Abstract
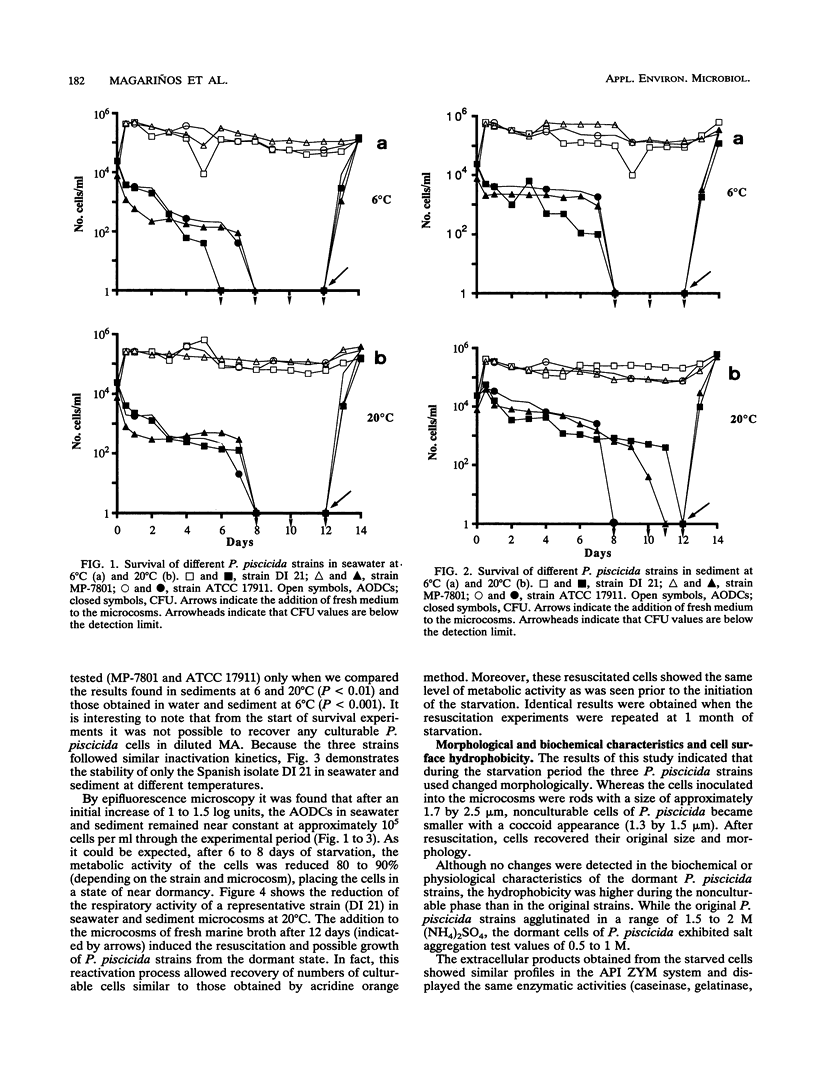
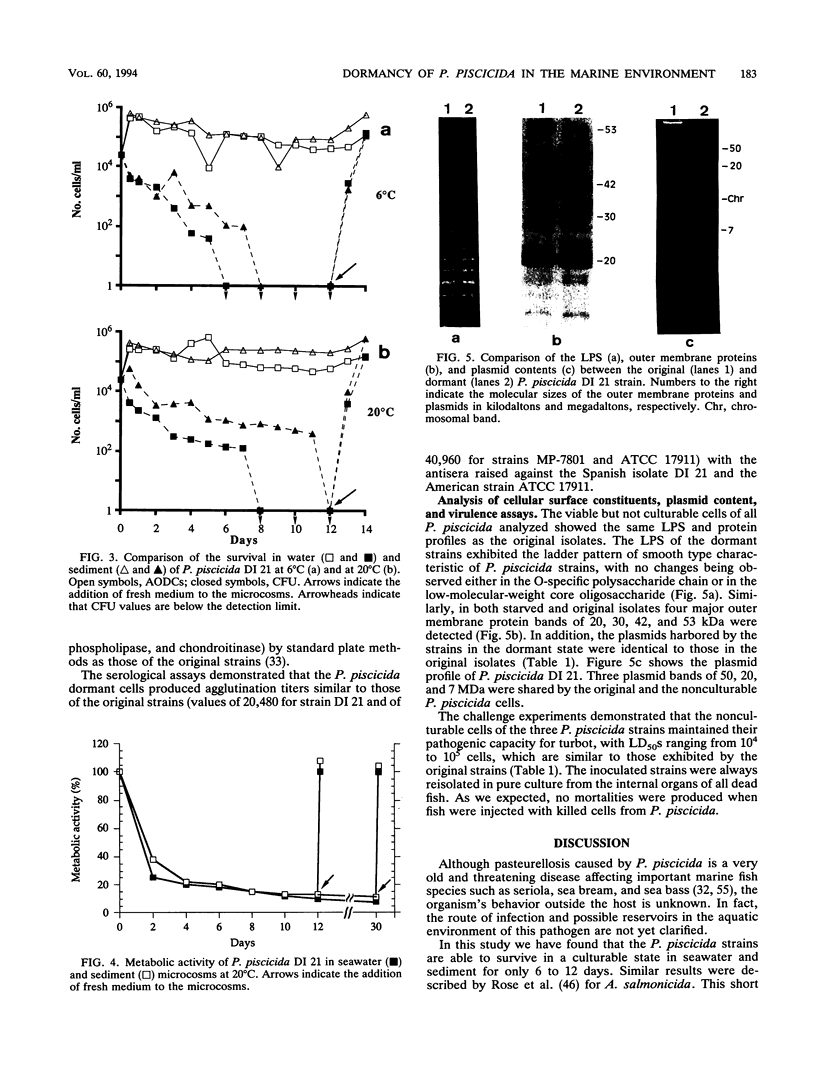
The stability of Pasteurella piscicida strains in seawater and sediment microcosms at different temperatures (6 and 20 degrees C) was investigated during a 1-month period. Three strains of P. piscicida showed similar survival kinetics. By a standard plate count method they survived in water and sediment for only 6 to 12 days, depending on the strain and type of microcosm. During this starvation period, the metabolic activity of the cells was reduced by more than 80%. Culturable cells of each P. piscicida strain persisted better in sediment than in water, as well as at 20 degrees C compared to 6 degrees C. However, in all the microcosms, the acridine orange direct counts remained at about 10(5) cells per ml during the experimental period, which demonstrated that P. piscicida possesses a capacity to enter a viable but not culturable state. Moreover, dormant cells were always resuscitated by the addition of fresh medium to the microcosms, since we recovered numbers of culturable cells similar to the acridine orange direct counts. These resuscitated cells exhibited the same respiration rate as that seen prior to the start of the experiments. Although the biochemical, physiological, and serological characteristics; lipopolysaccharides; membrane proteins; and plasmid content of P. piscicida strains were unaffected during the starvation conditions, the dormant cells were smaller (dwarf cells) and had increased surface hydrophobicity. The starved cells maintained their infectivity and pathogenic potential for fish, with 50% lethal doses similar to those of the original strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcina I., González J. M., Iriberri J., Egea L. Effect of visible light on progressive dormancy of Escherichia coli cells during the survival process in natural fresh water. Appl Environ Microbiol. 1989 Jan;55(1):246–251. doi: 10.1128/aem.55.1.246-251.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcina I., González J. M., Iriberri J., Egea L. Survival strategy of Escherichia coli and Enterococcus faecalis in illuminated fresh and marine systems. J Appl Bacteriol. 1990 Feb;68(2):189–198. doi: 10.1111/j.1365-2672.1990.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L. Outer membrane proteins induced under conditions of iron limitation in the marine fish pathogen Vibrio anguillarum 775. Infect Immun. 1981 Jan;31(1):223–227. doi: 10.1128/iai.31.1.223-227.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopazo C. P., Lemos M. L., Lodeiros C., Bolinches J., Barja J. L., Toranzo A. E. Inhibitory activity of antibiotic-producing marine bacteria against fish pathogens. J Appl Bacteriol. 1988 Aug;65(2):97–101. doi: 10.1111/j.1365-2672.1988.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Flärdh K., Cohen P. S., Kjelleberg S. Ribosomes exist in large excess over the apparent demand for protein synthesis during carbon starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1992 Nov;174(21):6780–6788. doi: 10.1128/jb.174.21.6780-6788.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. J., Atwell R. W., Brayton P. R., Palmer L. M., Rollins D. M., Roszak D. B., Singleton F. L., Tamplin M. L., Colwell R. R. The fate of enteric pathogenic bacteria in estuarine and marine environments. Microbiol Sci. 1986 Nov;3(11):324–329. [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff K. A. Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl Environ Microbiol. 1989 Jul;55(7):1775–1786. doi: 10.1128/aem.55.7.1775-1786.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaan A. J., Dahllöf B., Kjelleberg S. Changes in Protein Composition of Three Bacterial Isolates from Marine Waters during Short Periods of Energy and Nutrient Deprivation. Appl Environ Microbiol. 1986 Dec;52(6):1419–1421. doi: 10.1128/aem.52.6.1419-1421.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen W. A., Surgalla M. J. Morphology, physiology, and serology of a Pasteurella species pathogenic for white perch. (Roccus americanus). J Bacteriol. 1968 Nov;96(5):1606–1610. doi: 10.1128/jb.96.5.1606-1610.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar C. W., Tamplin M. L. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol. 1993 Aug;59(8):2425–2429. doi: 10.1128/aem.59.8.2425-2429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M. Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol. 1984 Sep;48(3):497–503. doi: 10.1128/aem.48.3.497-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen L. K., Martikainen P. J. Survival of Escherichia coli and Campylobacter jejuni in untreated and filtered lake water. J Appl Bacteriol. 1991 Oct;71(4):379–382. doi: 10.1111/j.1365-2672.1991.tb03804.x. [DOI] [PubMed] [Google Scholar]
- Kramer J. G., Singleton F. L. Variations in rRNA content of marine Vibrio spp. during starvation-survival and recovery. Appl Environ Microbiol. 1992 Jan;58(1):201–207. doi: 10.1128/aem.58.1.201-207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños B., Romalde J. L., Bandín I., Fouz B., Toranzo A. E. Phenotypic, antigenic, and molecular characterization of Pasteurella piscicida strains isolated from fish. Appl Environ Microbiol. 1992 Oct;58(10):3316–3322. doi: 10.1128/aem.58.10.3316-3322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños B., Santos Y., Romalde J. L., Rivas C., Barja J. L., Toranzo A. E. Pathogenic activities of live cells and extracellular products of the fish pathogen Pasteurella piscicida. J Gen Microbiol. 1992 Dec;138(12):2491–2498. doi: 10.1099/00221287-138-12-2491. [DOI] [PubMed] [Google Scholar]
- Morgan J. A., Cranwell P. A., Pickup R. W. Survival of Aeromonas salmonicida in lake water. Appl Environ Microbiol. 1991 Jun;57(6):1777–1782. doi: 10.1128/aem.57.6.1777-1782.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Olsson R. M., Kjelleberg S. Survival, stress resistance, and alterations in protein expression in the marine vibrio sp. strain S14 during starvation for different individual nutrients. Appl Environ Microbiol. 1992 Jan;58(1):55–65. doi: 10.1128/aem.58.1.55-65.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Nilsson L., Kjelleberg S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol. 1991 Sep;57(9):2640–2644. doi: 10.1128/aem.57.9.2640-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985 Sep 3;82(1):131–140. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Oliver J. D. Starvation Response of the Marine Barophile CNPT-3. Appl Environ Microbiol. 1992 Aug;58(8):2432–2437. doi: 10.1128/aem.58.8.2432-2437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. S., Ellis A. E., Munro A. L. Evidence against dormancy in the bacterial fish pathogen Aeromonas salmonicida subsp. salmonicida. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):105–107. doi: 10.1016/0378-1097(90)90133-b. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Singh A., McFeters G. A. Survival and virulence of copper- and chlorine-stressed Yersinia enterocolitica in experimentally infected mice. Appl Environ Microbiol. 1987 Aug;53(8):1768–1774. doi: 10.1128/aem.53.8.1768-1774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Yeager R., McFeters G. A. Assessment of in vivo revival, growth, and pathogenicity of Escherichia coli strains after copper- and chlorine-induced injury. Appl Environ Microbiol. 1986 Oct;52(4):832–837. doi: 10.1128/aem.52.4.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita H., Asai T., Hayashi K., Mitsuya T., Amanuma K., Maruyama C., Deguchi Y. Application of ozone disinfection to remove Enterococcus seriolicida, Pasteurella piscicida, and Vibrio anguillarum from seawater. Appl Environ Microbiol. 1992 Dec;58(12):4072–4075. doi: 10.1128/aem.58.12.4072-4075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Potter S. A., Colwell R. R., Hetrick F. M., Crosa J. H. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect Immun. 1983 Mar;39(3):1220–1227. doi: 10.1128/iai.39.3.1220-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]




