Abstract
The malignant cells of acute promyelocytic leukemia (APL) contain a reciprocal chromosomal translocation that fuses the promyelocytic leukemia gene (PML) with the retinoic acid receptor α gene (RARα). To test the hypothesis that the chimera PMLRARα plays a role in leukemogenesis, we expressed a PMLRARα cDNA in myeloid cells of transgenic mice. PMLRARα transgenic mice exhibited impaired neutrophil maturation early in life, which progressed at a low frequency over the course of several months to overt APL. Both the preleukemic state and the leukemia could be transplanted to nontransgenic mice, and the transplanted preleukemia could progress to APL. The APL recapitulated features of the human disease, including a response to retinoic acid. Retinoic acid caused the leukemic cells to differentiate in vitro and in vivo, eliciting remissions of both the preleukemic state and APL in mice. Our results demonstrate that PMLRARα impairs neutrophil differentiation and initiates the development of APL. The transgenic mice described here provide an apparently accurate model for human APL that includes clear evidence of tumor progression. The model should be useful for exploring the molecular pathogenesis of APL and the mechanisms of the therapeutic response to retinoic acid, as well as for preclinical studies of therapeutic regimens.
Keywords: [retinoic acid, t15;17, transgenic mice, hematopoiesis]
The malignant cells of acute promyelocytic leukemia (APL) carry a reciprocal chromosomal translocation, t(15;17)(q24;q21) (1–3). The translocation fuses two genes (4, 5): RARα, which encodes the retinoic acid receptor α (Rarα); and promelocytic leukemia (PML), which encodes Pml, a nuclear phosphoprotein of unknown function (6). Prior to the identification of the genes at the t(15;17) breakpoint, all-trans retinoic acid (ATRA) had been shown to induce complete remission in APL patients by differentiation of the leukemic cells (7, 8). This convergence of a novel therapeutic strategy and the molecular analysis of the translocation focused interest on how the resulting PMLRARα and RARαPML fusion genes functioned in the pathogenesis and therapeutic response of APL.
The PMLRARα fusion encodes the bulk of both gene products. PmlRarα retains activities of both Pml and Rarα, including transcriptional activation in response to retinoic acid, and can inhibit differentiation of cultured cells (5, 9–18). In contrast, the coding domain of the reciprocal RARαPML fusion contains little of note and has not been shown to have any phenotypic effects. While PMLRARα is expressed in virtually all cases of APL, RARαPML is frequently not expressed (19). For these reasons it has been assumed that PMLRARα is responsible for the initiation of leukemogenesis, but this view has not been directly authenticated.
Here we report the establishment of a transgenic mouse model that documents the ability of PMLRARα to initiate leukemogenesis. The mice develop two apparently unrelated abnormalities. The first is a severe papillomatosis of the skin that we will describe elsewhere. The second is a disturbance of hematopoiesis that presented as a partial block of differentiation in the neutrophil lineage of the transgenic mice and then progressed at low frequency to overt APL. The leukemias appear to be a faithful reproduction of the human disease, including a therapeutic response to ATRA that reflects differentiation of the leukemic cells. Both the preleukemic state and the overt leukemia can be transplanted into nontransgenic hosts. The mouse model described here can be used to explore the pathogenesis and treatment of APL.
MATERIALS AND METHODS
Generation of Transgenic Mice.
A human PMLRARα cDNA (10) [whose chromosome 15 breakpoint lies in breakpoint cluster region 1 as defined in Pandolfi 1992 (20)] was cloned into the hMRP8 expression cassette (21). Transgenic animals were prepared following standard procedures (22) from inbred FVB/N mice (23).
Isolation of Cells from Tissues.
Blood was obtained from anesthetized animals by venipuncture of the retro-orbital venous plexus. Bone marrow was obtained by flushing buffered saline through mouse long bones. Tissues were suspended in buffered saline and filtered through nylon mesh. Red cells were lysed as necessary (24). Blood and bone marrow smears and cytospins were prepared according to standard hematological techniques.
Immunofluorescence.
Cytospins prepared as above were fixed for 5 min in −20°C methanol and air dried. Samples were blocked for 15 min in PBS with 10% normal goat serum (PBS/NGS), and incubated for 1 h with rabbit polyclonal antiserum prepared against a glutathione S-transferase Pml fusion protein diluted 1:4000 in PBS/NGS (the antiserum was a kind gift of K. S. Chang, M. D. Anderson Cancer Center, University of Texas). Washing with PBS/NGS was followed by 30 min of incubation with Texas Red-labeled goat anti-rabbit IgG (Southern Biotechnology Associates) at 1:400 in PBS/NGS. The slides were mounted in Prolong medium (Molecular Probes) plus 1 μM 4,6-diamidino-2-phenylindole (DAPI) (Sigma).
Fluorescence-Activated Cell Sorting (FACS).
Single cell suspensions prepared as above were stained with the indicated antibodies and analyzed on a FACScan Flow Cytometer (Becton Dickinson). Cells were incubated with mAbs for 20–30 min on ice, then allowed to settle through a calf serum cushion to remove unbound antibodies. Cells were resuspended in staining medium (buffered saline with 2% fetal calf serum) containing propidium iodide at 4 μg/ml. At least 10,000 cells were analyzed per sample. Dead cells that stained with propidium iodide were gated out at the time of analysis. FACS data were analyzed with the cellquest program (Becton Dickinson). Anti-Gr-1–fluorescein isothiocyanate and anti-MAC-1–phycoerythrin were obtained from PharMingen.
Methylcellulose Cultures of Bone Marrow.
Duplicate cultures of 5 × 104 viable bone marrow cells per 35-mm tissue culture dish were incubated for 7 days in Methocult M3230 methylcellulose medium (Stem Cell Technologies, Vancouver, British Columbia) supplemented with 2% pokeweed mitogen spleen-conditioned media (Stem Cell Technologies) with or without 1 μM ATRA (Sigma).
Western Blot Analysis.
Western blot analysis was performed as described (25) with rabbit polyclonal antiserum raised against a glutathione S-transferase fusion protein encompassing amino acids 420–462 of the human Rarα protein (26). Before use, the antiserum was affinity purified with the analogous maltose binding protein fusion.
Transplantation of Leukemia.
Viable cells (107) isolated from bone marrow, spleen, or lymph nodes of animals with leukemia were resuspended in 200 μl of buffered saline and injected into the tail veins of 5- to 8-week-old FVB/N mice.
Transplantation of Bone Marrow.
Total bone marrow isolated from the tibiae and femurs of a single donor was divided for intravenous injection into six recipient mice. Five- to 12-week-old FVB/N mice were prepared for transplantation by cesium irradiation totaling 10.5 Gray, divided in two doses 3–6 h apart.
ATRA Treatment.
At the indicated times after transplantation, ATRA was administered to mice via one of two routes: intraperitoneal injection of 100 μl of ATRA (Sigma) or ethanol vehicle suspended in canola or peanut oil at 54 mg/kg per day or subcutaneous implantation of a 21-day-release pellet containing 5 mg ATRA or placebo (Innovative Research of America).
RESULTS
Expression of PMLRARα in Transgenic Mice.
To examine the effect of PMLRARα on granulocyte development, we created mice that expressed a human PMLRARα cDNA (10) from the hMRP8 promoter cassette, which drives transgene expression in myeloid cells (21). Injections into FVB/N embryos yielded nine transgenic lines.
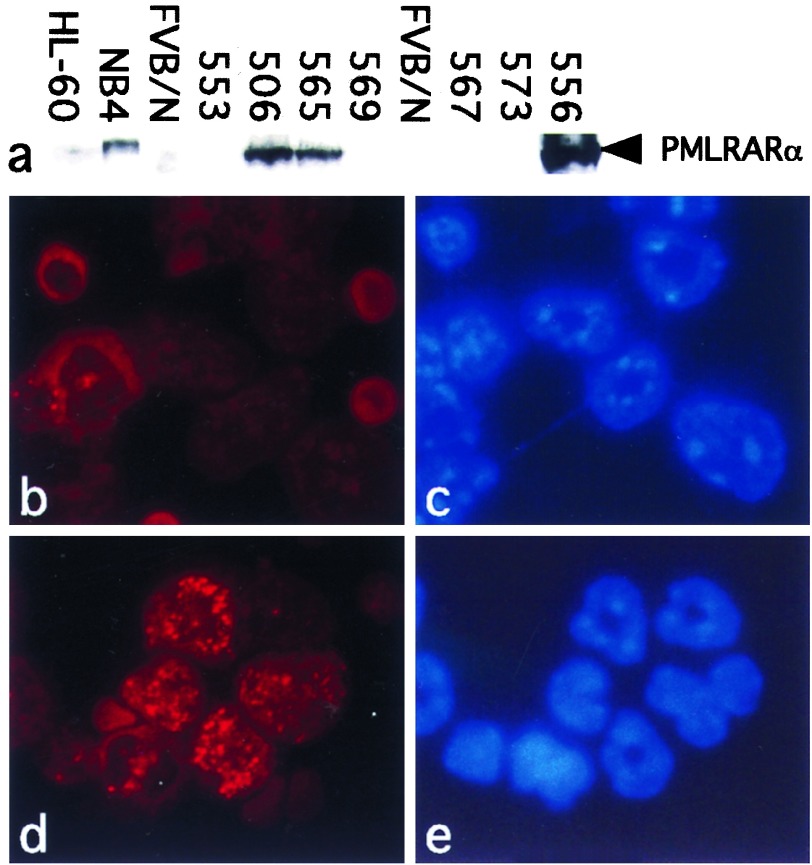
Levels of PMLRARα transgene expression in the bone marrow of transgenic mice varied from virtually undetectable to several-fold higher than NB4 human promyelocytic leukemia cells (4, 27) (Fig. 1a). To determine which cells in the transgenic bone marrow expressed PMLRARα, bone marrow cells or peripheral blood leukocytes were analyzed by immunofluorescence using antiserum directed against human Pml. PmlRarα was distributed in a speckled nuclear pattern, consistent with previous reports (11, 12) (Fig. 1d). Simultaneous staining with DAPI highlighted the distinctive indented, horseshoe- or doughnut-shaped nuclei characteristic of murine granulocytes (Fig. 1e), verifying that the transgene was expressed in the granulocyte lineage. In lines where transgene expression was undetectable by Western blot, very few cells were stained by the anti-Pml antibody; in lines with high levels of expression, many more of the cells were stained (data not shown). Altogether, we detected PmlRarα expression in granulocytes from eight of nine transgenic lines.
Figure 1.
PMLRARα expression in bone marrow of transgenic mice. (a) Western blot. Total cell lysates from equal numbers of unfractionated bone marrow cells from the indicated hMRP8–PMLRARα transgenic lines or control cell lines were loaded into each lane. The blot was probed with antibodies against human Rarα. NB4 human APL cells (27) express PMLRARα (4), but HL-60 human myeloid leukemia cells do not (28). FVB/N samples were from nontransgenic mice. (b–e) Immunofluorescence. Bone marrow cells from healthy 8-week-old mice were cytospun and incubated with antiserum specific for human Pml (b and d) and the DNA-intercalating dye DAPI (c and e). (b and c) Control FVB/N. (d and e) Transgenic line 556. (×250.)
Neutrophil Maturation Is Impaired in PMLRARα Transgenic Mice.
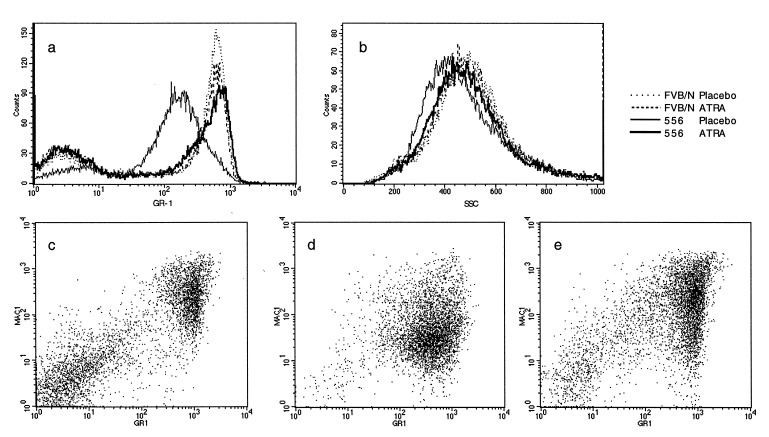
When examined microscopically, the peripheral blood and bone marrow of PMLRARα transgenic mice resembled the controls (data not shown). However, although neutrophilic cells from transgenic mice appeared morphologically normal, when we subjected cells isolated from peripheral blood or bone marrow to FACS using markers for myeloid differentiation, subtle aberrations were apparent. The transgenic samples displayed lower levels of the Gr-1 antigen (29) (Fig. 2a), expression of which increases as neutrophils mature (30). The transgenic samples also had lower than normal levels of side-scatter (Fig. 2b), reflecting a low cytoplasmic granularity, which is typical of immature granulocytes. Both side-scatter and Gr-1 expression were inversely related to the level of transgene expression. In contrast, levels of CD11b, a cell-surface marker that also increases with myeloid differentiation, were not diminished (data not shown). We examined at least five mice each from three transgenic lines (553, 556, and 565), and the aberrations were consistent within each line, regardless of age. Granulocytic cells from the transgenic mice therefore combined features of both immature and mature cells. These data reveal that PMLRARα blocks some aspects of neutrophil maturation but not others. For convenience, we refer to this condition as the “preleukemic state.”
Figure 2.
FACS analysis of bone marrow. Bone marrow cells were stained with Gr-1 and Mac-1 (murine Cd11b), markers for differentiation of murine myeloid cells. (a and b) Placebo or 5 mg ATRA pellets were implanted into 3-week-old FVB/N or line 556 mice; marrow was analyzed after 21 days. Each curve combines data from two mice. FVB/N–placebo, dotted line; line 556–placebo, thin line; FVB/N–ATRA, dashed line; line 556–ATRA, thick line. (a) Gr-1 antigen expression. (b) Side-scatter profile of Gr-1-positive cells from samples in a. (c–e) Murine APL responds to ATRA. (c) Age-matched FVB/N control. (d and e) Samples from second passage by transplantation of leukemia 935 (line 569). On days 14–21 after transplantation, mice were given placebo (d) or 1.35 mg ATRA (e) by intraperitoneal injection of oil suspension. Cells were isolated on day 28 after transplantation.
PMLRARα Transgenic Mice Develop APL.
Twelve PMLRARα mice from five independent transgenic lines have developed APL when aged 3–9 months (median, 174 days) (Table 1). Assessment of lifetime penetrance of the leukemia has been precluded by the epidermal papillomatosis, which occurs in varying degrees in all the mice and makes it difficult to maintain mice for a normal life span (to be reported elsewhere). Nevertheless, it appears that penetrance of the leukemia may correlate with overall levels of transgene expression: although leukemias have been seen in lines with relatively low levels of PMLRARα expression, the disease has been more frequent in mice from the high-expressing 556 line (Table 1).
Table 1.
Transgene expression and leukemia incidence
| Line | PmlRarα expression* | APL cases | Age at diagnosis, days | Mice reaching age 174 days† |
|---|---|---|---|---|
| 506 | ++ | 2 | 174, 195 | 45 |
| 553 | + | 3 | 78, 174, 265 | 79 |
| 556 | +++ | 5 | 92, 143, 149, 176, 203 | 16 |
| 565 | ++ | 0 | 31 | |
| 569 | + | 1 | 155 | 12 |
| 608 | + | 1 | 247 | 34 |
Relative levels based on visual comparison of western blot and immunofluorescence.
Includes living mice and those aged at least 174 days at death (median age at diagnosis of APL).
The leukemic mice were abnormally susceptible to bleeding after injections, and a few mice hemorrhaged spontaneously. In some mice, fibrin thrombi were present in pulmonary blood vessels but not in other organs. Peripheral blood counts were notable for anemia and thrombocytopenia in the absence of increased numbers of white blood cells (0.8–7.9 × 106 cells/ml in leukemias vs. 1.1–8.3 × 106 cells/ml in controls). A bleeding diathesis in the presence of normal or even low white counts is typical of human APL (31).
The pathology of the leukemia was consistent among the various transgenic lines. Pale bone marrow was accompanied by lymphadenopathy and hepatosplenomegaly. Microscopically, the mixed hematopoiesis of normal bone marrow was completely replaced by leukemic cells, splenic and lymphatic architecture was effaced, and leukemia extended throughout the hepatic parenchyma. In some cases, extensive invasion of the kidneys, reproductive organs, or the meninges was also observed.
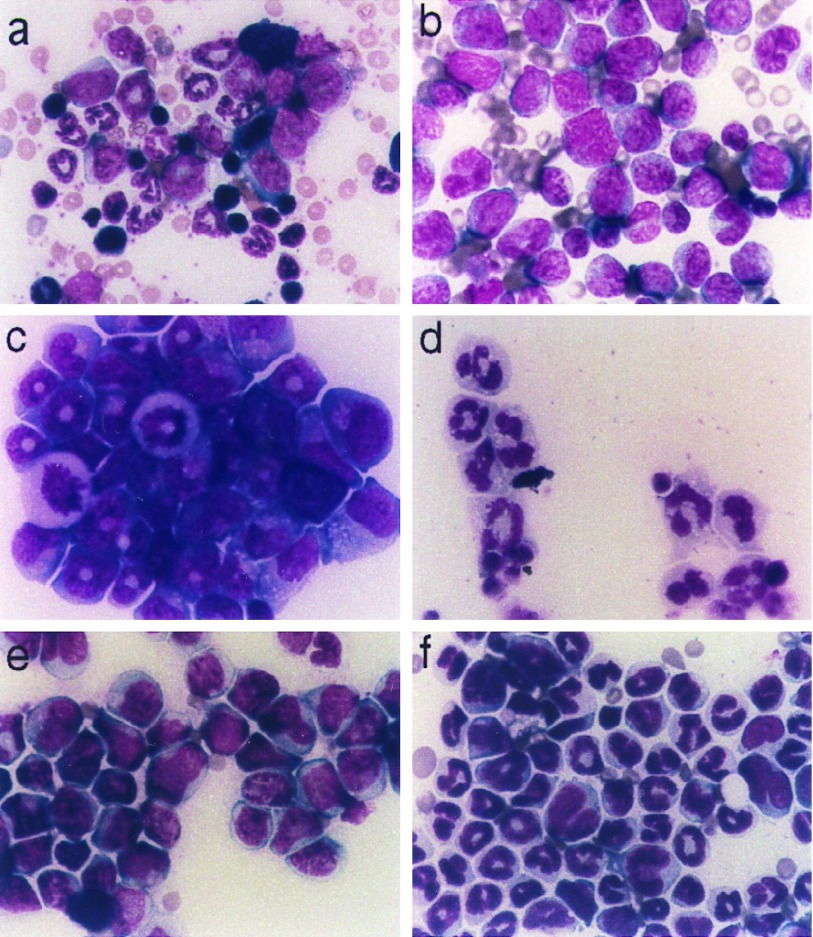
Morphologically, the leukemic cells were promyelocytes (Fig. 3b): they were large, had oval to indented nuclei and abundant basophilic cytoplasm with many primary granules. Mitotic figures and dysplastic nuclei were present. Although we have not observed Auer rods or faggot cells in these leukemias, both of which are characteristic of human APL (31), we note that maturing human and murine granulocytes are morphologically distinct, and morphological variations might be expected between human and murine leukemias arising from this lineage. Like human promyelocytic leukemia cells, the murine leukemia cells stained strongly with Sudan Black B (data not shown), which marks the myeloperoxidase found in the primary granules of cells in the promyelocyte/granulocyte lineage (32). The cells expressed low levels of Gr-1 and Mac-1 antigens (Fig. 2d); low expression of CD11b, the surface marker detected by the Mac-1 antibody, is characteristic of APL cells (33). In 7-day methylcellulose culture, leukemic bone marrow gave rise to abundant compact colonies. Cytospins of cells isolated from these cultures principally contained promyelocytes (Fig. 3c). We conclude that the transgenic mice had developed APL.
Figure 3.
Murine APL. (a and b) Bone marrow isolates. (a) Control FVB/N. (b) Leukemia (mouse 1333, line 556). (c and d) Cytospins of 7-day methylcellulose cultures from passage 1 of leukemia 877 (line 553) cultured without (c) or with (d) 1 μM ATRA. (e and f) Bone marrow isolates from third passage of leukemia 877. On day 11 after transplantation, pellets containing placebo (e) or 5 mg ATRA (f) were implanted. Samples were prepared on day 16 after transplantation. Cells were stained with Wright’s Giemsa with Azure B. (×250.)
Murine APL and the Preleukemic State Are Transplantable.
To further verify the malignant character of the myeloid leukemia, we transplanted leukemic cells into unirradiated FVB/N mice. We intravenously injected cells isolated from the bone marrow or spleen of leukemic donors. To date, five leukemias have been tested with similar results: 3–5 weeks after transplantation, the recipient mice developed leukemia. Clinical and pathological features resembled the disease in the donors. Morphologically and by FACS analysis, the leukemic cells were identical to the donor cells. When analyzed by immunofluorescence, samples from the transplant recipients stained intensely with anti-Pml antibodies (data not shown). Because the recipient mice did not carry the transgene, this verified that the leukemic cells themselves expressed PMLRARα.
We were also able to transplant the preleukemic state by injecting bone marrow from young, healthy transgenic mice into lethally irradiated FVB/N mice. We have tested 6 of 45 recipient mice and all displayed marrow and blood FACS profiles characteristic of the transgenic line of the donor (data not shown). We have seen 9 cases of APL in the recipients to date. These results demonstrated that the observed hematological abnormalities were not a reaction to the papillomas, and provide the opportunity to define the incidence of leukemia and study tumor progression in a setting free of the epidermal disease.
Murine APL Differentiates in Response to ATRA in Vitro and in Vivo.
We wanted to determine whether the murine leukemia responded to ATRA like the human leukemia; the ability to passage the leukemias enabled us to generate additional material for these studies. When cultured for 7 days in the presence of ATRA, the compact colonies seen in untreated cultures were almost entirely replaced by colonies with an open, spreading appearance (data not shown). This suggested that ATRA was inducing differentiation, since mature neutrophils are mobile. Cytospins of treated cultures chiefly consisted of mature neutrophils (Fig. 3d). These experiments demonstrated that the leukemic cells could be differentiated by ATRA treatment.
Next we examined the ATRA response in mice. We first used a suspension of ATRA in oil for intraperitoneal injection. The dose was calculated to approximate the blood levels achieved in human patients, with corrections for different route of administration and more rapid murine metabolism (34, 35). ATRA and placebo injections were begun in four and two animals, respectively, on day 15 after transplantation, when promyelocytes were present in the peripheral circulation; on day 21 after transplantation, injections were stopped and one mouse killed due to onset of vitamin A toxicity.
On day 28, a moribund placebo-treated mouse and a healthy ATRA-treated mouse were killed and their bone marrows were analyzed by FACS. Comparison of cells stained with Gr-1 and Mac-1 antibodies showed two main clusters of cells in the FVB/N control marrow (Fig. 2c): nonmyeloid cells, negative for both markers, and maturing neutrophils, strongly positive for both markers. In the placebo-treated mouse (Fig. 2d), the bulk of the marrow was composed of leukemic cells expressing low levels of Gr-1 and Mac-1. ATRA treatment (Fig. 2e) restored a near-normal distribution of Gr-1 and Mac-1 staining.
The FACS changes were accompanied by the appearance of mature neutrophils in blood and bone marrow and by lessened hepatosplenomegaly and lymphadenopathy in treated mice. The two remaining ATRA-treated mice were monitored to examine their long-term response to therapy. They continued in apparent remission until one relapsed 47 days after the end of treatment (68 days after transplantation), while the other remains in good health 7 months after transplantation.
Subsequently, we have subcutaneously implanted 21-day release pellets (containing either 5 mg ATRA or placebo) 10–20 days after transplantation. These obviated the need for daily injections and did not lead to overdose. In trials involving 6–12 animals each, we have tested recipients of transplants from 4 cases of leukemia, and have observed consistent responses to ATRA pellets. Within 3–5 days of initiating treatment, increasing numbers of neutrophils appeared in the peripheral blood and bone marrow (Fig. 3f). The mice treated with the ATRA pellets generally did not survive much longer than those treated with placebos, but we suspect that their deaths were related to the pulse of differentiating neutrophils rather than to the leukemia itself, since maturing neutrophils were the predominant cell type in the peripheral blood and bone marrows of treated mice. These adverse results of ATRA treatment may represent a murine version of the “retinoic acid syndrome,” which is thought to be related to the rapid expansion of the neutrophil population induced by differentiation of the leukemic cells (36).
In addition to treating the leukemia with ATRA, we implanted ATRA or placebo pellets into transgenic mice that did not have leukemia. The distribution of Gr-1 expression and side-scatter was shifted toward normal levels in five of five ATRA-treated transgenic mice, while control FVB/N were unaffected by the treatment (Fig. 2 a and b). It thus appears that the drug overcomes the preneoplastic changes as well as the acute leukemia, reinforcing the hypothesis that ATRA can correct abnormalities elicited by expression of PMLRARα in neutrophils.
DISCUSSION
PMLRARα Impairs Neutrophil Differentiation.
We have shown that expression of a human PMLRARα cDNA in murine granulocyte precursor cells alters their differentiation and initiates the development of APL. Some aspects of neutrophilic differentiation were sensitive to inhibition by PmlRarα, others were not. In particular, expression of the myeloid differentiation marker CD11b was not inhibited by PmlRarα, in accord with a previous report in which PMLRARα was expressed in U937 cells (13). Our in vivo results confirm the hypothesis that expression of PMLRARα in otherwise normal granulocytic cells impairs their maturation, and define a preleukemic state elicited by PMLRARα.
It could be argued that the severe epidermal papillomatosis seen in the transgenic mice might have secondary effects that could account for a disturbance in granulopoiesis. We do not believe that this is the case: we have seen consistent FACS abnormalities in transgenic mice as young as 3 weeks, in the absence of visible skin lesions, and preleukemic marrow transplanted into nontransgenic mice retains its characteristic phenotypic abnormalities and propensity to develop APL.
PMLRARα Initiates APL.
In addition to the preneoplastic changes in granulopoiesis, hMRP8–PMLRARα transgenic mice develop acute leukemia. We believe that expression of PMLRARα alone is not sufficient to cause leukemia because the leukemic phenotype combines incomplete penetrance with delayed onset. While the epidermal papillomatosis has limited median survival in the most severely afflicted transgenic lines to 3–5 months, we suspect the incidence of leukemia in most of the transgenic lines may be only a few percent over the normal life span of the mice (Table 1). However, results with a small number of bone marrow transplants suggest an incidence approaching 30% at 1 year in the high expressing line 556. Initial results indicate that crossing transgenic lines 556 and 565 yields higher expression of the transgene and a greater incidence of leukemia, without changing the latency. The 3- to 9-month latency of the disease, taken together with the low penetrance of leukemia, suggests that leukemogenesis by PMLRARα requires additional genetic changes.
Expression of PMLRARα may facilitate the acquisition of additional genetic changes. Although the transgenic mice did not show a clear expansion of the myeloid compartment prior to the development of APL, increased proliferation might be balanced by increased destruction, the balance between differentiation and self-renewal might be altered, or genomic instability might be increased. These mechanisms could enhance accumulation of mutations that further block differentiation, promote proliferation, or decrease apoptosis of the promyelocytic cells. In collaboration with PmlRarα, these genetic changes could then release promyelocytes from normal growth constraints, resulting in the uncontrolled proliferation of malignancy.
The Murine Leukemia Is an Accurate Model for Human APL.
We believe that the murine leukemia described here is a true analog of the human disease. APL in both species shares the promyelocytic character of the leukemic cells and differentiation in response to ATRA (with both therapeutic and adverse effects). Our data further authenticate the role of PMLRARα in the specific pathogenesis of APL.
Also similar to human APL is the presence of a bleeding tendency in leukemic mice. The etiology of bleeding in the mice remains uncertain, however, since we have not observed disseminated intravascular thrombi.
Other investigators have prepared mice in which transgenic PMLRARα is expressed by transcriptional controls for either the CD11b gene (37) or the human cathepsin G gene (hCG) (38). The CD11b mice failed to develop either preleukemia or leukemia. Their only apparent abnormality was an increased sensitivity to doses of irradiation that are normally sublethal, succumbing to infections because of protracted bone marrow suppression. Thus, it is not immediately apparent that these mice offer any access to the pathogenesis of APL.
In contrast, the hCG mice did display a preleukemic state that eventually progressed to overt leukemia, but they nevertheless differed in several substantive regards from the hMRP8 mice described in the present communication. The leukemias in hCG mice were accompanied by elevated white blood cell counts and the persistence of maturing neutrophils in the bone marrow, and treatment with ATRA caused apoptosis rather than differentiation of the leukemic cells. Several factors may figure in these differences. The hMRP8 gene is expressed more broadly in the myeloid lineage than hCG, which is restricted to promyelocytes and promonocytes (39). The hCG transgene may be down-regulated following ATRA treatment, while expression from the hMRP8 promoter is maintained during neutrophilic differentiation. The genetic backgrounds of the transgenic mice also differ, which could affect the response to PMLRARα expression: the hCG mice were made in (C57BL/6 × C3H/He)F2 cross mice, whereas the hMRP8 mice were made in inbred FVB/N mice.
In yet another experimental approach, an avian retrovirus was used to express PMLRARα in chicken embryos (40). The result was an acute leukemia composed of multipotent blast cells, which were far less mature than the promyelocytes of APL (40). The blasts did not respond to ATRA, and their occurrence was associated with a consistent pair of mutations in the PML moiety of the fusion gene, mutations that have not been reported to occur in human patients. While this system did reveal that PMLRARα has leukemogenic potential outside a promyelocytic context, the recurrent mutations and nonpromyelocytic character of the leukemic blasts distinguish it from human APL.
The strong correspondence between human APL and the murine leukemia described here indicates that the hMRP8–PMLRARα system can be used to obtain information that is directly relevant to the human disease. The transgenic mice provide a valuable tool for further exploring the mechanisms of PmlRarα action, for isolating the additional genetic events that collaborate with PMLRARα to induce acute disease, and for exploring therapeutic approaches that may improve the survival of patients with APL and other leukemias.
Acknowledgments
We thank K. Chang for anti-Pml antiserum and M. Lanotte for providing NB4 cells, members of the Bishop lab for helpful advice and discussion, D. Hanahan for teaching D.B. how to make and care for transgenic mice, M. Pallavicini for teaching murine bone marrow transplantation to S.K, and T. Ley for communication of results prior to publication. This work was supported by Grant CA 44338 from the National Institutes of Health and by funds from the G. W. Hooper Research Foundation (to J.M.B.). S.K. was supported by physician fellowships from the American Cancer Society and the Howard Hughes Medical Institute.
ABBREVIATIONS
- APL
acute promyelocytic leukemia
- PML
promyelocytic leukemia gene
- RARα
retinoic acid receptor α
- ATRA
all-trans retinoic acid
- DAPI
4,6-diamidino-2-phenylindole
- FACS
fluorescence-activated cell sorting
References
- 1.Rowley J D, Golomb H M, Dougherty C. Lancet. 1977;i:549–550. doi: 10.1016/s0140-6736(77)91415-5. [DOI] [PubMed] [Google Scholar]
- 2.Warrell R P, Jr, de The H, Wang Z Y, Degos L. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 3.Grignani F, Fagioli M, Alcalay M, Longo L, Pandolfi P P, Donti E, Biondi A, Lo Coco F, Grignani F, Pelicci P G. Blood. 1994;83:10–25. [PubMed] [Google Scholar]
- 4.de The H, Chomienne C, Lanotte M, Degos L, Dejean A. Nature (London) 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 5.Kakizuka A, Miller W, Jr, Umesono K, Warrell R, Jr, Frankel S R, Murty V V, Dmitrovsky E, Evans R M. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 6.Chang K S, Fan Y H, Andreeff M, Liu J, Mu Z M. Blood. 1995;85:3646–3653. [PubMed] [Google Scholar]
- 7.Huang M E, Ye Y C, Chen S R, Zhao J C, Gu L J, Cai J R, Zhao L, Xie J X, Shen Z X, Wang Z Y. Chin Med J (Engl) 1987;100:949–953. [PubMed] [Google Scholar]
- 8.Warrell R J, Frankel S R, Miller W J, Scheinberg D A, Itri L M, Hittelman W N, Vyas R, Andreeff M, Tafuri A, Jakubowski A. N Engl J Med. 1991;324:1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 9.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfi P P, Grignani F, Alcalay M, Mencarelli A, Biondi A, LoCoco F, Grignani F, Pelicci P G. Oncogene. 1991;6:1285–1292. [PubMed] [Google Scholar]
- 11.Kastner P, Perez A, Lutz Y, Rochette E C, Gaub M P, Durand B, Lanotte M, Berger R, Chambon P. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel M T, Koken M, Romagne O, Barbey S, Bazarbachi A, Stadler M, Guillemin M C, Degos L, Chomienne C, de The H. Blood. 1993;82:1858–1867. [PubMed] [Google Scholar]
- 13.Grignani F, Ferrucci P F, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Grignani F, Peschle C, Nicoletti I, Pelicci P G. Cell. 1993;74:423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- 14.Perez A, Kastner P, Sethi S, Lutz Y, Reibel C, Chambon P. EMBO J. 1993;12:3171–3182. doi: 10.1002/j.1460-2075.1993.tb05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyck J A, Maul G G, Miller W H, Jr, Chen J D, Kakizuka A, Evans R M. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 16.Rousselot P, Hardas B, Patel A, Guidez F, Gaken J, Castaigne S, Dejean A, de The H, Degos L, Farzaneh F, Chomienne C. Oncogene. 1994;9:545–551. [PubMed] [Google Scholar]
- 17.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 18.Grignani F, Testa U, Fagioli M, Barberi T, Masciulli R, Mariani G, Peschle C, Pelicci P G. Cancer Res. 1995;55:440–443. [PubMed] [Google Scholar]
- 19.Borrow J, Goddard A D, Gibbons B, Katz F, Swirsky D, Fioretos T, Dube I, Winfield D A, Kingston J, Hagemeijer A, Rees J K H, Lister T A, Solomon E. Br J Haematol. 1992;82:529–540. doi: 10.1111/j.1365-2141.1992.tb06463.x. [DOI] [PubMed] [Google Scholar]
- 20.Pandolfi P P, Alcalay M, Fagioli M, Zangrilli D, Mencarelli A, Diverio D, Biondi A, Lo Coco F, Rambaldi A, Grignani F. EMBO J. 1992;11:1397–1407. doi: 10.1002/j.1460-2075.1992.tb05185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagasse E, Weissman I L. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan B, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 23.Taketo M, Schroeder A C, Mobraaten L E, Gunning K B, Hanten G, Fox R R, Roderick T H, Stewart C L, Lilly F, Hansen C T, Overbeek P A. Proc Natl Acad Sci USA. 1991;88:2065–2969. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current Protocols in Immunology. New York: Greene/Wiley Interscience; 1991. pp. 3.1.3–3.1.5. [Google Scholar]
- 25.Robbins S M, Quintrell N A, Bishop J M. Mol Cell Biol. 1995;15:3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaub M P, Rochette-Egly C, Lutz Y, Ali S, Matthes H, Scheuer I, Chambon P. Exp Cell Res. 1992;201:335–346. doi: 10.1016/0014-4827(92)90282-d. [DOI] [PubMed] [Google Scholar]
- 27.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 28.Dalton W T, Jr, Ahearn M J, McCredie K B, Freireich E J, Stass S A, Trujillo J M. Blood. 1988;71:242–247. [PubMed] [Google Scholar]
- 29.Fleming T J, Fleming M L, Malek T R. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 30.Hestdal K, Ruscetti F W, Ihle J N, Jacobsen S E, Dubois C M, Kopp W C, Longo D L, Keller J R. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 31.Knowles D M. Neoplastic Hematopathology. Baltimore: Williams & Wilkins; 1992. [Google Scholar]
- 32.Davey F R, Davis R B, MacCallum J M, Nelson D A, Mayer R J, Ball E D, Griffin J D, Schiffer C A, Bloomfield C D. Am J Hematol. 1989;30:221–227. doi: 10.1002/ajh.2830300406. [DOI] [PubMed] [Google Scholar]
- 33.Paietta E, Andersen J, Gallagher R, Bennett J, Yunis J, Cassileth P, Rowe J, Wiernik P H. Leukemia. 1994;8:1108–1112. [PubMed] [Google Scholar]
- 34.Muindi, J. R., Young, C. W. & Warrell, R. P., Jr. (1994) Leukemia 8 (Suppl. 3), S16–S21. [PubMed]
- 35.Achkar C C, Bentel J M, Boylan J F, Scher H I, Gudas L J, Miller W H., Jr Drug Metab Dispos. 1994;22:451–458. [PubMed] [Google Scholar]
- 36.Frankel S R, Eardley A, Lauwers G, Weiss M, Warrell R P., Jr Ann Intern Med. 1992;117:292–296. doi: 10.7326/0003-4819-117-4-292. [DOI] [PubMed] [Google Scholar]
- 37.Early E, Moore M A S, Kakizuka A, Nason-Burchenal K, Martin P, Evans R M, Dmitrovsky E. Proc Natl Acad Sci USA. 1996;93:7900–7904. doi: 10.1073/pnas.93.15.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grisolano J L, Wesselschmidt R L, Pelicci P G, Ley T L. Blood. 1997;89:376–387. [PubMed] [Google Scholar]
- 39.Grisolano J L, Sclar G M, Ley T J. Proc Natl Acad Sci USA. 1994;91:8989–8993. doi: 10.1073/pnas.91.19.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altabef M, Garcia M, Lavau C, Bae S-C, Dejean A, Samarut J. EMBO J. 1996;15:2707–2716. [PMC free article] [PubMed] [Google Scholar]