Abstract
The growth rate of Chromohalobacter salexigens DSM 3043 can be stimulated in media containing 0.3 M NaCl by a 0.7 M concentration of other salts of Na+, K+, Rb+, or NH4+, Cl−, Br−, NO3−, or SO42− ions. To our knowledge, growth rate stimulation by a general high ion concentration has not been reported for any organism previously.
The halophilic gram-negative bacterium Chromohalobacter salexigens (1) has been reported to require at least 0.5 M NaCl for growth (2). In this study, we carried out a more comprehensive characterization of the ion requirements of this organism and made the unexpected finding that while this organism requires moderate concentrations of Na+ and Cl− ions, its growth rate was stimulated by a number of other salts, indicating that C. salexigens requires a combination of NaCl and high ionic strength for optimal growth.
Characterization of the cation requirements of C. salexigens DSM 3043.
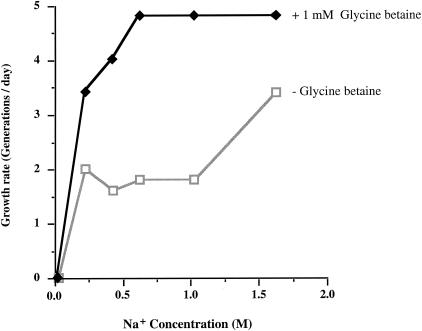
For growth rate studies, C. salexigens DSM 3043 was cultured aerobically at 37°C in a modified form of M63 medium (3), consisting of 0.10 M KH2PO4, 0.075 M KOH, and 0.015 M (NH4)2SO4, supplemented with MgSO4 and FeSO4 · 7H2O, whose original concentrations in M63 were increased to 8.0 and 0.1 mM, respectively, as suggested by Martin et al. (9). The Na+ requirement of C. salexigens was determined in media containing 2.0 M Cl− salts, made up of various combinations of NaCl and KCl (Fig. 1). The strain was unable to grow with 0.03 M Na+ plus 1.97 M K+ but was able to do so with 0.23 M Na+ plus 1.77 M K+. Thus, although strain DSM 3043 needs Na+, its requirement for this cation can be reduced below the 0.5 M concentration suggested previously (2) if the total monovalent-ion concentration is maintained at 2 M with KCl. The growth rate was 35 to 65% higher in the presence of 1.77 M Na+ plus 0.23 M K+ than in the presence of 0.23 M Na+ plus 1.77 M K+, indicating that high concentrations of Na+ are more beneficial than high concentrations of K+. As has been observed previously (2), glycine betaine was stimulatory at all Na+ and K+ concentrations, with the exception of 0.03 M Na+ (which was inadequate to support growth).
FIG. 1.
Effect of Na+ on the growth rate of C. salexigens DSM 3043 in the presence of a constant 2.0 M concentration of Cl− salts. The growth rate of the strain was determined in modified M63 medium (M63-5× Mg-Fe; see text) supplemented with 10 mM glucose, the indicated concentrations of Na+ (supplied as NaCl), and KCl, added at concentrations such that the sum of the NaCl and KCl was constant at 2.0 M. When used, glycine betaine was added at 1 mM. Cells were first grown to saturation in liquid Luria-Bertani medium (4) with 1 M NaCl, subcultured at a 1:100 dilution into M63-5× Mg-Fe-10 mM glucose-2.0 M NaCl, and grown to saturation. The cells were subcultured at a 1:50 dilution into M63-5× Mg-Fe-10 mM glucose containing various combinations of NaCl and KCl, and the growth rates were determined from the increases in cell density, measured as light scattering at 600 nm (A600), as a function of time. The final density in all cultures containing ≥0.23 M Na+ was ∼2 × 109 cells/ml (A600 = 1.2 to 1.5).
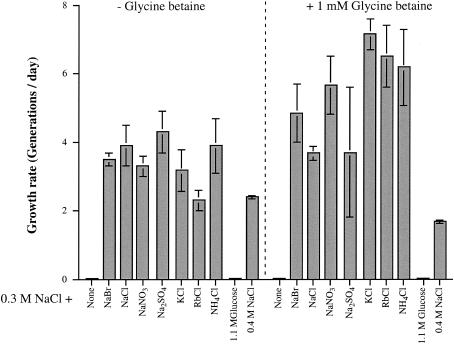
To address whether the optimal growth of C. salexigens seen in the presence of high concentrations of Na+ is dependent specifically on this cation, we determined the organism's growth rate in media containing 0.3 M NaCl and higher concentrations of other salts or glucose (Fig. 2). The strain could not grow rapidly with 0.3 M NaCl in the absence of additional salts. However, augmentation of this medium with 0.7 M NaCl, NaBr, NaNO3, Na2SO4, KCl, RbCl, or NH4Cl resulted in a marked stimulation of growth. Glucose at a concentration of 1.1 M (osmotically equivalent to 0.7 M NaCl) did not support growth, indicating that the growth stimulation seen with the salts was not due to high osmolality alone. Thus, the results presented in Fig. 1 and 2 suggest that in addition to 0.2 to 0.3 M Na+ and/or Cl− ions, for optimum growth, C. salexigens has a requirement for a high ion concentration, which can be satisfied by a 0.7 M concentration of a number of ionic solutes, including the cations Na+, K+, Rb+, and NH4+ and the anions Cl−, Br−, NO3−, and SO42−.
FIG. 2.
Effect of various salts on the growth rate of C. salexigens DSM 3043. Cells were grown as described in the legend to Fig. 1, except that they were subcultured from Luria broth with 1 M NaCl into M63-5× Mg-Fe-10 mM glucose-1.0 M NaCl. Growth rates were determined after a second subculture into M63-5× Mg-Fe-10 mM glucose-0.3 M NaCl-0.7 M salt or 1.1 M glucose. The final density of all cultures was ∼2 × 109 cells/ml (A600 = 1.2 to 1.5), except for those grown in 0.3 M NaCl or 0.3 M NaCl-1.1 M glucose (in which case there was no growth).
C. salexigens DSM 3043 requires Cl− ions.
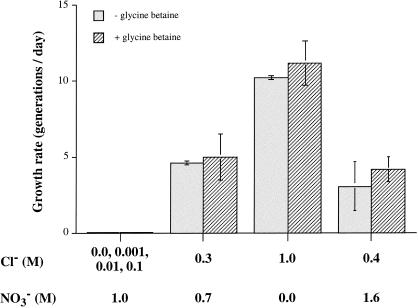
We investigated whether C. salexigens DSM 3043 has a specific requirement for Cl− in experiments in which the strain was grown in the presence of various combinations of NaCl and NaNO3. Figure 3 shows that the organism was not able to grow in media containing ≤0.1 M Cl− plus 1.0 M NaNO3 but was able to grow at a rate of ∼5 generations/day in the presence of 0.3 M Cl− plus 0.7 M NaNO3. The growth rate was increased to 10 and 11 generations/day in medium containing 1 M Cl− in the absence and presence of glycine betaine, respectively. The lack of growth at a Cl− concentration of ≤0.1 M in the presence of 1.0 M NO3− could not be attributed to inhibitory effects of the latter anion, not only because 0.7 M NaNO3 was stimulatory in the presence of 0.3 M NaCl (Fig. 2) but also because the organism could grow in medium containing 1.6 M NaNO3 and 0.4 M NaCl at rates of 3.1 and 4.2 generations/day in the absence and presence of glycine betaine, respectively (Fig. 3). These results show that C. salexigens DSM 3043 has a Cl− ion requirement of >0.1 M. We found that SO42− could not substitute for Cl− ions (data not shown), but we have not investigated whether the requirement for Cl− ions could be met by Br− or I− as entire sources of anions.
FIG. 3.
C. salexigens requires Cl− ions for growth. The cells were grown as described in the legend to Fig. 2. Growth rates were determined after a second subculture into M63-5× Mg-Fe-10 mM glucose containing the indicated concentrations of Cl− and NO3−, added as Na+ salts. The final density of all cultures was ∼2 × 109 cells/ml (A600 = 1.2 to 1.5), except for those containing ≤0.1 M NaCl (in which case there was no growth).
Conclusions.
The major new observation we made is that C. salexigens DSM 3043 does not need high concentrations of NaCl. Provided that the medium contained 0.2 to 0.3 M concentrations of Na+ and Cl− ions, the growth rate of this organism was enhanced by a number of salts of other ions, such as K+, Rb+, NH4+, Br−, NO3−, and SO42−. Thus, C. salexigens DSM 3043 seems to grow optimally in a highly ionic environment, and not necessarily in the presence of high concentrations of NaCl alone. Vreeland and Martin reported that the moderate halophile Halomonas elongata 1H9 has a specific requirement for Na+ which cannot be met by other cations, including K+, Li+, Mg2+, or NH4+ added as Cl− salts (13). Thus, the response of C. salexigens DSM 3043 to high concentrations of ions is very different from those of other H. elongata strains. To our knowledge, this is the first time that growth stimulation by nonspecific high ion concentrations has been reported for any organism. However, a generalized high ion concentration is not sufficient for C. salexigens DSM 3043; in addition, this organism requires moderate concentrations of Na+, which may be used to drive Na+ gradient-dependent processes (5, 6, 8, 12).
In addition to requiring Na+ for growth, C. salexigens DSM 3043 needs Cl− ions at a concentration of >0.1 M, and NO3− cannot be used in place of Cl− ions. This observation points to a second important difference between C. salexigens DSM 3043 and H. elongata 1H9, because unlike C. salexigens DSM 3043, the latter organism was able to use NO3− instead of Cl− (13). It has been reported that Halobacillus halophilus has a requirement for Cl− ions (10). However, that organism was able to adapt to use NO3− instead of Cl− (10), unlike C. salexigens, for which NO3− could not replace Cl− (Fig. 3). Like most other eubacteria, C. salexigens excludes Cl− from the cytoplasm and accumulates the zwitterionic organic compounds ectoine, hydroxyectoine, and glycine betaine as compatible solutes (7, 11); therefore, the biochemical function of Cl− in C. salexigens needs to be elucidated.
Acknowledgments
This work was supported by the National Science Foundation Life in Extreme Environments program (grant MCB-9978253).
REFERENCES
- 1.Arahal, D. R., M. T. García, C. Vargas, D. Cánovas, J. J. Nieto, and A. Ventosa. 2001. Chromohalobacter salexigens sp. nov., a moderately halophilic species that includes Halomonas elongata DSM 3043 and ATCC 33174. Int. J. Syst. E vol. Microbiol. 51:1457-1462. [DOI] [PubMed] [Google Scholar]
- 2.Cánovas, D., C. Vargas, L. N. Csonka, A. Ventosa, and J. Nieto. 1996. Osmoprotectants in Halomonas elongata: high affinity betaine transport system and choline-betaine pathway. J. Bacteriol. 178:7221-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, G. N., and R. H. Rickenberg. 1956. Concentration specifique reversible des amino acides chez E. coli. Ann. Inst. Pasteur Paris 91:693-720. [PubMed] [Google Scholar]
- 4.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
- 5.Dimroth, P. 1997. Primary sodium ion translocating enzymes. Biochim. Biophys. Acta 1318:11-51. [DOI] [PubMed] [Google Scholar]
- 6.Häse, C. C., N. D. Fedorova, M. Y. Galperin, and P. A. Dibrov. 2001. Sodium ion cycle in bacterial pathogens: evidence from cross-genome comparisons. Microbiol. Mol. Biol. Rev. 65:353-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraegeloh, A., and H. J. Kunte. 2002. Novel insights into the role of potassium for osmoregulation in Halomonas elongata. Extermophiles 6:453-462. [DOI] [PubMed] [Google Scholar]
- 8.Maloy, S. R. 1990. Sodium-coupled cotransport, p. 203-224. In T. A. Krulwich (ed.), Bacterial energetics. Academic Press, Inc., San Diego, Calif.
- 9.Martin, E. L., T. Duryea-Rice, R. H. Vreeland, L. Hilsabek, and C. Davis. 1983. Effects of NaCl on the uptake of α-[14C]aminoisobutyric acid by the halotolerant bacterium Halomonas elongata. Can. J. Microbiol. 29:1424-1429. [DOI] [PubMed] [Google Scholar]
- 10.Roeβler, M., and V. Müller. 2001. Quantitative and physiological analyses of chloride dependence of growth of Halobacillus halophilus. Appl. Environ. Microbiol. 64:3813-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Severin, J., A. Wohlfarth, and E. Galinski. 1992. The predominant role of recently discovered tetrahydropyrimidines for the osmoadaptation of halophilic eubacteria. J. Gen. Microbiol. 138:1629-1638. [Google Scholar]
- 12.Skulachev, V. P. 1994. The latest news from the sodium world. Biochim. Biophys. Acta 1187:216-221. [Google Scholar]
- 13.Vreeland, R. H., and E. L. Martin. 1980. Growth characteristics, effects of temperature, and ion specificity of the halotolerant bacterium Halomonas elongata. Can. J. Microbiol. 26:746-752. [Google Scholar]