Abstract
One strategy for delaying evolution of resistance to Bacillus thuringiensis crystal (Cry) endotoxins is the production of multiple Cry toxins in each transgenic plant (gene stacking). This strategy relies upon the assumption that simultaneous evolution of resistance to toxins that have different modes of action will be difficult for insect pests. In B. thuringiensis-transgenic (Bt) cotton, production of both Cry1Ac and Cry2Ab has been proposed to delay resistance of Heliothis virescens (tobacco budworm). After previous laboratory selection with Cry1Ac, H. virescens strains CXC and KCBhyb developed high levels of cross-resistance not only to toxins similar to Cry1Ac but also to Cry2Aa. We studied the role of toxin binding alteration in resistance and cross-resistance with the CXC and KCBhyb strains. In toxin binding experiments, Cry1A and Cry2Aa toxins bound to brush border membrane vesicles from CXC, but binding of Cry1Aa was reduced for the KCBhyb strain compared to susceptible insects. Since Cry1Aa and Cry2Aa do not share binding proteins in H. virescens, our results suggest occurrence of at least two mechanisms of resistance in KCBhyb insects, one of them related to reduction of Cry1Aa toxin binding. Cry1Ac bound irreversibly to brush border membrane vesicles (BBMV) from YDK, CXC, and KCBhyb larvae, suggesting that Cry1Ac insertion was unaffected. These results highlight the genetic potential of H. virescens to become resistant to distinct Cry toxins simultaneously and may question the effectiveness of gene stacking in delaying evolution of resistance.
Transgenic crops producing insecticidal Cry toxins from the bacterium Bacillus thuringiensis (Bt crops) have demonstrated significant advantages over chemical pesticides for insect pest control in the field (2). B. thuringiensis toxins' mode of action includes ingestion by a susceptible insect, solubilization and proteolytic activation to an active toxin core in the gut juice, binding to receptors on the brush border membrane of midgut cells, insertion and oligomerization to form pores, and finally cell death by osmotic shock (25).
Transgenic Bt plants developed to date produce Cry toxins constitutively, conferring continuous pest insect resistance without affecting nontarget insects or vertebrates (2). For example, Bt cotton producing Cry1Ac toxin was commercialized to control Heliothis virescens (tobacco budworm) in the field, and this technology proved efficient in target control, chemical insecticide reduction, and environmental safety (2). One of the main issues related to use of this technology is the potential for development of resistance by target insect pests due to intense selection pressure (6). Although altered toxin binding is the best-characterized mechanism of resistance, alteration of any step in the toxin mode of action can result in decreased susceptibility (8).
Based on the frequency of resistance genes in wild populations of H. virescens, Gould et al. (15) predicted evolution of resistance to Bt plants after 10 years of use if the effective refuge size was consistently 4%. No resistance to Bt plants in the field has been reported after more than 6 years of less-intensive usage. However, resistance studies with laboratory-selected H. virescens strains (14, 32) have demonstrated that the genetic potential for resistance exists.
To delay development of resistance against Bt crops, different strategies have been proposed (16). One of these strategies, called gene stacking or pyramiding, consists in the simultaneous expression of combinations of distinct toxins in transgenic plants. The success of this approach depends on heightened evolutionary challenge to an insect selected simultaneously for resistance to multiple biologically distinct toxins in the presence of a refuge (16). To gain the benefits of this approach, toxins to be used in gene stacking must be selected based on having different mechanisms of action and metabolism in the insect.
Several Cry1-resistant H. virescens strains have been developed through laboratory selection with Cry1Ac (8). Cry1Ac-selected H. virescens strains developed cross-resistance to Cry1A and Cry1Fa toxins (14, 32), a phenomenon explained by alteration of shared toxin binding sites (20, 21, 27). Therefore, toxins that do not share binding sites in brush border membrane vesicles (BBMV) from H. virescens should be used in gene stacking to delay development of resistance due to alteration of toxin binding in this insect. Both Cry1A and Cry1Fa toxins share binding sites in BBMV from H. virescens (20, 44). Several binding studies with BBMV from different lepidopteran pests have demonstrated that Cry2A toxins do not share binding sites with Cry1A toxins (7, 22, 23, 24). Consequently, Cry1Ac and Cry2A are good candidates for gene stacking in Bt cotton to control H. virescens; since they do not share binding sites in H. virescens, they share low sequence homology and have distinct modes of action (7, 35). Furthermore, this combination of toxins broadens the toxicity spectra of the cultivars, because Cry2A toxins are active against some lepidopteran pests that are unaffected by Cry1Ac (1, 39, 43). Bt cotton plants expressing both Cry1Ac and Cry2Ab toxins will be the first commercial plants developed based on the gene stacking strategy.
After laboratory selection with Cry1Ac, the CP73-3 and KCB strains of H. virescens developed cross-resistance to Cry2A (13; F. Gould, unpublished data). Adults from these strains were crossed to susceptible moths, and the resulting strains (CXC from CP73-3 and KCBhyb from KCB) were further selected with Cry2Aa (26). The mechanism of resistance in the CP73-3, CXC, KCB, and KCBhyb strains has been previously studied (9, 10, 13, 33). Cry1Ab and Cry1Ac toxin binding was not affected in the CP73-3 strain compared to the case with susceptible insects (13). Different patterns of midgut proteases were described for larvae from susceptible, CP73-3, CXC, and KCB strains, although no direct correlation between midgut protease pattern and resistance could be established (9, 10). Additionally, it was proposed that insects from the CP73-3 and KCB strains displayed enhanced epithelium recovery after challenge with sublethal doses of Cry1Ac (10, 33).
In the present study we investigated the characteristics of binding of Cry1A, Cry2Aa, and Cry1Fa toxins to BBMV from CXC and KCBhyb larvae. The goal was to examine any potential resistance mechanism related to alteration of toxin binding in these insects. Our results are evidence that alteration of Cry1Aa binding is a resistance mechanism in the KCBhyb strain of H. virescens that may explain resistance to Cry1Ac and other Cry1 toxins. We hypothesize that resistance to Cry2Aa in larvae from both KCBhyb and CXC strains is due to an additional resistance mechanism related to alteration of toxin processing in the larval midgut. Our results imply the existence of at least two mechanisms of resistance in KCBhyb insects developed after selection with a single toxin that result in resistance to both Cry1Ac and Cry2Aa toxins. These results demonstrate the wide array of mechanisms that can evolve simultaneously in susceptible H. virescens to result in cross-resistance to very different Cry toxins.
MATERIALS AND METHODS
Insect strains and bioassays.
Both strains CXC and KCBhyb were generated by backcrossing adults of parental resistant strains to susceptible insects to increase the gene pool of the colonies. The resistant H. virescens strain CXC was founded by mating the Cry1Ac-resistant strain CP73-3 to susceptible insects (10). The parental strain CP73-3 was mainly resistant to Cry1Ac (50-fold), Cry1Ab (12-fold), and Cry2Aa (52-fold), although low levels of resistance to Cry1Aa, Cry1B, and Cry1C were also detected (13). Strain CXC was further selected with Cry2Aa in the laboratory for more than 24 generations to increase resistance against this toxin (26).
Strain KCBhyb was developed by crossing adults of the resistant strain KCB with susceptible moths followed by selection with Cry2Aa. The parental strain, KCB, was resistant to Cry1Ac and cross-resistant to Cry2A (10), among other toxins (F. Gould, unpublished observations).
Derivation of strains YDK and YHD2 and bioassay protocols with Cry1Ac and Cry2Aa toxins are described by Gould et al. (14). Briefly, the YDK strain is a susceptible population of insects that served as a base strain for selecting resistant YHD2 larvae. The YHD2 strain was selected with Cry1Ac and became cross-resistant to Cry1A and Cry1Fa toxins and only slightly cross-resistant to Cry2A (14). The YHD2 strain was further selected with the MVP formulation of Cry1Ac.
Preparation of BBMV.
Midguts were dissected from fifth-instar H. virescens CXC, KCBhyb, YDK, and YHD2 larvae, washed in ice-cold MET buffer (250 mM mannitol, 17 mM Tris [pH 7.5], 5 mM EGTA), and kept at −80°C until used. BBMV were prepared from isolated midguts of all the strains by differential centrifugation according to the method of Wolfersberger et al. (45) with minor modifications (21). Briefly, midguts were homogenized in SET buffer (250 mM sucrose, 17 mM Tris [pH 7.5], 5 mM EGTA) containing protease inhibitors (complete tablets; Roche), mixed with one volume of 24 mM MgCl2, 250 mM sucrose, and BBMV purified by differential centrifugation. Final purified BBMV pellets were suspended in ice-cold PBS buffer (135 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1.7 mM KH2PO4 [pH 7.5]) with protease inhibitors (complete tablets; Roche). BBMV protein concentration was determined by the method of Bradford (3) using bovine serum albumin (BSA) as a standard, and aminopeptidase activity was used as a marker of enrichment for brush border membranes, using leucine-ρ-nitroanilide as substrate. Typical activity enrichment in the BBMV preparations was five to seven times the activity measured in the initial midgut homogenates. BBMV were kept at −80°C until used.
Bacterial toxin production, purification, and labeling.
B. thuringiensis strains HD-37 and HD-73, producing Cry1Aa and Cry1Ac, respectively, were obtained from the Bacillus Genetic Stock Collection (Columbus, Ohio). A B. thuringiensis strain producing Cry1Fa was obtained from Ecogen Inc. (Langhorne, Pa.). An Escherichia coli strain carrying the B. thuringiensis NRD-12 cry1Ab toxin gene was kindly provided by Luke Masson (National Research Council of Canada, Montreal, Canada). Inclusion bodies produced in Pseudomonas fluorescens bacteria containing Cry2Aa toxin were obtained from Dow Agrosciences (Indianapolis, Ind.).
Methods for Cry1 toxin production, activation with trypsin, and purification were as described in Luo et al. (31). Cry2Aa was extracted from P. fluorescens inclusion bodies and solubilized in 12 mM KOH (pH 12) for 5 h at 37°C. Nonsolubilized debris was eliminated by centrifugation, and the solubilized toxin samples were adjusted to pH 10 before storage at −80°C. Cry2Aa toxin was used without trypsin activation, as previously reported (7, 24). Purity of Cry1 and Cry2 toxins was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (data not shown), and the toxin concentration was determined by the method of Bradford (3) with BSA as a standard. Purified toxins were kept at −80°C until used.
Cry1A and Cry2A toxins (10 or 1 μg) were radiolabeled with 0.5 μCi of 125I using the chloramine T method of Garczynski et al. (12) or Iodobeads (Pierce) following instructions from the manufacturer. Specific activities of the radio-iodinated toxins were 13 to 27 μCi/μg based on input toxin. To measure the fraction of labeled toxin able to bind specifically to BBMV proteins, we performed toxin bindability assays as described in the work of Schumacher and Von Tscharner (38). Briefly, 1 nM labeled Cry1A or Cry2A toxins were saturated with increasing amounts of BBMV proteins, and the maximum amount of labeled toxin able to bind specifically was obtained by plotting the reciprocal of the amount of ligand bound against the reciprocal of BBMV protein concentration. Bindable fractions were used to calculate specific activities to obtain binding affinity constants and concentration of receptors from the binding saturation results.
Cry1Ac and Cry1Fa toxins (0.5 mg) were biotinylated (1:30 molar ratio) with EZ-Link sulfo-NHS-LC-Biotin (Pierce) as described by Jurat-Fuentes and Adang (20). Biotinylated toxins were quantified as described above for purified toxins and stored at −80°C until used.
Toxin binding to BBMV.
For 125I-Cry toxin binding saturation assays, increasing concentrations of labeled Cry1A or Cry2Aa toxins in binding buffer (PBS [pH 7.5]-0.1% BSA) were incubated with BBMV proteins (100 μg/ml) for 1 h at room temperature. Binding reactions were stopped by centrifugation, and pellets containing BBMV and bound toxins were washed once with 1 ml of ice-cold binding buffer. Radioactivity of the final pellets was measured in a Beckman model 4000 gamma detector. No differences in pellet activity were observed when the pellets were washed once, two, or three times (data not shown). Specific binding was calculated as total 125I-Cry1A toxin bound minus nonspecific binding, determined by including 1,000 nM unlabeled homologous competitor in the reaction.
Methods for 125I-Cry1A toxin binding competition experiments were as described by Jurat-Fuentes and Adang (20). Briefly, 10 μg (for Cry1Aa and Cry1Ab) or 5 μg (for Cry1Ac) of BBMV proteins were incubated with 0.1 nM labeled toxins for an hour at room temperature. Increasing amounts of unlabeled homologous competitors were used to compete binding. Competition reactions were stopped by centrifugation, and the pellets washed twice with ice-cold binding buffer.
From the results of both toxin binding saturation and competition experiments, a value of the dissociation constant (Kcom) and concentration of receptors (Bmax) for each toxin in BBMV from each of the strains was calculated using the KELL software package (BIOSOFT, Cambridge, United Kingdom).
Irreversible binding of 125I-Cry1Ac to BBMV from CXC, KCBhyb, and YDK insects was measured as the amount of toxin dissociated from BBMV through time after addition of an excess unlabeled competitor as described by Luo et al. (31). Toxin binding reactions were conducted for 1 h prior to the addition of the competitor. Reactions were stopped by centrifugation, and pellets were washed twice with binding buffer before their activity was measured.
Western blotting.
Binding of biotinylated Cry1Fa and Cry1Ac toxins to BBMV from KCBhyb, YDK, and YHD2 larvae was analyzed using Western blot analysis as in the work of Jurat-Fuentes et al. (21). BBMV proteins (20 μg) were incubated with 12 nM biotinylated Cry1Ac or Cry1Fa in 0.1 ml of binding buffer (PBS [pH 7.5] containing 0.1% BSA) at room temperature for 1 h. Binding was stopped by centrifugation, and pellets were washed twice with 0.5 ml of ice-cold binding buffer. Final BBMV pellets were solubilized and electrophoresed in SDS-10% PAGE and transferred to polyvinylidene difluoride Q membrane filters (Millipore) in transfer buffer (48 mM Tris, 390 mM glycine, 0.1% [wt/vol] SDS, 20% methanol [pH 8.3]). After blocking for 1 h in PBS plus 0.1% Tween 20 containing 3% BSA, membranes were incubated with streptavidin-peroxidase conjugate (Sigma) in PBS plus 0.1% Tween 20 plus 0.1% BSA for 1 h. After washing, biotinylated toxins were visualized using ECL (Amersham-Pharmacia) reagents following the manufacturer's instructions.
RESULTS
Cry1Ac and Cry2Aa activity against H. virescens strains.
Both Cry1Ac and Cry2Aa toxins were highly active against YDK larvae (Table 1). The 50% lethal concentrations (LC50s) obtained for both toxins were similar to those in previous reports (14, 26).
TABLE 1.
Toxicity and resistance ratio against Cry1Ac and Cry2Aa toxins in susceptible (YDK) and resistant (CXC and KCBhyb) H. virescens strains
| Strain | Toxin
|
|||||
|---|---|---|---|---|---|---|
| Cry1Ac
|
Cry2Aa
|
|||||
| Slope | LC50a (95% fiducial limits) | RRb | Slope | LC50 (95% fiducial limits) | RR | |
| YDK | 0.93 | 0.73 (0.33-1.39) | NAc | 3.31 | 4.30 (2.33-6.36) | NA |
| CXC | 1.46 | 211.20 (104.40-343.60) | 289.31 | NA | No mortality at 1,000 μg/ml | >250 |
| KCBhyb | 2.00 | 137.00 (84.89-200.00) | 187.67 | NA | No mortality at 1,000 μg/ml | >250 |
LC50 values are expressed in micrograms of toxin per milliliter of diet.
Resistance ratio (LC50 for resistant strain/LC50 for YDK).
NA, not applicable.
Levels of resistance to Cry1Ac observed for the CXC and KCBhyb larvae were statistically the same. Resistance ratios for CXC and KCBhyb larvae were about 289- and 187-fold, respectively. Compared to results in previous reports (13, 10, 26), the resistance ratio for Cry1Ac in CXC larvae is high, suggesting that after selection with Cry2Aa resistance to both Cry1Ac and Cry2Aa toxins was increased. In the case of KCBhyb larvae, compared to the resistance ratio of its parental strain (KCB) (10), resistance to Cry1Ac appears to have decreased after selection with Cry2Aa. However, because resistance ratios can vary over time due to laboratory conditions, the differences between the present results and the previous bioassays must be interpreted cautiously.
Both the CXC and KCBhyb strains were highly resistant to Cry2Aa compared to larvae from YDK. Since no mortality was observed at the highest Cry2Aa toxin concentration used, we were unable to obtain accurate LC50s for this toxin for CXC and KCBhyb larvae.
Although other Cry1 toxins were not tested in our bioassays, larvae from the CXC and KCBhyb strains are cross-resistant to Cry1Aa, Cry1Ab, and Cry1Fa (F. Gould, unpublished observation).
Saturation of toxin binding to BBMV.
To investigate the specificity of 125I-Cry1A and 125I-Cry2Aa toxin binding to BBMV from the CXC, KCBhyb, and YDK strains, we performed binding saturation assays (Fig. 1). All 125I-Cry1A toxins bound specifically and in a saturable manner to BBMV from YDK and CXC larvae. Toxin binding affinities and concentrations of receptors for all the toxins were similar between these strains (Table 2).
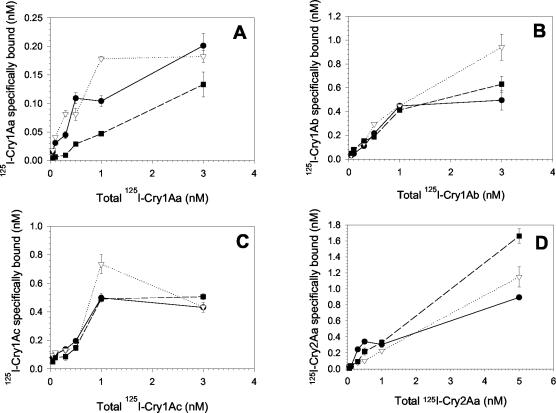
FIG. 1.
Specific binding saturation of 125I-Cry1Aa (A), 125I-Cry1Ab (B), 125I-Cry1Ac (C), and 125I-Cry2Aa (D) to BBMV proteins from YDK (•), CXC (▿) or KCBhyb (▪) larvae. BBMV proteins (10 μg) were incubated with increasing amounts of labeled toxins for 1 h. Nonspecific binding was calculated in the presence of 1,000 nM of the respective unlabeled homologous toxin and was subtracted from total binding to obtain specific binding. Binding reactions were stopped by centrifugation. Bound labeled toxin (nanomolar concentration) was calculated using the RADLIG software. Each data point is a mean based on at least two experiments done in quadruplicate. Error bars depict standard deviation of the mean values.
TABLE 2.
Dissociation constants (Kcom) and concentrations of receptors (Bmax) calculated from 125I-Cry1A toxin binding saturation assays with BBMV from YDK, CXC, and KCBhyb H. virescens strains
| Toxin | Result for strainsc
|
|||||
|---|---|---|---|---|---|---|
| YDK
|
CXC
|
KCBhyb
|
||||
| Kcoma | Bmaxb | Kcom | Bmax | Kcom | Bmax | |
| Cry1Aa | 0.39 ± 0.19 | 0.90 ± 0.30 | 0.83 ± 0.29 | 2.70 ± 0.70 | 26.67 ± 18.39 | 7.20 ± 4.30 |
| Cry1Ab | 0.30 ± 0.16 | 1.90 ± 0.60 | 0.14 ± 0.08 | 1.90 ± 0.50 | 0.63 ± 0.21 | 4.30 ± 0.80 |
| Cry1Ac | 0.42 ± 0.15 | 5.00 ± 0.60 | 0.40 ± 0.31 | 5.80 ± 3.10 | 0.86 ± 0.43 | 8.60 ± 1.30 |
Expressed in nanomolar units.
Expressed in picomoles per milligram of BBMV protein.
Expressed as value ± standard error.
In the case of BBMV from KCBhyb larvae, binding of 125I-Cry1Aa was altered from that of YDK and CXC (Fig. 1A). At all the 125I-Cry1Aa concentrations tested, vesicles from KCBhyb bound less 125I-Cry1Aa than BBMV from YDK or CXC, evidencing a reduction of high-affinity Cry1Aa binding sites in BBMV from KCBhyb larvae. According to the calculated toxin binding affinities, Cry1Aa had about 68-fold less affinity for BBMV from KCBhyb than for CXC or YDK vesicles. Neither 125I-Cry1Ab nor 125I-Cry1Ac binding was altered in the BBMV from KCBhyb larvae compared to YDK and CXC binding (Table 2).
As previously described for other insect BBMV preparations (7, 22, 23, 24), binding of Cry2Aa was nonsaturable in the range of ligand concentrations tested. Nevertheless, Cry2Aa binding was not reduced in any of the resistant strains from that for YDK vesicles (Fig. 1D). The nonsaturability of Cry2Aa binding limited our ability to accurately calculate binding affinities for this toxin.
Taken together, these results are evidence that only Cry1Aa binding was affected in BBMV from KCBhyb larvae, while no alteration of Cry1A toxin binding was detected in CXC vesicles. Binding of Cry2Aa toxin was unaffected in BBMV from CXC and KCBhyb compared to results for YDK vesicles, suggesting that resistance to Cry2Aa is not related to altered toxin binding.
Competition of 125I-Cry1A binding to BBMV.
To confirm the differences detected in the saturation binding assays, we performed homologous competition binding experiments. 125I-Cry1A toxins were incubated with BBMV in the presence of increasing concentrations of homologous unlabeled toxin. Binding parameters were calculated for each of the 125I-Cry1A toxins in BBMV from YDK, CXC, and KCBhyb strains and are presented in Table 3.
TABLE 3.
Dissociation constants (Kcom) and concentrations of receptors (Bmax) calculated from homologous 125I-Cry1A toxin binding competition assays with BBMV from H. virescens strains YDK, CXC, and KCBhyb
| Toxin | Result for straind
|
|||||
|---|---|---|---|---|---|---|
| YDK
|
CXC
|
KCBhyb
|
||||
| Kcoma | Bmaxb | Kcom | Bmax | Kcom | Bmax | |
| Cry1Aa | 1.25 ± 0.51 | 1.20 ± 0.30 | 1.47 ± 0.71 | 0.90 ± 0.30 | NAc | NA |
| Cry1Ab | 2.26 ± 0.15 | 7.60 ± 0.40 | 3.97 ± 0.78 | 8.70 ± 1.60 | 2.31 ± 0.42 | 6.50 ± 1.10 |
| Cry1Ac | 0.15 ± 0.09 | 0.50 ± 0.10 | 0.26 ± 0.06 | 0.40 ± 0.10 | 0.04 ± 0.01 | 0.50 ± 0.10 |
Expressed in nanomolar units.
Expressed in picomoles per milligram of BBMV protein.
NA, not applicable.
Expressed as value ± standard error.
In agreement with the results of toxin binding saturation experiments, binding of 125I-Cry1Aa was highly reduced in BBMV from KCBhyb (Fig. 2A). We were not able to detect Cry1Aa toxin binding competition at the competitor concentrations tested, suggesting that most of the binding of Cry1Aa to BBMV from KCBhyb was nonspecific. Unlabeled Cry1Aa totally competed 125I-Cry1Aa binding to BBMV from the YDK and CXC strains (Fig. 2A), and this is reflected in the Cry1Aa binding affinity being the same for both strains.
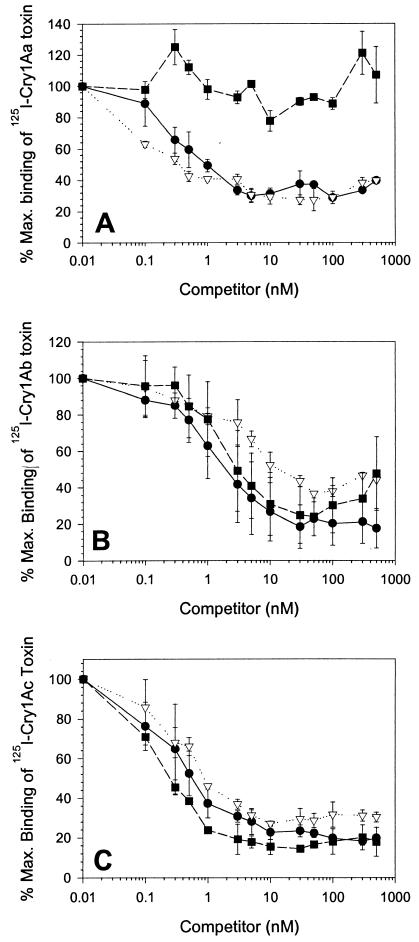
FIG. 2.
Homologous binding competition of 125I-Cry1Aa (A), 125I-Cry1Ab (B), and 125I-Cry1Ac (C) to BBMV proteins from YDK (•), CXC (▿), or KCBhyb (▪) larvae. BBMV proteins (10 μg) were incubated with labeled toxins (0.1 nM) in the presence of increasing concentrations of homologous unlabeled competitor for 1 h. Binding reactions were stopped by centrifugation. Binding was expressed as a percentage of the amount of toxin bound in the absence of competitor. Each data point is a mean based on at least two independent trials done in duplicate. Error bars depict standard deviation of the mean values.
As observed in binding saturation assays, no differences in binding of either Cry1Ab or Cry1Ac were observed between BBMV from YDK, CXC, and KCBhyb, suggesting that binding of these toxins is not affected in the resistant strains. Toxin binding affinities as well as concentration of binding sites for Cry1Ab and Cry1Ac toxins for YDK were in agreement with previous reports (27) and were alike for CXC and KCBhyb vesicles.
These results demonstrate that at the time these assays were conducted, the BBMV from KCBhyb larvae could be differentiated from YDK and CXC vesicles by 125I-Cry1Aa toxin-binding assays. Cry1Aa binding sites in KCBhyb were modified to reduce toxin-binding affinity. Since Cry1Ab and Cry1Ac bind to the Cry1Aa binding site (20), reduced Cry1Aa binding together with decreased susceptibility to Cry1Ab and Cry1Ac suggests that resistance to Cry1Ab and Cry1Ac is probably linked to the modification of the Cry1Aa binding site. Unexpectedly, quantitative binding parameters for Cry1Ab and Cry1Ac were the same for BBMV from KCBhyb-resistant and YDK-susceptible larvae. This observation may be explained by the binding assays not being sensitive enough to detect reduced binding of Cry1Ab and Cry1Ac to the shared Cry1A binding site.
Irreversibility of 125I-Cry1Ac binding to BBMV.
Cry1 toxins undergo a reversible binding phase before toxin insertion in the membrane (28). To study the possibility that BBMV from YDK, CXC, and KCBhyb differed in irreversibility of 125I-Cry1Ac binding, we performed dissociation binding assays for Cry1Ac. In these experiments, reversibly bound 125I-Cry1Ac was competed with an excess of unlabeled Cry1Ac after reaching binding equilibrium. As shown in Fig. 3, Binding of 125I-Cry1Ac to BBMV from YDK, CXC, and KCBhyb larvae was not competed by an excess of unlabeled Cry1Ac, demonstrating that most of the 125I-Cry1Ac toxin was irreversibly bound to the BBMV. These results suggest that resistance to Cry1Ac in CXC and KCBhyb larvae is not related to altered toxin insertion on the BBMV.
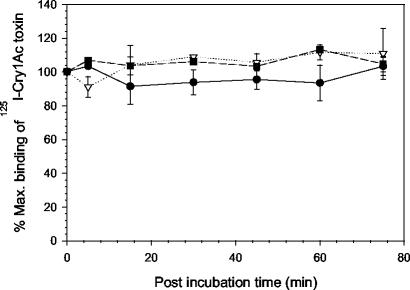
FIG. 3.
Irreversible binding of 125I-Cry1Ac toxin to BBMV from YDK (•), CXC (▿), or KCBhyb (▪) larvae. Binding reactions were started by mixing BBMV proteins (10 μg) with 0.1 nM 125I-Cry1Ac. One hour after initiation of the binding reaction, 1,000 nM unlabeled Cry1Ac was added to the mixture. The time on the x axis represents postincubation time after addition of the unlabeled competitor. Binding was expressed as a percentage of the amount of toxin bound before addition of competitor. Each data point is a mean based on data from two independent trials done in dupliacte. Error bars depict standard deviation of the mean values.
Binding of biotinylated Cry1Fa to BBMV.
Larvae from the KCBhyb and CXC strains were cross-resistant to Cry1Fa (F. Gould, unpublished observation). Cry1Aa shares its only binding site in H. virescens BBMV with Cry1Ab, Cry1Ac, and Cry1Fa toxins (20). Since binding of Cry1Aa was decreased in the BBMV from the KCBhyb strain, we studied the potential role of altered toxin binding in cross-resistance to Cry1Fa in this strain. Because iodination inactivates Cry1Fa, binding of this toxin was studied using biotinylated Cry1Fa and Western blotting as previously described (21). BBMV from the YHD2-resistant strain were included in the analysis as a control, since these vesicles have decreased Cry1Fa binding (21). As an internal control, we also studied binding of biotinylated Cry1Ac to BBMV from YDK, KCBhyb, and YHD2 insects.
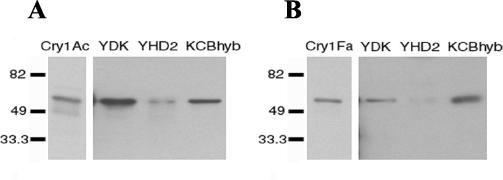
As expected from the radiolabeled toxin binding experiments, biotinylated Cry1Ac bound BBMV from YDK and KCBhyb strains, but binding to BBMV from the YHD2 strain was highly reduced (Fig. 4A), as previously shown (21). Biotinylated Cry1Fa bound similarly to YDK and KCBhyb but not to YHD2 vesicles (Fig. 4B). Although these results need to be taken with caution due to the toxin detection limits of Western blotting, they suggest that cross-resistance to Cry1Fa in the KCBhyb strain is not related to alteration of toxin binding.
FIG. 4.
Binding of biotinylated Cry1Ac (A) and Cry1Fa (B) toxins to BBMV from YDK, YHD2, and KCBhyb larvae. Toxins (12 nM) were incubated with BBMV proteins (20 μg) for 1 h. Binding reactions were stopped by centrifugation, and washed pellets were separated by SDS-10% PAGE and transferred to polyvinylidene difluoride filters. Biotinylated toxins were detected with streptavidin-peroxidase conjugate and enhanced chemiluminescence.
DISCUSSION
Cry2A toxins show low sequence homology with Cry1 toxins (41), have distinct receptor binding epitopes (35), and do not share binding sites with Cry1A toxins in BBMV from lepidopteran larvae (20, 22, 23, 24). Consequently, Cry1-selected strains of H. virescens, Plutella xylostella, and Pectinophora gossypiella developed low cross-resistance or no cross-resistance to Cry2A toxins (14, 41, 42, 48). These features make Cry2A toxins ideal candidates for use in combination with Cry1Ac in a gene stacking approach for delaying the onset of H. virescens resistance in the field.
In this work, we have studied the resistance mechanisms in two independently selected laboratory strains of H. virescens: CXC and KCBhyb. The most relevant feature of both resistant strains was that after selection with Cry1Ac, both developed cross-resistance to Cry2Aa, among other toxins. After becoming resistant due to selection with Cry1Ac, both strains were backcrossed to susceptible individuals and further selected with Cry2Aa. Our bioassays with larvae from the CXC strain suggest that resistance against both Cry1Ac and Cry2Aa had increased after further selection with Cry2Aa compared to results previously reported (10, 26). This observation indicates that the mechanism conferring resistance to Cry2Aa may also be involved in resistance to Cry1Ac. On the other hand, comparison of our bioassay data with KCBhyb insects to results of bioassays with larvae from its parental strain, KCB (10), suggested that selection with Cry2Aa had not increased resistance to Cry1Ac in this case. Although this observation has to be taken with caution as indicated above, it suggests the existence of at least two mechanisms of resistance in KCBhyb larvae.
Resistance to Cry1A toxins in several strains of H. virescens has been associated with altered toxin binding (21, 27, 32). In both toxin binding saturation and competition assays, BBMV from the CXC larvae were similar to those of YDK larvae in binding of 125I-Cry1A and 125I-Cry2Aa toxins. These results demonstrate that resistance in CXC larvae was not related to an alteration of toxin binding. Changes affecting a common step in the mode of action of both Cry1A and Cry2A toxins previous to toxin binding are probably responsible for resistance in this strain.
Toxin binding saturation experiments demonstrated a 68-fold decrease in 125I-Cry1Aa binding affinity for BBMV from the KCBhyb strain, together with a slight increase in the concentration of binding sites, compared to results with YDK vesicles. The use of toxin binding saturation curves allowed us to confirm toxin-binding saturability and to obtain a ratio of Cry1Aa binding reduction for BBMV from KCBhyb compared to YDK and CXC vesicles. The toxin binding differences observed in saturation assays were confirmed in toxin binding competition assays, in which no homologous competition of Cry1Aa binding to BBMV from KCBhyb larvae was detected. The small amounts of BBMV used in competition assays explain the absence of Cry1Aa binding competition for KCBhyb vesicles, since similar vesicle amounts in saturation assays resulted in low levels of Cry1Aa toxin binding. Decreased toxin binding affinity has been previously reported for other resistant H. virescens strains (27, 32). In H. virescens BBMV, the Cry1Aa population of receptors (receptor A) also binds Cry1Ab and Cry1Ac (20). We did not detect any significant difference in the binding of 125I-Cry1Ab, 125I-Cry1Ac, or 125I-Cry2Aa toxins to BBMV from KCBhyb compared to YDK vesicles. These results suggested that alteration of binding is not involved in resistance to these toxins. An alternative explanation for these results would be that the binding assays performed may not have been sensitive enough to detect elimination of a fraction of the available Cry1Ab and Cry1Ac toxin binding sites (receptor A) due to the high affinity of the additional Cry1Ab and Cry1Ac binding sites present in BBMV.
Since Cry1Fa also binds to receptor A (20), and a H. virescens strain cross-resistant to Cry1Fa showed reduced Cry1Fa toxin binding (21), we studied the possibility that alteration of Cry1Aa binding had an effect on binding of Cry1Fa to KCBhyb vesicles. Western blotting results showed that binding of Cry1Fa was not reduced in BBMV from KCBhyb compared to the case with YDK vesicles. Even though results from Western blotting experiments need to be taken carefully due to detection limits of this technique, they indicate that cross-resistance to Cry1Fa is not related to alteration of Cry1Aa binding.
Interestingly, BBMV from YDK, CXC, and KCBhyb larvae bound more than 90% of the 125I-Cry1Ac toxin irreversibly, evidencing toxin insertion on the membrane of BBMV from all three strains. Lee et al. (27) made a similar observation after comparing irreversible binding of Cry1Ac to BBMV from YDK and the H. virescens resistant strain YHD2. These authors also reported for BBMV from YHD2 larvae the same pattern of reduced Cry1Aa but not Cry1Ab or Cry1Ac toxin binding that we have observed for BBMV from KCBhyb. To explain toxin binding not leading to toxicity, Lee et al. (27) proposed the existence of “null receptors” in the YHD2 strain that would allow irreversible binding of the toxins but not toxicity. In this respect, Cry1Ac has been shown to bind specifically to BBMV from Spodoptera frugiperda and Lymantria dispar without conferring susceptibility (12, 46).
From previous results with YHD2 (27) and our results with KCBhyb BBMV, alteration of Cry1Aa binding sites seems critical for resistance in H. virescens. Absence of a cadherin-like protein (HevCaLP) that may function as a toxin-binding site was proposed as a resistance mechanism (11) to explain the results obtained by Lee et al. (27) with YHD2 vesicles. Our laboratory is currently addressing the potential existence of this mechanism in KCBhyb larvae. Although altered glycosylation of specific BBMV proteins has also been proposed as mechanism of resistance for the YHD2 strain (21), this mechanism resulted in a lack of Cry1Ab, Cry1Ac, and Cry1Fa toxin binding to BBMV from YHD2, a phenomenon not observed for BBMV from KCBhyb larvae.
Interestingly, cross-resistance to Cry2Aa in the YHD2 strain was low (14). Since the YHD2 larvae lacking HevCaLP had little cross-resistance to Cry2Aa, HevCaLP is probably not a binding site for this toxin, and the absence of HevCadLP would not explain resistance to Cry2Aa for the KCBhyb strain. Based on this information, we hypothesize the existence of at least two resistance mechanisms in KCBhyb insects. One mechanism would be responsible for resistance to Cry1Ac and cross-resistance to Cry1Aa, Cry1Ab, and Cry1Fa, while a second mechanism would mainly result in resistance to Cry2Aa, although we cannot deny potential involvement of the second mechanism in resistance to Cry1A toxins. Based on the similarities between YHD2 and KCBhyb toxin binding features, we hypothesize that the first mechanism of resistance in KCBhyb is similar to the HevCaLP alteration observed in YHD2 larvae.
The second mechanism of resistance in KCBhyb larvae would be similar to the one observed for CXC insects. This resistance mechanism is not related to altered toxin binding but must involve a modification of a step in toxin action shared by both Cry1A and Cry2A toxins. Although these toxins have different modes of action at the membrane level, they both undergo the same initial steps of solubilization and activation in the insect midgut juice. Defects in toxin activation have been proposed as mechanisms of resistance for Plodia interpunctella (4, 36), Plutella xylostella (37), and Leptinotarsa decemlineata (30). Differential midgut protease activity was proposed as a mechanism of resistance for the CXC strain and the parental strains of CXC (CP73-3) and KCBhyb (KCB) (9, 10). However, a correlation between expression of specific proteases and resistance could not be established for any of the strains (10). The potential role of altered proteolytic activity in resistance for both CXC and KCBhyb insects is currently being addressed by another group (B. Oppert, personal communication).
Furthermore, enhanced midgut epithelium regeneration after Cry toxin challenge was also proposed as a potential mechanism of resistance for the CXC and KCB strains (10). Faster rates of midgut epithelium renewal have been associated with resistance against B. thuringiensis in Corcyra cephalonica (5) and baculovirus infection (19) in H. virescens. Furthermore, challenge of H. virescens midgut cell cultures with sublethal doses of Cry1Ac induced an increase in the number of stem and differentiating cells compared to results for controls (29). The potential role of this mechanism in H. virescens resistance needs further study.
Resistance to Cry toxins due to a combination of resistance genes in the same insect strain has been previously reported for H. virescens (17), P. xylostella (47), and P. interpunctella (18). More specifically, in a Cry1Ab-resistant strain of P. interpunctella, reduced toxin binding and a protease-mediated mechanism were observed (18).
Our results are evidence for the existence of at least two resistance mechanisms in the KCBhyb strain of H. virescens. This highlights the broad variety of potential resistance mechanisms that H. virescens may develop to cope with very different Cry toxins. Dual resistance to Cry1Ac and Cry2Aa in the CXC and KCBhyb laboratory strains may raise questions as to how H. virescens in the field will respond to transgenic cotton producing Cry1Ac and Cry2Ab proteins. In this regard, it is important to notice that the original cross-resistant strains that gave rise to the CXC and KCBhyb strains were generated by selection with a single toxin. Evolution of resistance to a high dose/refuge strategy with Bt cotton that produces two toxins is expected to be difficult, especially if cross-resistance between the toxins is an unlikely event as it has been in the case of Cry1Ac and Cry2A toxins (14, 42). Strains of P. xylostella and P. interpunctella selected with a commercial mixture of Cry1A and Cry2A toxins developed high levels of resistance to Cry1A toxins, while resistance to Cry2A toxins was much lower, probably due to smaller amounts of Cry2Aa present in the mixtures (34, 40). Cotton plants expressing Cry2Ab alone or in combination with Cry1Ac resulted in efficient control of Cry1Ac-resistant strains of P. gossypiella (43) as well as YHD2 and CXC larvae (26). These observations suggest the difficulty of H. virescens simultaneously developing high levels of resistance to both Cry1A and Cry2A toxins after exposure to Bt plants producing both toxins.
Our results demonstrate the possibility of cross-resistance development between Cry1Ac and Cry2A by co-occurrence of different mechanisms of resistance. This information is extremely important when designing and implementing strategies aimed at delaying resistance and cross-resistance to insecticides based on these toxins and Bt crops.
REFERENCES
- 1.Adamczyk, J. J., L. C. Adams, and D. D. Hardee. 2001. Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. J. Econ. Entomol. 94:1589-1593. [DOI] [PubMed] [Google Scholar]
- 2.Betz, F. S., B. G. Hammond, and R. L. Fuchs. 2000. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharm. 32:156-173. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Candas, M., O. Loseva, B. Oppert, P. Kosaraju, and L. A. Bulla, Jr. 2003. Insect resistance to Bacillus thuringiensis: alterations in the Indianmeal moth larval gut proteome. Mol. Cell Proteomics 2:19-28. [DOI] [PubMed] [Google Scholar]
- 5.Chiang, A. S., D. F. Yen, and W. K. Peng. 1986. Defense reaction of midgut epithelial cells in the rice moth larva (Corcyra cephalonica) infected with Bacillus thuringiensis. J. Invertebr. Pathol. 47:333-339. [Google Scholar]
- 6.De Maagd, R. A., D. Bosch, and W. Stiekema. 1999. Bacillus thuringiensis toxin-mediated insect resistance in plants. Trends Plant Sci. 4:9-13. [DOI] [PubMed] [Google Scholar]
- 7.English, L., H. L. Robbins, M. A. Von Tersch, C. A. Kulesza, D. Ave, D. Coyle, C. S. Jany, and S. Slatin. 1994. Mode of action of CryIIA: a Bacillus thuringiensis delta-endotoxin. Insect Biochem. Mol.. Biol. 24:1025-1035. [Google Scholar]
- 8.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 9.Forcada, C., E. Alcácer, M. D. Garcerá, and R. Martínez. 1996. Differences in the midgut proteolityc activity of two Heliothis virescens strains, one susceptible and one resistant to Bacillus thuringiensis toxins. Arch. Insect Biochem. Physiol. 31:257-272. [Google Scholar]
- 10.Forcada, C., E. Alcácer, M. D. Garcerá, A. Tato, and R. Martínez. 1999. Resistance to Bacillus thuringiensis Cry1Ac toxin in three strains of Heliothis virescens: proteolytic and SEM study of the larval midgut. Arch. Insect Biochem. Physiol. 42:51-63. [DOI] [PubMed] [Google Scholar]
- 11.Gahan, L. J., F. Gould, and D. G. Heckel. 2001. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293:857-860. [DOI] [PubMed] [Google Scholar]
- 12.Garczynski, S. F., J. W. Crim, and M. J. Adang. 1991. Identification of putative insect brush border membrane-binding molecules specific to Bacillus thuringiensis δ-endotoxin by protein blot analysis. Appl. Environ. Microbiol. 57:2816-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould, F., A. Martínez-Ramírez, A. Anderson, J. Ferré, F. J. Silva, and W. J. Moar. 1992. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl. Acad. Sci. USA 89:7986-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould, F., A. Anderson, A. Reynolds, L. Bumgarner, and W. Moar. 1995. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 88:1545-1559. [Google Scholar]
- 15.Gould, F., A. Anderson, A. Jones, D. Sumerford, D. G. Heckel, J. Lopez, S. Micinski, R. Leonard, and M. Laster. 1997. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proc. Natl. Acad. Sci. USA 94:3519-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould, F. 1998. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43:701-726. [DOI] [PubMed] [Google Scholar]
- 17.Heckel, D. G., L. C. Gahan, F. Gould, and A. Anderson. 1997. Identification of a linkage group with a major effect on resistance to Bacillus thuringiensis Cry1Ac endotoxin in the tobacco budworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 90:75-86. [Google Scholar]
- 18.Herrero, S., B. Oppert, and J. Ferré. 2001. Different mechanisms of resistance to Bacillus thuringiensis toxins in the Indianmeal moth. Appl. Environ. Microbiol. 67:1085-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoover, K., J. O. Washburn, and L. E. Volkman. 2000. Midgut-based resistance of Heliothis virescens to baculovirus infection mediated by phytochemicals in cotton. J. Insect Physiol. 46:999-1007. [DOI] [PubMed] [Google Scholar]
- 20.Jurat-Fuentes, J. L., and M. J. Adang. 2001. Importance of Cry1 δ-endotoxin domain II loops for binding specificity in Heliothis virescens. Appl. Environ. Microbiol. 67:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurat-Fuentes, J. L., F. L. Gould, and M. J. Adang. 2002. Altered glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Appl. Environ. Microbiol. 68:5711-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karim, S., and D. H. Dean. 2000. Toxicity and receptor binding properties of Bacillus thuringiensis δ-endotoxins to the midgut brush border membrane vesicles of the rice leaf folders, Cnaphalocrocis medinalis and Marasmia patnalis. Curr. Microbiol. 41:276-283. [DOI] [PubMed] [Google Scholar]
- 23.Karim, S., and D. H. Dean. 2000. Pesticidal and receptor binding properties of Bacillus thuringiensis Cry1Ab and Cry1Ac δ-endotoxin mutants to Pectinophora gossypiella and Helicoverpa zea. Curr. Microbiol. 41:430-440. [DOI] [PubMed] [Google Scholar]
- 24.Karim, S., S. Riazuddin, F. Gould, and D. H. Dean. 2000. Determination of receptor binding properties of Bacillus thuringiensis δ-endotoxins to cotton bollworm (Helicoverpa zea) and pink bollworm (Pectinophora gossypiella) midgut brush border membrane vesicles. Pestic. Biochem. Physiol. 67:198-216. [Google Scholar]
- 25.Knowles, B. 1994. Mechanism of action of Bacillus thuringiensis insecticidal delta-endotoxins. Adv. Insect Physiol. 24:275-308. [Google Scholar]
- 26.Kota, M., H. Daniell, S. Varma, S. F. Garczynski, F. Gould, and W. J. Moar. 1999. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc. Natl. Acad. Sci. USA 96:1840-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, M. K., F. Rajamohan, F. Gould, and D. H. Dean. 1995. Resistance to Bacillus thuringiensis Cry1A δ-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl. Environ. Microbiol. 61:3836-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, Y., S. S. Patel, and D. H. Dean. 1995. Irreversible binding kinetics of Bacillus thuringiensis Cry1A δ-endotoxins to gypsy moth brush border membrane vesicles is directly correlated to toxicity. J. Biol. Chem. 270:24719-24724. [DOI] [PubMed] [Google Scholar]
- 29.Loeb, M. J., P. A. W. Martin, R. S. Hakim, S. Goto, and M. Takeda. 2001. Regeneration of cultured midgut cells after exposure to sublethal doses of toxin from two strains of Bacillus thuringiensis. J. Insect Physiol. 47:599-606. [DOI] [PubMed] [Google Scholar]
- 30.Loseva, O., M. Ibrahim, M. Candas, C. N. Koller, L. S. Bauer, and L. A. Bulla, Jr. 2002. Changes in protease activity and Cry3Aa toxin binding in the Colorado potato beetle: implications for insect resistance to Bacillus thuringiensis toxins. Insect Biochem. Mol. Biol. 32:567-577. [DOI] [PubMed] [Google Scholar]
- 31.Luo, K., D. J. Banks, and M. J. Adang. 1999. Toxicity, binding and permeability analyses of four Bacillus thuringiensis Cry1 δ-endotoxins using brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl. Environ. Microbiol. 65:457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacIntosh, S. C., T. B. Stone, R. S. Jokerst, and R. L. Fuchs. 1991. Binding of Bacillus thuringiensis proteins to a laboratory-selected line of Heliothis virescens. Proc. Natl. Acad. Sci. USA 88:8930-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Ramírez, A. C., F. Gould, and J. Ferré. 1999. Histopathological effects and growth reduction in a susceptible and a resistant strain of Heliothis virescens (Lepidoptera: Noctuidae) caused by sublethal doses of pure Cry1A crystal proteins from Bacillus thuringiensis. Biocontrol Sci. Technol. 9:239-246. [Google Scholar]
- 34.McGaughey, W. M., and D. E. Johnson. 1987. Toxicity of different serotypes and toxins of Bacillus thuringiensis to resistant and susceptible Indianmeal moths (Lepidoptera: Pyralidae). J. Econ. Entomol. 80:1122-1126. [Google Scholar]
- 35.Morse, R. J., T. Yamamoto, and R. M. Stroud. 2001. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 9:409-417. [DOI] [PubMed] [Google Scholar]
- 36.Oppert, B., K. J. Kramer, R. W. Beeman, D. Johnson, and W. H. McGaughey. 1997. Proteinase-mediated insect resistance to Bacillus thuringiensis toxins. J. Biol. Chem. 38:23473-23476. [DOI] [PubMed] [Google Scholar]
- 37.Sayyed, A. H., R. Gatsi, T. Kouskoura, D. J. Wright, and N. Crickmore. 2001. Susceptibility of a field-derived, Bacillus thuringiensis-resistant strain of diamondback moth to in vitro-activated Cry1Ac toxin. Appl. Environ. Microbiol. 67:4372-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacher, C., and V. von Tscharner. 1994. Practical instructions for radioactively labeled ligand receptor binding studies. Anal. Biochem. 222:262-269. [DOI] [PubMed] [Google Scholar]
- 39.Stewart, S. D., J. J. Adamczyk, Jr., K. S. Knighten, and F. M. Davis. 2001. Impact of Bt cottons expressing one or two insecticidal proteins of Bacillus thuringiensis Berliner on growth and survival of noctuid (Lepidoptera) larvae. J. Econ. Entomol. 94:752-760. [DOI] [PubMed] [Google Scholar]
- 40.Tabashnik, B. E., N. Finson, M. W. Johnson, and W. J. Moar. 1993. Resistance to toxins from Bacillus thuringiensis subsp. kurstaki causes minimal cross-resistance to B. thuringiensis subsp. aizawai in the diamondback moth (Lepidoptera: Plutellidae). Appl. Environ. Microbiol. 59:1332-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabashnik, B. E., T. Malvar, Y. B. Liu, N. Finson, D. Borthakur, B. S. Shin, S. H. Park, L. Masson, R. A. De Maagd, and D. Bosch. 1996. Cross-resistance of the diamondback moth indicates altered interaction with domain II of Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 62:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabashnik, B. E., Y.-B. Liu, R. A. De Maagd, and T. J. Dennehy. 2000. Cross-resistance of pink bollworm (Pectiniphora gossypiella) to Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 66:4582-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabashnik, B. E., T. J. Dennehy, M. A. Sims, K. Larkin, G. P. Head, W. J. Moar, and Y. Carrière. 2002. Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl. Environ. Microbiol. 68:3790-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Rie, J., S. Jansens, H. Hofte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis δ-endotoxins: importance of specific receptors on the brush border membrane of the midgut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 45.Wolfersberger, M. G., P. Luthy, A. Maurer, P. Parenti, V. F. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86:301-308. [Google Scholar]
- 46.Wolfersberger, M. G. 1990. The toxicity of two Bacillus thuringiensis delta-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membrane for the toxins. Experientia 46:475-477. [DOI] [PubMed] [Google Scholar]
- 47.Wright, D. J., M. Iqbal, F. Granero, and J. Ferré. 1997. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for field resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl. Environ. Microbiol. 63:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, J.-Z., Y.-X. Li, H. L. Collins, J. Cao, E. D. Earle, and A. M. Shelton. 2001. Different cross-resistance patterns in the diamondback moth (Lepidoptera: Plutellidae) resistant to Bacillus thuringiensis toxin Cry1C. J. Econ. Entomol. 94:1547-1552. [DOI] [PubMed] [Google Scholar]