indicates that some of the sulfur is only partially oxidized and that the residual oil contains sulfones and sultines, suggesting that enzymes occurring later in the desulfurization pathway may not have such a broad specificity. These enzymes are a promising target for future efforts to maximize biodesulfurization effectiveness. Nevertheless, biodesulfurization remains a promising technology that may have a role in meeting present and future regulations on the sulfur content of mid-distillate fuels, perhaps especially in combination with current hydrotreatment technology, which tends to reduce the range of sulfur compounds present to those most susceptible to biodesulfurization.
REFERENCES
- 1.Denis-Larose, C., D. Labbé, H. Bergeron, A. M. Jones, C. W. Greer, J. Al-Hawari, M. J. Grossman, B. M. Sankey, and P. C. K. Lau. 1997. Conservation of plasmid-encoded dibenzothiophene desulfurization genes in several rhodococci. Appl. Environ. Microbiol. 63:2915-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denome, S. A., C. Oldfield, L. J. Nash, and K. D. Young. 1994. Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. J. Bacteriol. 176:6707-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas, G. S., K. J. McCarthy, D. T. Dahlen, J. A. Seavey, W. G. Steinhauer, R. C. Prince, and D. L. Elmendorf. 1992. The use of hydrocarbon analyses for environmental assessment and remediation. J. Soil Contam. 1:197-216. [Google Scholar]
- 4.Federal Register. 2001. Regulation of fuels and fuel additives. Fed. Regist. 66:5135-5193. [Google Scholar]
- 5.Folsom, B. R., D. R. Schieche, P. M. DiGrazia, J. Werner, and S. Palmer. 1999. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Appl. Environ. Microbiol. 65:4967-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray, K. A., O. S. Pogrebinsky, G. T. Mrachko, L. Xi, D. J. Monticello, and C. H. Squires. 1996. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat. Biotechnol. 14:1705-1709. [DOI] [PubMed] [Google Scholar]
- 7.Grossman, M. J. 2002. Biodesulfurization. In I. T. Horváth (ed.), Encyclopedia of catalysis. John Wiley & Sons. [Online.] http://www.mrw.interscience.wiley.com/enccat/index.html. Accessed 29 August 2003.
- 8.Grossman, M. J., M. K. Lee, R. C. Prince, K. K. Garrett, G. N. George, and I. J. Pickering. 1999. Microbial desulfurization of a crude oil middle-distillate fraction: analysis of the extent of sulfur removal and the effect of removal on remaining sulfur. Appl. Environ. Microbiol. 65:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman, M. J., M. K. Lee, R. C. Prince, V. Minak-Bernero, G. N. George, and I. J. Pickering. 2001. Deep desulfurization of extensively hydrodesulfurized middle distillate oil by Rhodococcus sp. strain ECRD-1. Appl. Environ. Microbiol. 67:1949-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabe, T., A. Ishihara, and H. Tajima. 1992. Hydrodesulfurization of sulfur-containing polyaromatic compounds in light oil. Ind. Eng. Chem. Res. 31:1577-1580. [Google Scholar]
- 11.Lee, M. K., J. D. Senius, and M. J. Grossman. 1995. Sulfur-specific microbial desulfurization of sterically hindered analogs of dibenzothiophene. Appl. Environ. Microbiol. 61:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland, B. L., D. J. Boron, W. Deever, J. A. Meyer, A. R. Johnson, and R. Atlas. 1998. Biocatalytic sulfur removal from fuels: applicability for producing low sulfur gasoline. Crit. Rev. Microbiol. 24:99-147. [DOI] [PubMed] [Google Scholar]
- 13.Monticello, D. J. 2000. Biodesulfurization and the upgrading of petroleum distillates. Curr. Opin. Biotechnol. 11:540-546. [DOI] [PubMed] [Google Scholar]
- 14.Mössner, S. G., and A. A. Wise. 1999. Determination of polycyclic aromatic sulfur heterocycles in fossil fuel-related samples. Anal. Chem. 71:58-69. [DOI] [PubMed] [Google Scholar]
- 15.Ohshiro, T., T. Kojima, K. Torii, H. Kawasoe, and Y. Izumi. 1999. Purification and characterization of dibenzothiophene (DBT) sulfone monooxygenase, an enzyme involved in DBT desulfurization, from Rhodococcus erythropolis D-1. J. Biosci. Bioeng. 88:610-616. [DOI] [PubMed] [Google Scholar]
- 16.Piddington, C. S., B. R. Kovacevich, and J. Rambosek. 1995. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl. Environ. Microbiol. 61:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speight, J. G. 1981. The desulfurization of heavy oils and residua. Marcel Dekker, New York, N.Y.