Abstract
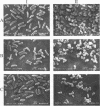
Investigations of psychrotrophic microorganisms have been limited even though the dominant environment of the Earth is cold and enzymes with high activities at low temperatures could have commercial uses. We have isolated and characterized three psychrotrophic strains with beta-galactosidase activities. The isolates, B7, D2, and D5, were gram-positive, catalase-positive, obligate aerobes. Cells observed with a scanning electron microscope appeared as rods during the early stages of growth but became coccoid during the stationary phase. An analysis of the amino acid composition of the cell walls demonstrated the presence of lysine as the predominant diamino acid in all three isolates. The cell cycle morphology and cell wall composition suggest that the three isolates are members of the genus Arthrobacter. The beta-galactosidase activities in whole cells were labile when incubated at 40 degrees C and had temperature optima about 20 degrees C below that of the enzyme encoded by the lacZ gene of Escherichia coli. Electrophoresis of extracts from the isolates in nondenaturing polyacrylamide gels detected at least two protein bands that hydrolyzed 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-Gal), suggesting the presence of beta-galactosidase isozymes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gounot A. M. Bacterial life at low temperature: physiological aspects and biotechnological implications. J Appl Bacteriol. 1991 Nov;71(5):386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Hirata H., Negoro S., Okada H. Molecular basis of isozyme formation of beta-galactosidases in Bacillus stearothermophilus: isolation of two beta-galactosidase genes, bgaA and bgaB. J Bacteriol. 1984 Oct;160(1):9–14. doi: 10.1128/jb.160.1.9-14.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAHAM J. L. Growth of psychrophilic bacteria. J Bacteriol. 1958 Jul;76(1):75–80. doi: 10.1128/jb.76.1.75-80.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inniss W. E. Interaction of temperature and psychrophilic microorganisms. Annu Rev Microbiol. 1975;29:445–465. doi: 10.1146/annurev.mi.29.100175.002305. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]