Abstract
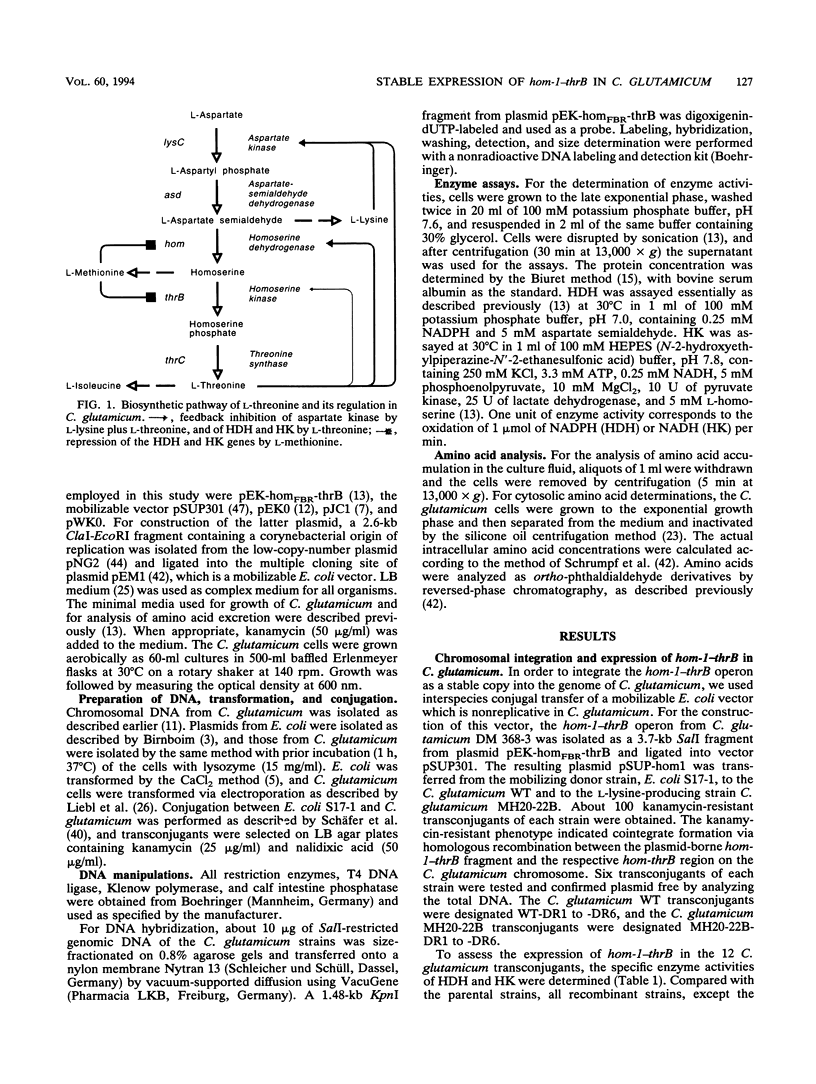
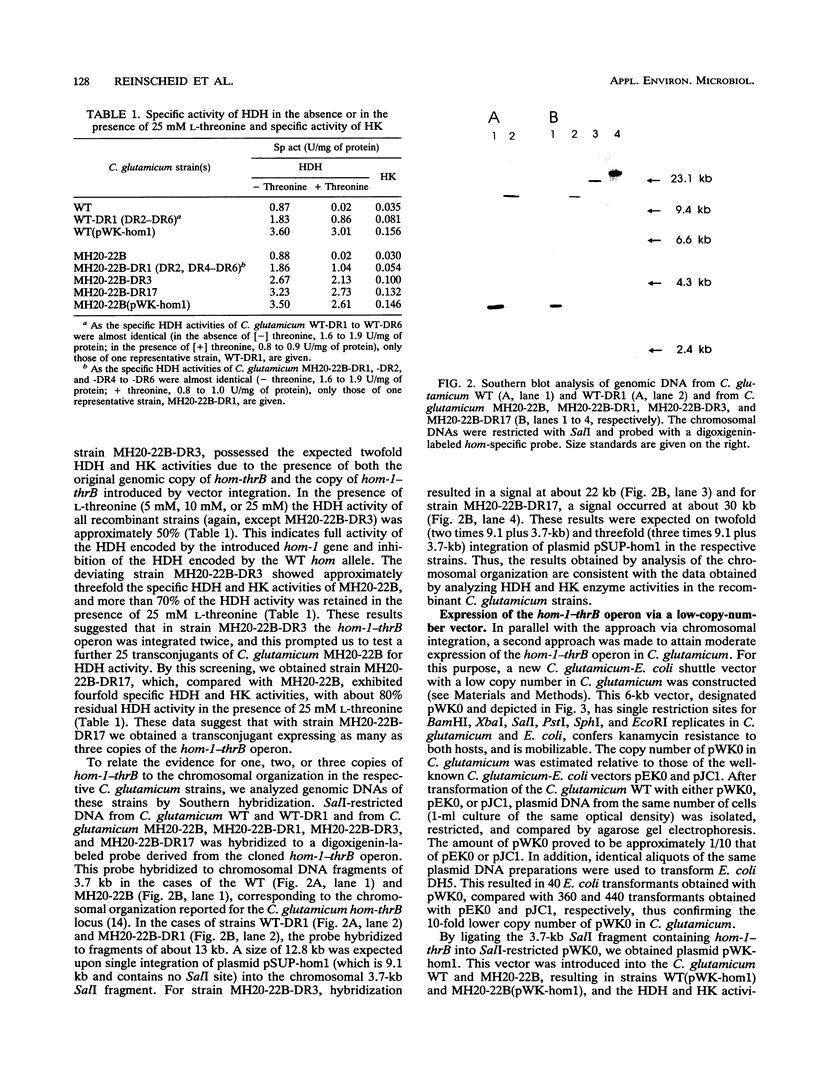
The hom-1-thrB operon encodes homoserine dehydrogenase resistant to feedback inhibition by L-threonine and homoserine kinase. Stable expression of this operon has not yet been attained in different Corynebacterium glutamicum strains. We studied the use of chromosomal integration and of a low-copy-number vector for moderate expression of the hom-1-thrB operon to enable an analysis of the physiological consequences of its expression in C. glutamicum. Strains carrying one, two, or three copies of hom-1-thrB were obtained. They showed proportionally increased enzyme activity of feedback-resistant homoserine dehydrogenase and of homoserine kinase. This phenotype was stably maintained in all recombinants for more than 70 generations. In a lysine-producing C. glutamicum strain which does not produce any threonine, expression of one copy of hom-1-thrB resulted in the secretion of 39 mM threonine. Additional copies resulted in a higher, although not proportional, accumulation of threonine (up to 69 mM). This indicates further limitations of threonine production. As the copy number of hom-1-thrB increased, increasing amounts of homoserine (up to 23 mM) and isoleucine (up to 34 mM) were secreted. Determination of the cytosolic concentration of the respective amino acids revealed an increase of intracellular threonine from 9 to 100 mM and of intracellular homoserine from 4 to 74 mM as the copy number of hom-1-thrB increased. These results suggest that threonine production with C. glutamicum is limited by the efflux system for this amino acid. Furthermore, the results show the successful use of moderate and stable hom-1-thrB expression for directing the carbon flux from aspartate to threonine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Solow-Cordero D. E., Sinskey A. J. A C-terminal deletion in Corynebacterium glutamicum homoserine dehydrogenase abolishes allosteric inhibition by L-threonine. Gene. 1991 Oct 30;107(1):53–59. doi: 10.1016/0378-1119(91)90296-n. [DOI] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. Bacterial catabolism of threonine. Threonine degradation initiated by L-threonine-NAD+ oxidoreductase. Biochem J. 1976 May 15;156(2):449–458. doi: 10.1042/bj1560449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bröer S., Krämer R. Lysine excretion by Corynebacterium glutamicum. 1. Identification of a specific secretion carrier system. Eur J Biochem. 1991 Nov 15;202(1):131–135. doi: 10.1111/j.1432-1033.1991.tb16353.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes C., Möckel B., Eggeling L., Sahm H. Cloning, organization and functional analysis of ilvA, ilvB and ilvC genes from Corynebacterium glutamicum. Gene. 1992 Mar 1;112(1):113–116. doi: 10.1016/0378-1119(92)90311-c. [DOI] [PubMed] [Google Scholar]
- Cremer Josef, Eggeling Lothar, Sahm Hermann. Control of the Lysine Biosynthesis Sequence in Corynebacterium glutamicum as Analyzed by Overexpression of the Individual Corresponding Genes. Appl Environ Microbiol. 1991 Jun;57(6):1746–1752. doi: 10.1128/aem.57.6.1746-1752.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikmanns B. J., Kircher M., Reinscheid D. J. Discrimination of Corynebacterium glutamicum, Brevibacterium flavum and Brevibacterium lactofermentum by restriction pattern analysis of DNA adjacent to the hom gene. FEMS Microbiol Lett. 1991 Aug 1;66(2):203–207. doi: 10.1016/0378-1097(91)90333-6. [DOI] [PubMed] [Google Scholar]
- Eikmanns B. J., Kleinertz E., Liebl W., Sahm H. A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene. 1991 Jun 15;102(1):93–98. doi: 10.1016/0378-1119(91)90545-m. [DOI] [PubMed] [Google Scholar]
- Eikmanns B. J., Metzger M., Reinscheid D., Kircher M., Sahm H. Amplification of three threonine biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains. Appl Microbiol Biotechnol. 1991 Feb;34(5):617–622. doi: 10.1007/BF00167910. [DOI] [PubMed] [Google Scholar]
- Follettie M. T., Shin H. K., Sinskey A. J. Organization and regulation of the Corynebacterium glutamicum hom-thrB and thrC loci. Mol Microbiol. 1988 Jan;2(1):53–62. doi: 10.1111/j.1365-2958.1988.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Gutmann M., Hoischen C., Krämer R. Carrier-mediated glutamate secretion by Corynebacterium glutamicum under biotin limitation. Biochim Biophys Acta. 1992 Nov 23;1112(1):115–123. doi: 10.1016/0005-2736(92)90261-j. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Murphy G. P., Stephens C. M. Two mechanisms for growth inhibition by elevated transport of sugar phosphates in Escherichia coli. J Gen Microbiol. 1992 Oct;138(10):2007–2014. doi: 10.1099/00221287-138-10-2007. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Labarre J., Reyes O., Guyonvarch A., Leblon G. Gene replacement, integration, and amplification at the gdhA locus of Corynebacterium glutamicum. J Bacteriol. 1993 Feb;175(4):1001–1007. doi: 10.1128/jb.175.4.1001-1007.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl W., Bayerl A., Schein B., Stillner U., Schleifer K. H. High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol Lett. 1989 Dec;53(3):299–303. doi: 10.1016/0378-1097(89)90234-6. [DOI] [PubMed] [Google Scholar]
- Marcel T., Archer J. A., Mengin-Lecreulx D., Sinskey A. J. Nucleotide sequence and organization of the upstream region of the Corynebacterium glutamicum lysA gene. Mol Microbiol. 1990 Nov;4(11):1819–1830. doi: 10.1111/j.1365-2958.1990.tb02030.x. [DOI] [PubMed] [Google Scholar]
- Miyajima R., Otsuka S., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. I. Inhibition by amino acids of the enzymes in threonine biosynthesis. J Biochem. 1968 Feb;63(2):139–148. doi: 10.1093/oxfordjournals.jbchem.a128754. [DOI] [PubMed] [Google Scholar]
- Miyajima R., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. VI. Effects of isoleucine and valine on threonine dehydratase activity and its formation. J Biochem. 1972 Jun;71(6):951–960. doi: 10.1093/oxfordjournals.jbchem.a129866. [DOI] [PubMed] [Google Scholar]
- Mollet B., Knol J., Poolman B., Marciset O., Delley M. Directed genomic integration, gene replacement, and integrative gene expression in Streptococcus thermophilus. J Bacteriol. 1993 Jul;175(14):4315–4324. doi: 10.1128/jb.175.14.4315-4324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Liebl W., Bodis M., Maeng P. J., Follettie M. T., Archer J. A., Sinskey A. J. Nucleotide sequence and fine structural analysis of the Corynebacterium glutamicum hom-thrB operon. Mol Microbiol. 1988 Jan;2(1):63–72. doi: 10.1111/j.1365-2958.1988.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Pátek M., Hochmannová J., Nesvera J. Production of threonine by Brevibacterium flavum containing threonine biosynthesis genes from Escherichia coli. Folia Microbiol (Praha) 1993;38(5):355–359. doi: 10.1007/BF02898754. [DOI] [PubMed] [Google Scholar]
- Reinscheid D. J., Eikmanns B. J., Sahm H. Analysis of a Corynebacterium glutamicum hom gene coding for a feedback-resistant homoserine dehydrogenase. J Bacteriol. 1991 May;173(10):3228–3230. doi: 10.1128/jb.173.10.3228-3230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes O., Guyonvarch A., Bonamy C., Salti V., David F., Leblon G. 'Integron'-bearing vectors: a method suitable for stable chromosomal integration in highly restrictive corynebacteria. Gene. 1991 Oct 30;107(1):61–68. doi: 10.1016/0378-1119(91)90297-o. [DOI] [PubMed] [Google Scholar]
- Schrumpf B., Schwarzer A., Kalinowski J., Pühler A., Eggeling L., Sahm H. A functionally split pathway for lysine synthesis in Corynebacterium glutamicium. J Bacteriol. 1991 Jul;173(14):4510–4516. doi: 10.1128/jb.173.14.4510-4516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer A., Pühler A. Manipulation of Corynebacterium glutamicum by gene disruption and replacement. Biotechnology (N Y) 1991 Jan;9(1):84–87. doi: 10.1038/nbt0191-84. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Kalinowski J., Simon R., Seep-Feldhaus A. H., Pühler A. High-frequency conjugal plasmid transfer from gram-negative Escherichia coli to various gram-positive coryneform bacteria. J Bacteriol. 1990 Mar;172(3):1663–1666. doi: 10.1128/jb.172.3.1663-1666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwold-Davis T. M., Groman N. B., Kao C. C. Localization of an origin of replication in Corynebacterium diphtheriae broad host range plasmid pNG2 that also functions in Escherichia coli. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):119–123. doi: 10.1016/0378-1097(90)90268-u. [DOI] [PubMed] [Google Scholar]
- Shiio I., Miyajima R. Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem. 1969 Jun;65(6):849–859. doi: 10.1093/oxfordjournals.jbchem.a129089. [DOI] [PubMed] [Google Scholar]
- Yeh P., Sicard A. M., Sinskey A. J. General organization of the genes specifically involved in the diaminopimelate-lysine biosynthetic pathway of Corynebacterium glutamicum. Mol Gen Genet. 1988 Apr;212(1):105–111. doi: 10.1007/BF00322451. [DOI] [PubMed] [Google Scholar]
- Young M., Cullum J. A plausible mechanism for large-scale chromosomal DNA amplification in streptomycetes. FEBS Lett. 1987 Feb 9;212(1):10–14. doi: 10.1016/0014-5793(87)81547-8. [DOI] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]




