Abstract
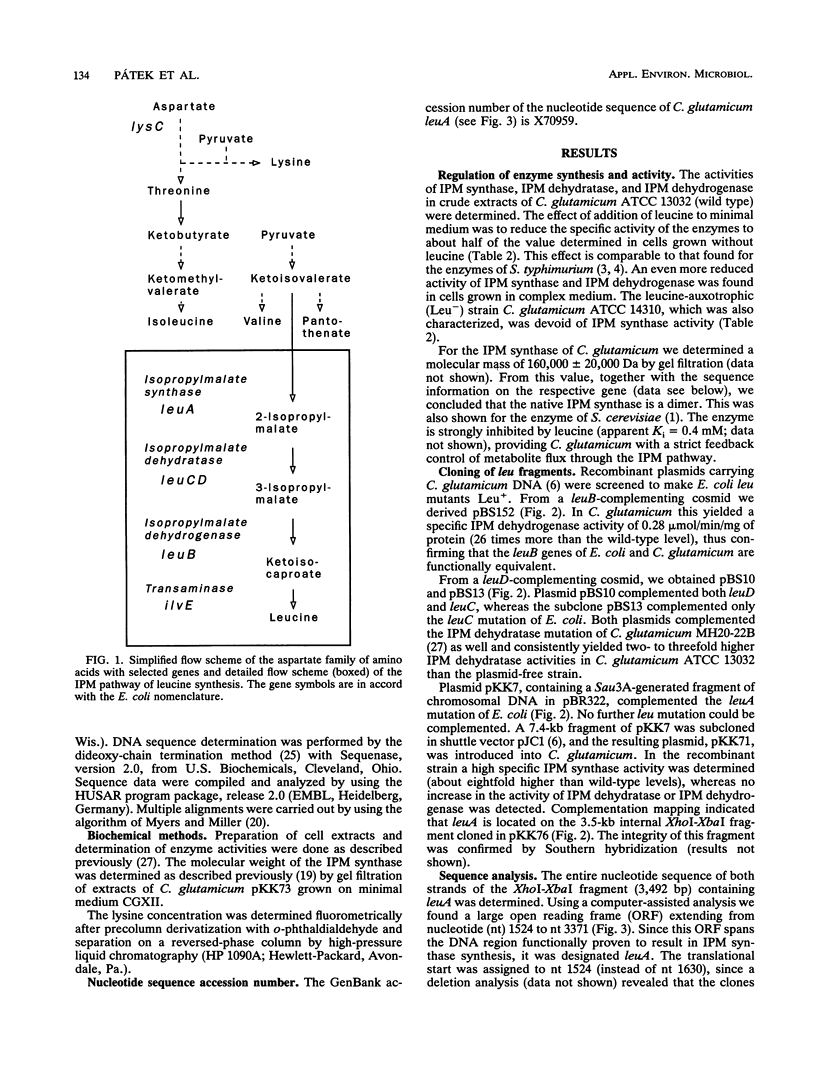
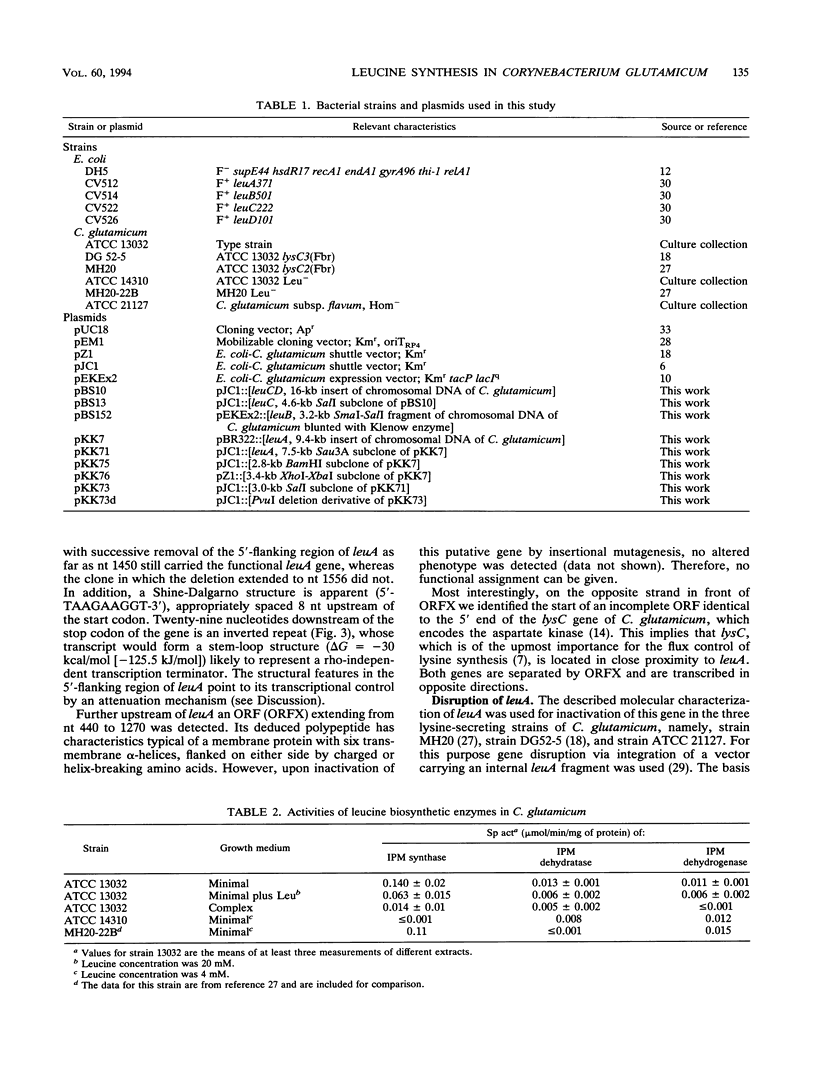
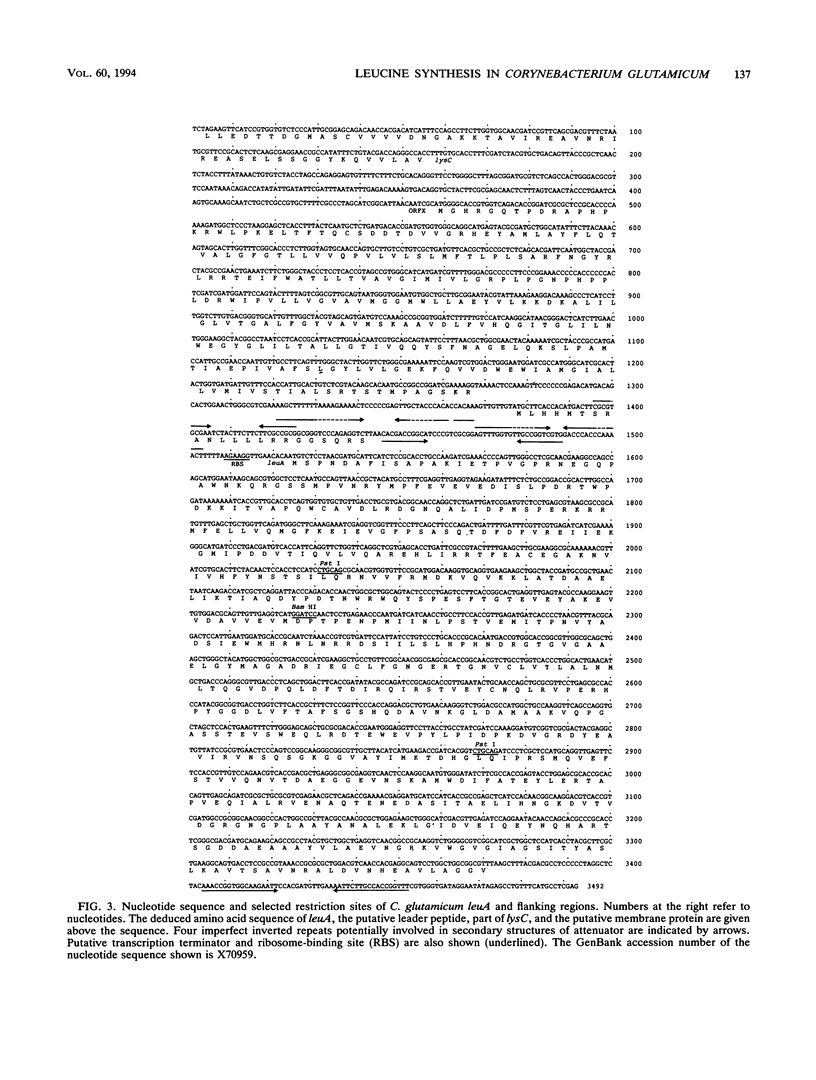
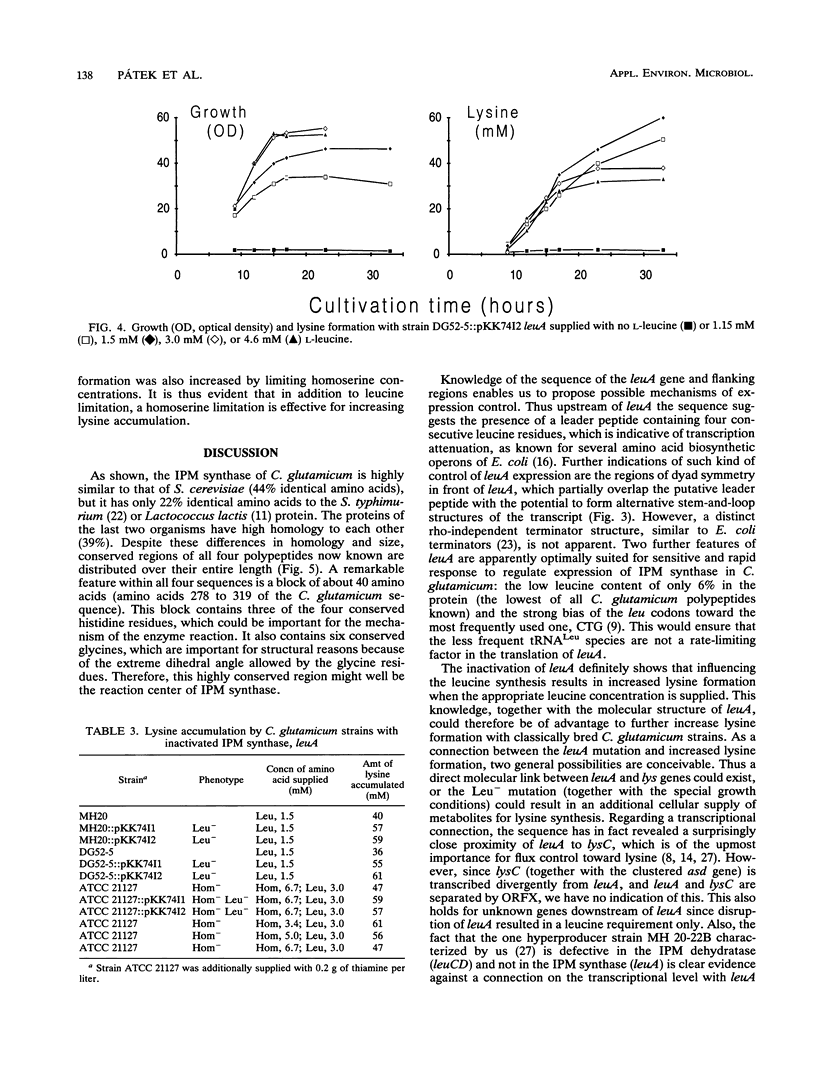
Enzymes and genes of the isopropylmalate pathway leading to leucine in Corynebacterium glutamicum were studied, and assays were performed to unravel their connection to lysine oversynthesis. The first enzyme of the pathway is inhibited by leucine (Ki = 0.4 mM), and all three enzyme activities of the isopropylmalate pathway are reduced upon addition of this amino acid to the growth medium. Three different DNA fragments were cloned, each resulting in an oversynthesis of one of the three enzymes. The leuA complementing fragment encoding the isopropylmalate synthase was sequenced. The leuA gene is 1,848 bp in size, encoding a polypeptide with an M(r) of 68,187. Upstream of leuA there is extensive hyphenated dyad symmetry and a putative leader peptide, which are features characteristic of attenuation control. In addition to leuA, the sequenced fragment contains an open reading frame with high coding probability whose disruption did not result in a detectable phenotype. Furthermore, the sequence revealed that this open reading frame separates leuA from lysC, which encodes the aspartate kinase initiating the synthesis of all amino acids of the aspartate family. The leuA gene was inactivated in three lysine-secreting strains by insertional mutagenesis. Fermentations were performed, and a roughly 50% higher lysine yield was obtained when appropriate leucine concentrations limiting for growth of the constructed strains were used.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltzer J. P., Chang L. F., Hinkkanen A. E., Kohlhaw G. B. Structure of yeast LEU4. The 5' flanking region contains features that predict two modes of control and two productive translation starts. J Biol Chem. 1986 Apr 15;261(11):5160–5167. [PubMed] [Google Scholar]
- Bröer S., Krämer R. Lysine excretion by Corynebacterium glutamicum. 1. Identification of a specific secretion carrier system. Eur J Biochem. 1991 Nov 15;202(1):131–135. doi: 10.1111/j.1432-1033.1991.tb16353.x. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer J., Treptow C., Eggeling L., Sahm H. Regulation of enzymes of lysine biosynthesis in Corynebacterium glutamicum. J Gen Microbiol. 1988 Dec;134(12):3221–3229. doi: 10.1099/00221287-134-12-3221. [DOI] [PubMed] [Google Scholar]
- Cremer Josef, Eggeling Lothar, Sahm Hermann. Control of the Lysine Biosynthesis Sequence in Corynebacterium glutamicum as Analyzed by Overexpression of the Individual Corresponding Genes. Appl Environ Microbiol. 1991 Jun;57(6):1746–1752. doi: 10.1128/aem.57.6.1746-1752.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikmanns B. J. Identification, sequence analysis, and expression of a Corynebacterium glutamicum gene cluster encoding the three glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase, 3-phosphoglycerate kinase, and triosephosphate isomerase. J Bacteriol. 1992 Oct;174(19):6076–6086. doi: 10.1128/jb.174.19.6076-6086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikmanns B. J., Kleinertz E., Liebl W., Sahm H. A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene. 1991 Jun 15;102(1):93–98. doi: 10.1016/0378-1119(91)90545-m. [DOI] [PubMed] [Google Scholar]
- Godon J. J., Chopin M. C., Ehrlich S. D. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6580–6589. doi: 10.1128/jb.174.20.6580-6589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney S. A., Platko J. V., Oxender D. L., Calvo J. M. Lrp, a leucine-responsive protein, regulates branched-chain amino acid transport genes in Escherichia coli. J Bacteriol. 1992 Jan;174(1):108–115. doi: 10.1128/jb.174.1.108-115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski J., Cremer J., Bachmann B., Eggeling L., Sahm H., Pühler A. Genetic and biochemical analysis of the aspartokinase from Corynebacterium glutamicum. Mol Microbiol. 1991 May;5(5):1197–1204. doi: 10.1111/j.1365-2958.1991.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Liebl W., Bayerl A., Schein B., Stillner U., Schleifer K. H. High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol Lett. 1989 Dec;53(3):299–303. doi: 10.1016/0378-1097(89)90234-6. [DOI] [PubMed] [Google Scholar]
- Menkel E., Thierbach G., Eggeling L., Sahm H. Influence of increased aspartate availability on lysine formation by a recombinant strain of Corynebacterium glutamicum and utilization of fumarate. Appl Environ Microbiol. 1989 Mar;55(3):684–688. doi: 10.1128/aem.55.3.684-688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Möckel B., Eggeling L., Sahm H. Functional and structural analyses of threonine dehydratase from Corynebacterium glutamicum. J Bacteriol. 1992 Dec;174(24):8065–8072. doi: 10.1128/jb.174.24.8065-8072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Calvo J. M. The nucleotide sequence of leuA from Salmonella typhimurium. Nucleic Acids Res. 1990 Mar 11;18(5):1290–1290. doi: 10.1093/nar/18.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrumpf B., Schwarzer A., Kalinowski J., Pühler A., Eggeling L., Sahm H. A functionally split pathway for lysine synthesis in Corynebacterium glutamicium. J Bacteriol. 1991 Jul;173(14):4510–4516. doi: 10.1128/jb.173.14.4510-4516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer A., Pühler A. Manipulation of Corynebacterium glutamicum by gene disruption and replacement. Biotechnology (N Y) 1991 Jan;9(1):84–87. doi: 10.1038/nbt0191-84. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Kalinowski J., Simon R., Seep-Feldhaus A. H., Pühler A. High-frequency conjugal plasmid transfer from gram-negative Escherichia coli to various gram-positive coryneform bacteria. J Bacteriol. 1990 Mar;172(3):1663–1666. doi: 10.1128/jb.172.3.1663-1666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. M., Amzallag A., Middleton R. B. Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1268–1272. doi: 10.1128/jb.113.3.1268-1272.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T. S., Doellgast J., Kohlhaw G. B. Mechanism of feedback inhibition by leucine. Purification and properties of a feedback-resistant alpha-isopropylmalate synthase. Arch Biochem Biophys. 1976 Mar;173(1):362–374. doi: 10.1016/0003-9861(76)90271-x. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. Physiological and energetic aspects of bacterial metabolite overproduction. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):169–176. doi: 10.1111/j.1574-6968.1992.tb14036.x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]


