Abstract
Gamma-aminobutyric acid (GABA) is the main chemical inhibitory neurotransmitter in the brain. In the central nervous system (CNS) it acts on two distinct types of receptor: an ion channel, i.e., an “ionotropic” receptor permeable to Cl- and HCO3- (GABAA receptors) and a G-protein coupled “metabotropic” receptor that is linked to various effector mechanisms (GABAB receptors). This review will summarize novel developments in the physiology and pharmacology of GABAA receptors (GABAARs), specifically those found outside synapses. The focus will be on a particular combination of GABAAR subunits responsible for mediating tonic inhibition and sensitive to concentrations of ethanol legally considered to be sobriety impairing. Since the same receptors are also a preferred target for the metabolites of steroid hormones synthesized in the brain (neurosteroids), the ethanol-sensitive tonic inhibition may be a common pathway for interactions between the effects of alcohol and those of ovarian and stress-related neurosteroids.
Keywords: GABAARs, ethanol, inhibition
Introduction
Cell-to-cell chemical communication in the body can take three forms, each having various temporal and spatial limitations: 1) the relatively slow but spatially unrestricted neuroendocrine secretion, 2) the much faster volume transmission that reaches neighboring cells by diffusion of transmitter over hundreds of μm in the extracellular space, and 3) the ultra-fast synaptic transmission that requires specialized structures (synapses) between two communicating cell partners separated roughly by 20 nm. For the brain’s principal chemical inhibitory transmitter GABA, fast synaptic transmission has been long thought to be the sole mechanism for communication between cells. More recently, the non-synaptic localization of the metabotropic GABAB receptors and of a certain type of the ionotropic GABAARs has triggered a great interest in the diffusional inhibitory transmission (Semyanov et al., 2004;Farrant and Nusser, 2005;Kullmann et al., 2005;Cavelier et al., 2005;Vizi and Mike, 2006). To distinguish between the activation of GABAARs at synapses and of those on the outside or on the periphery of synapses one refers to phasic and tonic inhibitions to distinguish between the two types of inhibitory activity. The fast and local and slower but distant modes of GABAergic signaling is one of the principal reasons for the diversity of GABAergic action in the brain (Mody, 2001;Mody and Pearce, 2004). Many excellent reviews have been written on tonic inhibition and its role in the control of neuronal excitability (Semyanov et al., 2004;Farrant and Nusser, 2005;Kullmann et al., 2005;Cavelier et al., 2005;Vizi and Mike, 2006), therefore, our review will focus only on some methodological aspects related to its measurement. The main topic of the present review is the modulation of tonic inhibition by ethanol and by various endogenous steroid-hormone derived substances that are highly relevant to everyday life.
The δ subunit containing GABAA receptors
GABAA receptors are members of the superfamily of Cys-loop ligand gated ion channels in which five protein subunits (usually different proteins, and thus the name heteropentameric receptors) co-assemble to form a central aqueous pore through the lipid bilayer of the cell membrane (Sine and Engel, 2006). Ligand binding produces a conformational change in the receptor, and the central ion pore opens to allow the flow of ions. The channels open and close extremely fast until the ligand dissociates from the receptor. Some receptors, in spite of the continuing presence of the ligand, enter a closed conformational state known as the “desensitized” state. Desensitization is a characteristic property of many receptors in this super-family that in addition to the GABAARs include the nicotinic acetylcholine receptors (nAChR), the glycine receptors, and the ionotropic receptors for serotonin (5-HT3).
In the case of the GABAARs the five co-assembled subunits are different proteins. To date 19 different GABAAR subunits have been identified, and these include α1-6, β1-4, γ1-3, δ, ε, θ, and ρ1-2 (Sieghart and Sperk, 2002;Whiting, 2003). Depending on the assembled subunits, GABAARs have specific anatomical localization (Pirker et al., 2000) most likely as a result of various cell- or brain region-specific anchoring and trafficking mechanisms (Moss and Smart, 2001). Moreover, the physiological and pharmacological properties of the GABAARs also depend on their subunit composition (Hevers and Lüddens, 1998;Mody and Pearce, 2004). The random combinations of the 19 subunits taken five-by-five and considering orientation specificity would result in quite a large number of different GABAAR combinations. Yet, the total number of naturally occurring combinations is no more than a few dozen. This is reduction in the degrees of freedom is made possible by limiting the subunit partners that can assemble together, and by imposing strict rules on the number of different subunits of the same class in a given assembly (Sieghart and Sperk, 2002;Whiting, 2003). Thus, the most prevalent combination of GABAARs in the mammalian brain is that made of 2 α1, 2 β2 and 1 γ2 subunit arranged around the central pore in a particular order (the α1β2γ2 subunit combination). Specific GABAARs assembled from different subunit combinations have different developmental, physiological and pharmacological properties, and are also confined to specific compartments on a given cell (Hevers and Lüddens, 1998;Mody and Pearce, 2004). Therefore, these specific GABAARs are of great interest for developing highly specific drug targets for the brain (Whiting, 2003).
The focus of this review is a subclass of GABAARs that contain the δ subunit. The δ subunit was cloned many years ago, and was promptly shown to have a specific expression in the brain, specific pharmacological properties, most importantly lack of benzodiazepine sensitivity, and a mutual exclusion with γ subunits from receptor assemblies (Shivers et al., 1989). The preferred combination partners of δ subunits were the α6 and α4 subunits (from all the α’s) and the β2 and β3 subunits (from all the β’s). The δ subunits in combination with a6 subunits are mainly found in cerebellar granule cells, which constitute the highest density of δ subunits in the brain (Pirker et al., 2000). Outside of the cerebellum, the preferred partners of δ subunits are the α4 subunits. High densities of α4/δ subunit-containing GABAARs are found in the thalamus, striatum, hippocampal dentate gyrus, olfactory bulb, and layers 2/3 of the neocortex. Several studies have confirmed using pharmacological approaches, null mutant mice or both that in the neurons where δ subunits are present, these GABAARs are responsible for the mediation of tonic inhibition (Porcello et al., 2003;Wei et al., 2003;Stell et al., 2003;Jia et al., 2005;Drasbek and Jensen, 2006;Glykys and Mody, 2006a;Glykys et al., 2007).
The first indication about the peculiar subcellular localization of δ subunit containing GABAARs came from studies on cerebellar granule cells. In these neurons, δ subunit containing GABAARs are situated far from the synapses, scattered around the cell surface of the neurons (Nusser et al., 1998). In another area of the brain with high levels of δ subunits, in the granule cells of the dentate gyrus, these receptors are localized somewhat closer to the synapses, but still perisynaptically (Wei et al., 2003). This means that these receptors are ideally located to sense GABA overspilled after a synaptic release process from nearby boutons, or to be activated by the ambient levels of GABA present in the extracellular space. However, to function as receptors capable to detect low concentrations of extrasynaptic GABA, these receptors have to satisfy certain pharmacological criteria. First, they have to have a high affinity for GABA in order to be activated by the low concentration of transmitter in the extracellular space estimated to be in the range of one to a few μM (Kuntz et al., 2004;Nyitrai et al., 2006). Second, they have to be devoid of desensitization, as receptors in the continuing presence of agonist tend to desensitize and spend their time mainly in the closed configuration. Are these pharmacological properties met for the δ subunit containing GABAARs?.
Several studies have shown the high affinity for GABA of the receptors containing δ subunits in combination with either α4 or α6 subunits. Their half maximal activation by GABA (EC50) is in the tens of nM range, well within the range of GABA found in the extracellular space (Saxena and Macdonald, 1994); Wallner et al, 2003). The δ subunit containing GABAARs also have a low degree of desensitization in the continuous presence of agonist (Wohlfarth et al., 2002;Bianchi and Macdonald, 2003). This property is also essential for their role as mediators of a tonic (“always on”) conductance. One of the other interesting pharmacological properties of the δ subunit containing GABAARs is that GABA is not a very efficacious agonist. This means that the coupling between binding of GABA and the opening of the channel is not the most effective one, in spite of the fact that very low concentrations of GABA can open the channels. Other agonists, such as THIP or gaboxadol are more efficacious agonists than GABA itself at these receptors (Brown et al., 2002;Wafford and Ebert, 2006). Thus GABA is a high potency, but low efficacy agonist at the receptors mediating tonic inhibition in many central neurons. This interesting property means that the predominant mechanism for enhancing the function of these receptors may be through increasing the efficacy of GABA as an agonist instead of increasing their already exceptionally high affinity for GABA. This is precisely what neurosteroids appear to be doing to the δ subunit-containing GABAARs (Wohlfarth et al., 2002;Bianchi and Macdonald, 2003). Neurosteroids (also called neuroactive steroids) are metabolites of ovarian steroids such as progesterone and of corticosteroids such as corticosterone (Belelli and Lambert, 2005). They can be synthesized in the brain by specific enzymes present in neurons and glial cells. The most potent positive endogenous modulators of GABAA receptor function are the 3α-hydroxy ring A-reduced pregnane steroids, that have sedative-hypnotic, anticonvulsant, and anxiolytic effects (Majewska et al., 1986;Belelli and Lambert, 2005). Neurosteroids in the nM concentration range, i.e., the range assumed to be present in the extracellular space under various physiological and pathological conditions, selectively enhance the magnitude of tonic inhibition in cells in which this inhibition is mediated by δ subunit-containing GABAARs (Stell et al., 2003). The effect of neurosteroids on synaptic (phasic) inhibition does not occur until the neurosteroid reaches much higher concentrations than it is supposed to reach in the brain (Stell et al., 2003). The high potency of neurosteroids at δ subunit-containing GABAARs is interesting, as neurosteroids bind to α and β subunits (Hosie et al., 2006).
Ethanol sensitivity of GABA responses
The effects of alcohol on the human body and mind have been known for thousands of years. Late Stone Age beer jugs are proof for the existence of intentionally fermented beverages (around 10,000 B.C.), and it is possible that the consumption of beer may have preceded that of bread. Wine appears in Egyptian pictographs around 4,000 B.C. In spite of its long presence in human history, the mechanisms of action of ethanol on the brain are poorly understood. Although drinking and driving laws differ from country to country; in California the legal upper limit for blood alcohol level for operating a motor vehicle is 0.08%. This corresponds to approximately 14 mmol/l of ethanol in the blood. Commercial drivers are limited to a maximum of 0.04%, i.e., around 7 mmol/l of ethanol in the blood. Because of various factors such as age, gender, weight, metabolic rate, etc., not every individual becomes impaired to the same extent by these particular ethanol concentrations, nevertheless, at least legally, these ethanol concentrations are considered to be the impairing the operation of motor vehicles. According to the California Alcohol and Drug Programs (www.adp.ca.gov) the average BAC level of a convicted DUI offender in 2003, as reported by law enforcement on APS forms, was .161% (28 mM). Thus, for the purpose of this review we will consider ethanol concentrations in the range of 10-30 mM to be “sobriety-impairing”. Over the past 30 years several studies (Davidoff, 1973;Nestoros, 1980;Mereu and Gessa, 1985;Suzdak et al., 1986;Siggins et al., 1987;Celentano et al., 1988;Ticku, 1989;Aguayo, 1990;Akk and Steinbach, 2003;Kumar et al., 2004) have examined the effects of “low”, i.e., sobriety-impairing ethanol concentrations on various responses to GABAAR activation in neurons, but no consensus has been reached, and no specific GABAAR subunit combination could be identified as a specific ethanol target.
Recently however, δ subunit-containing GABAARs expressed in Xenopus laevis oocytes have been shown to be uniquely sensitive to low ethanol concentrations (Sundstrom-Poromaa et al., 2002;Wallner et al., 2003). These findings have not remained unchallenged. Recent experiments in various expression systems including oocytes, the stable L(tk-) cell linescell line expressing δ subunit-containing GABAARs (Borghese et al., 2005) and CHO cells (Yamashita et al., 2006) ethanol failed to enhance currents evoked by GABA, but the reason for the discrepancies between the results in various expression systems remains unknown. The reader should consult recent review articles summarizing the findings in expression systems (Hanchar et al., 2004;Wallner et al., 2006), in genetically altered mice (Boehm et al., 2006), and in brain slice preparations (Weiner and Valenzuela, 2006) from which it is clear that certain GABAAR combinations, including those containing δ subunits, may be responsible for specific actions of ethanol on the brain. In addition to the role of δ subunits, point mutations in the α1 subunit have also been shown to affect ethanol sensitivity, but only at higher (80 mM) ethanol concentrations (Werner et al., 2006). Moreover, enhancement of GABA release through presynaptic mechanisms has also been reported (Weiner and Valenzuela, 2006).
Ethanol sensitivity of tonic inhibition in neurons
The measurement of tonic inhibition
The term “tonic” in the case of inhibition is not meant to designate the carbonated beverage flavored with a small amount of quinine. By analogy to the tonic muscle contraction, tonic inhibition implies that the underlying conductance is “always-on”. Therefore, the presence of such a persistent conductance should be easily demonstrated if it generates a detectable amount of charge movement across the membrane by the use of a receptor antagonist. Indeed, the use of high resolution whole-cell recordings in neurons of brain slices and cell cultures allowed the recordings of persistent currents that could be blocked by the administration of GABAAR antagonists (Otis et al., 1991;Kaneda et al., 1995;Salin and Prince, 1996;Brickley et al., 1996;Bai et al., 2001). The difference in the holding current between that recorded in the presence of the antagonist and that before antagonist administration is commonly referred to as the tonic current. New methods have been developed based on all-points-histograms that allow the simultaneous measurement of tonic and phasic conductances at desired time intervals (Glykys and Mody, 2006b), but the essential approach of using saturating antagonist concentrations to block both tonic and phasic conductances has remained the same. It is important to emphasize that saturating antagonist concentrations should be used to block 100% of both tonic and phasic inhibitions, because the relative affinities for GABA and for the antagonist of the different receptors mediating the two types of inhibition may differentially affect the two types of inhibitory activity (Stell and Mody, 2002).
Unfortunately the different recording preparations and practices used by various laboratories make it difficult to come up with a consensus for the optimal recording conditions for tonic inhibition. As tonic inhibition is activated by the GABA levels present in the extracellular space, any factor that will control the presence of GABA in the extracellular space will have an impact on the magnitude of tonic inhibition. These include the activity of GABA uptake mechanisms, the level of spontaneous activity of GABAergic neurons, possible leakage of GABA from neurons or glial cells, the rate of perfusion of the slices, oxygenation levels, etc. It is also possible to record tonic current in the total absence of agonist-dependent activation of GABAARs, when just the spontaneous openings of the channels is responsible for a steady conductance (Birnir et al., 2000;McCartney et al., 2006). Interestingly the spontaneously opening receptors can be blocked by bicuculline but not gabazine (McCartney et al., 2006) thus introducing an added cautionary note when using various antagonists to reveal the magnitude of tonic inhibition (Bai et al., 2001).
Ethanol sensitivity of the tonic inhibition mediated by δ subunit-containing GABAARs
Tonic inhibition is present in various neurons in diverse brain regions. Thus far, various approaches including pharmacological, and genetic knockout techniques identified several GABAAR assemblies responsible for mediating tonic inhibition in the brain. Tonic inhibition can be mediated by GABAARs containing δ subunits in combination with α6 subunits in cerebellar granule cells (Brickley et al., 2001;Stell et al., 2003), by partnering with α4 subunits in dentate gyrus granule cells (Stell et al., 2003;Chandra et al., 2006;Glykys et al., 2007), thalamic neurons (Porcello et al., 2003;Cope et al., 2005;Jia et al., 2005;Chandra et al., 2006), layer 2/3 cortical pyramidal cells (Drasbek and Jensen, 2006), and by pairing up with α1 subunits in interneurons of the dentate gyrus (Glykys et al., 2007). Other GABAAR combinations without δ subunits known to mediate tonic inhibition in hippocampal pyramidal cells are those containing α5 subunits (Caraiscos et al., 2004;Glykys and Mody, 2006a) or simply αβ subunit combinations (Mortensen and Smart, 2006).
Only the tonic inhibition mediated by δ subunit-containing GABAARs has been shown to be sensitive to ethanol in the range of 20-30 mM (Wei et al., 2004;Hanchar et al., 2005;Liang et al., 2006;Glykys et al., 2007). Figure 1 shows the sensitivity of the tonic current to 30 mM ethanol in VB thalamic neurons, where this type of inhibition is also mediated by δ subunit-containing GABAARs (Porcello et al., 2003;Cope et al., 2005;Jia et al., 2005;Chandra et al., 2006). One report found no effect of 30 mM ethanol on the tonic current recorded in mouse dentate gyrus granule cells (Borghese et al., 2005). It has to be noted that in contrast to our recording conditions (Wei et al., 2004;Glykys et al., 2007) these authors used younger animals (P20-26, C57Bl/6 × 129SvJae mixed genetic background mice) and recorded a large tonic inhibitory current (mean = 19 pA) in the presence of TTX (Borghese et al., 2005), when this activity is significantly reduced by blocking action potentials in slices (Kaneda et al., 1995;Brickley et al., 1996;Glykys and Mody, 2006b). It is also curious that the size (mean = 72 pA) and the decay time constant (mean = 9.7 ms) of the mIPSCs recorded in the study (Borghese et al., 2005) are significantly larger than those reported by other investigators. It is also interesting to note that the tonic current recorded in cerebellar granule cells in culture does not respond to sobriety impairing ethanol concentrations (Yamashita et al., 2006). The reason for the discrepancies remain to be determined, but it should be kept in mind that there are several factors that can directly influence the magnitude of tonic inhibition and ethanol may act on any of these to enhance it.
Figure 1. Tonic inhibitory currents recorded from medium spiny thalamic neurons show potentiation by ethanol.
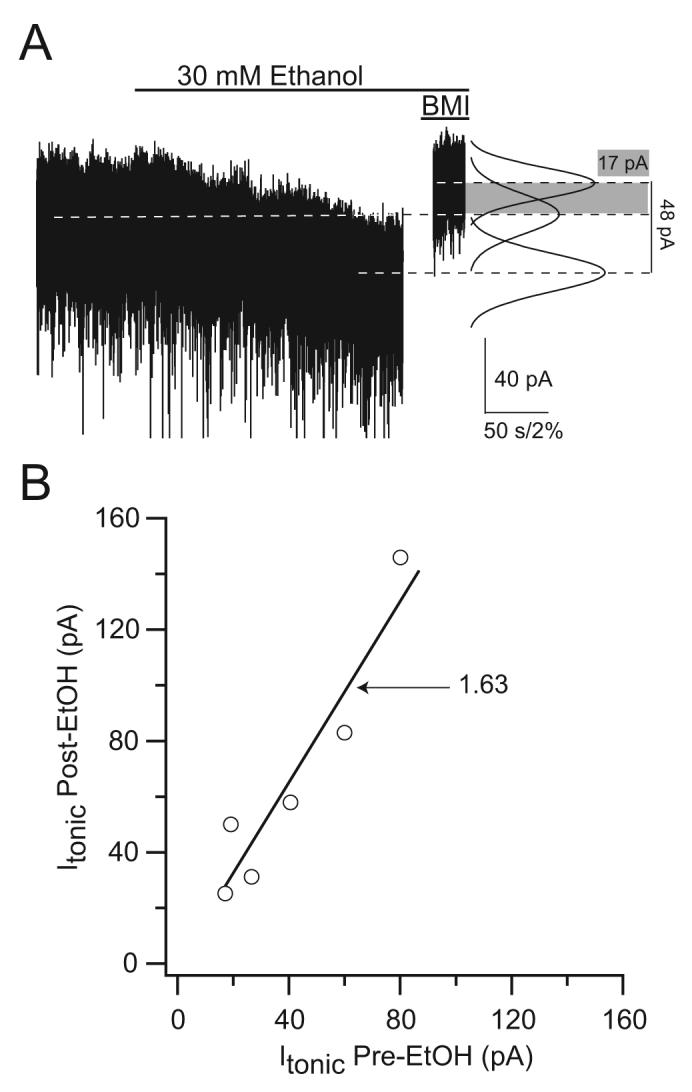
A) Voltage-clamp recording form a VB thalamic neuron (Vh, -70 mV). Tonic inhibitory current was enhanced by 30 mM ethanol (open bar). The magnitude of tonic inhibition was measured after the application of the GABAAR antagonist BMI (100 μM). The dashed lines represent the mean holding current during the three conditions. The average current increased from 40.5 ± 10.2 pA to 65.5 ± 18.1 pA (n=6). B) Scatter plot of the tonic inhibitory currents before plotted against those after perfusion of 30 mM EtOH (Linear fit, slope 1.63 is significantly different p<0.001 from s line with a slope of 1.0 that would indicate no effect, R=0.95).
One of the most important regulators of tonic inhibition is the uptake of GABA (Nusser and Mody, 2002;Semyanov et al., 2003;Jensen et al., 2003;Wu et al., 2006). It is therefore conceivable that ethanol could alter tonic inhibition by decreasing the activity of the GABA transporter, thus elevating the levels of extracellular GABA. Figure 2 shows an experiment in a dentate gyrus molecular layer interneuron that excludes this possibility. Ethanol enhanced the tonic current recorded in these neurons both in the absence and presence of NO711, a potent blocker of GABA uptake (Dalby, 2000). It is interesting to note that the potentiation by ethanol was similar regardless of the magnitude of the tonic current, which becomes quite large in the presence of the uptake blocker (Fig. 2). This is in contrast to the effects of benzodiazepines on tonic currents mediated by δ subunit-containing GABAARs, which contrary to a well established benzodiazepine-insensitive pharmacology, become potentiated by benzodiazepines when activated only by low GABA concentrations (Santhakumar et al., 2006). A point mutation in position 100 (Q/R) of the α6 subunit markedly affects benzodiazepine and ethanol sensitivities (Hanchar et al., 2005;Santhakumar et al., 2006). Therefore, we also wanted to know whether the H101R mutation that abolishes benzodiazepine sensitivity in α1 subunit-containing GABAARs (Wieland et al., 1992) results in a change of their modulation by ethanol. In α1H101R knock-in mice (Rudolph et al., 1999), a His residue in position 101 in α1 subunits is changed to an Arg as in α4 (Fig. 3), thus abolishing benzodiazepine sensitivity. Recordings in molecular layer interneurons, where the tonic current is mediated by α1β×δ GABAAR combinations (Glykys et al., 2007), in knock-in mice with this amino acid substitution showed a tonic inhibition similar to that in WT, and a comparable potentiation by 30 mM ethanol (CON 16.9 ± 1.9 vs. EtOH 26.9 ± 2.8 pA; p=0.0005 paired t-test; n=14; Fig. 3). The potentiation ratio was not different from WT (WT 2.05 ± 0.3 vs. α1H101R 1.74 ± 0.2; p=0.332 unpaired t-test assuming unequal variances; n=10 and 14 respectively) suggesting that the α1H101R mutation that renders α1 subunits at a residue critical for benzodiazepine sensitivity to be more like α4 subunits, is not a factor for the potentiating effects of ethanol.
Figure 2. Tonic inhibition enhancement by ethanol is present when GABA is added to the aCSF as well as when GABA transporters are blocked.
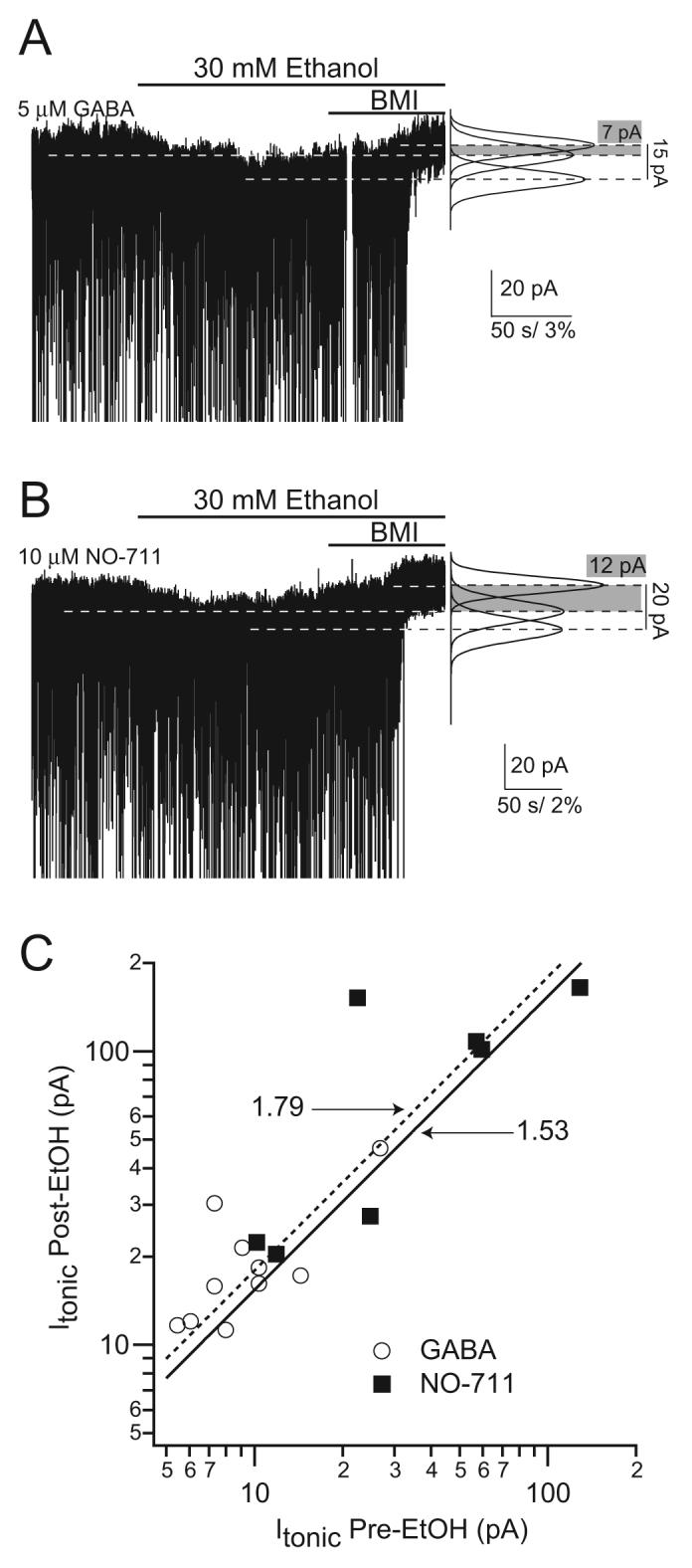
A) Voltage-clamp recordings from dentate gyrus molecular layer interneurons showing the ethanol-induced potentiation of the tonic inhibitory current in the presence of 5 μM GABA. (Vh = -70 mV, horizontal dashed lines indicate perfusion of ethanol and BMI); Right, Gaussian fits to the all-point histograms of the baseline current during each condition. B) Same as above but in the presence of 10 μM NO-711. C) Scatter plot of the tonic inhibitory currents before plotted against those after perfusion of 30 mM EtOH (Linear fit: GABA, (dashed line) slope 1.79 R=0.81; NO-711 (solid line) slope 1.53, R=0.72; both slopes are significantly different p<0.001 from line with a slope of 1.0 that would indicate no effect of ethanol). Note the logarithmic scale that was necessary to use for the very large tonic inhibitory currents in the presence of NO-711.
Figure 3. Tonic inhibition and its potentiation by ethanol are unaltered in α1H101R dentate gyrus molecular layer interneurons.
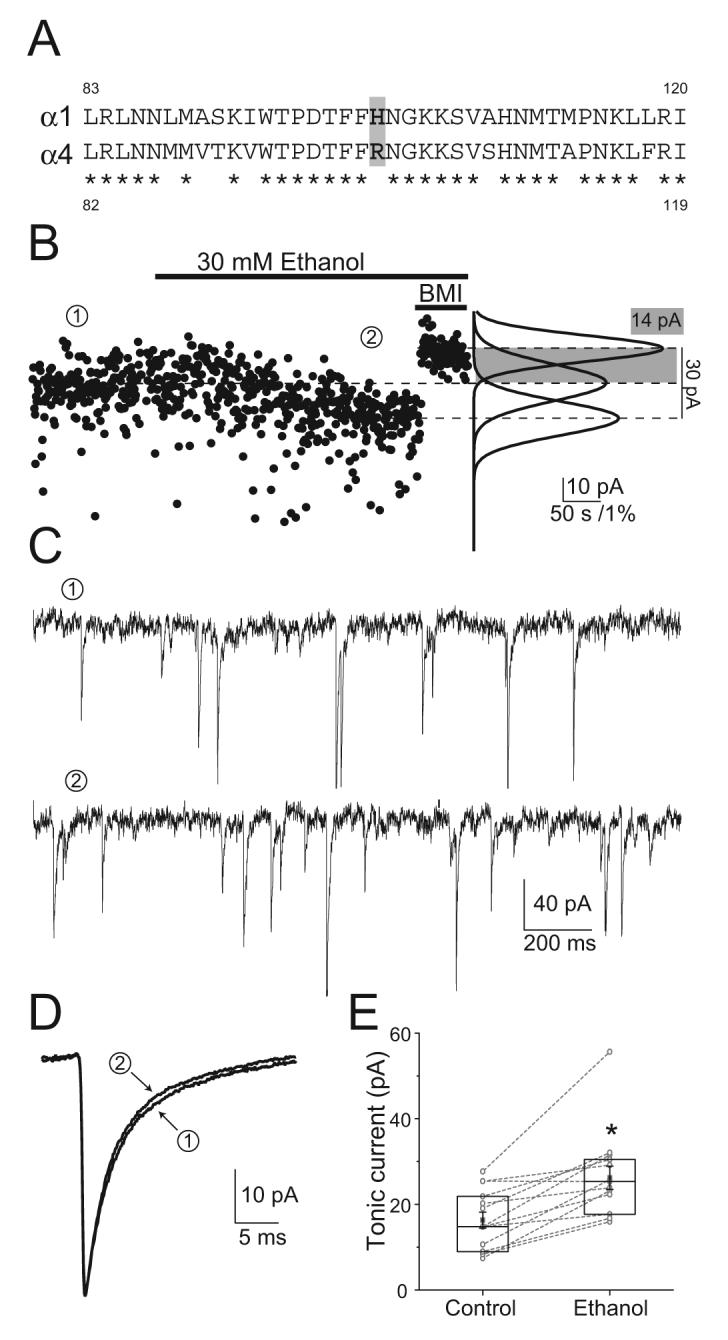
A) Amino acid alignment between α1 and α4 GABAAR subunits showing the position of the critical amino acid residue difference for BZ sensitivity. Asterisks indicate identical amino acids. B) Voltage-clamp recordings from α1H101R interneurons show a potentiation of the tonic inhibitory current by 30 mM ethanol. Left, baseline current plotted at 500-ms intervals in control (①), 30 mM ethanol (②) and BMI conditions (Vh= -70 mV, horizontal bars indicate perfusion of the drugs); Right, Gaussian fits to the all-point histograms of the baseline current recorded during each condition. C) Recording segments low-pass filtered at 1 KHz in control and ethanol conditions. D) Averaged sIPSC recorded in control and ethanol conditions from two different cells. E) Box-chart of all tonic currents recorded. Box represents the 25, 50, 75 percentiles, with the superimposed mean ± SEM. Circles connected by lines represent paired individual values of the tonic inhibitory currents recorded in a given cell under the two conditions. Asterisk represents p=0.0005 paired t-test; n=14.
From the data presented above, it is evident that tonic inhibition mediated by δ subunit-containing GABAARs is an important target for sobriety-impairing ethanol concentrations in the brain. It remains to be determined whether various intra- or extracellular factors modulate the action of ethanol on this type of tonic inhibition. Furthermore, it is presently unclear if the receptors underlying tonic inhibition also play a role in alcohol addiction and tolerance.
Tonic inhibition as a common target for ethanol and neurosteroids
We recently demonstrated dynamic, ovarian cycle-linked modifications in specific GABAAR expression and function (Maguire et al., 2005). During the stage of the estrous cycle in mice when levels of progesterone and of progesterone-derivatives locally synthesized in the brain (neurosteroids) are elevated, there is an increased expression of the GABAAR δ subunits in the membranes of hippocampal neurons and an increase in GABAAR δ subunit-mediated tonic inhibition in dentate gyrus granule cells. This increase in GABAAR δ subunits corresponds to a period of lowered seizure susceptibility and anxiety (Maguire et al., 2005). Other investigators have shown the same receptors to parallel ovarian cycle related changes in the periaqueductal grey matter (Griffiths and Lovick, 2005;Lovick et al., 2005;Lovick, 2006) or to be upregulated in a steroid withdrawal model of pre-menstrual dysphoric disorder (PMDD) (Smith et al., 2006). The changes in the GABAAR δ subunits during the ovarian cycle and the associated alterations in neuronal excitability and anxiety may be highly relevant to the common psychiatric and neurological disorders such as PMDD, its milder form PMS (pre-menstrual syndrome), and postpartum depression that affect women during fluctuating changes in ovarian steroid levels as take place during the menstrual cycle and pregnancy.
Consistent with the common involvement of the δ subunit-containing GABAARs in ovarian-cycle related anxiety and in mediating the effects of ethanol, women with PMS have increase their alcohol consumption during the luteal phase (Charette et al., 1990;Tobin et al., 1994;McLeod et al., 1994;Perry et al., 2004), which may be an indication of self-medication since, as shown above, the function of δ subunit-containing GABAARs and the tonic inhibition mediated by these receptors are enhanced by sobriety-impairing concentrations of ethanol.
A recent report highlighted that effect of the neurosteroid allopregnanolone on prolonging the long open-times of single α1β2γ2L GABAAR channels expressed in HEK 293 cells activated by 50 μM GABA was shifted to 100-fold lower neurosteroid concentrations in the presence of 17 mM ethanol (Akk et al., 2007). However, this effect was not evident in the whole-cell currents evoked by GABA, possibly because of the relatively small contribution of the long openings to the total current. Nevertheless, these findings illustrate that the δ subunit-containing GABAARs may not be the only receptor subtype to mediate interactions between neurosteroid and ethanol effects.
Summary
The δ subunit-containing GABAARs and consequently the tonic inhibition mediated by these receptors appears to be an important target for sobriety-impairing concentrations of ethanol. Stress- and ovarian steroid-derived neurosteroids also appear to act primarily on this type of inhibition, thus putting tonic inhibition mediated by δ subunit-containing GABAARs in the crosshairs for finding effective therapies against a large number of psychiatric and neurological disorders related to alcoholism, stress, ovarian cycle, and pregnancy.
Acknowledgements
This work was supported by NIH Grants NS30549 and the Coelho Endowment to I.M. J.G. was also supported by a fellowship from the Epilepsy Foundation of America and the Gonda Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl- current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- Akk G, Li P, Manion BD, Evers AS, Steinbach JH. Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the alpha1beta2gamma2L GABAA receptor. Mol Pharmacol. 2007;71:461–472. doi: 10.1124/mol.106.029942. [DOI] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Low doses of ethanol and a neuroactive steroid positively interact to modulate rat GABA(A) receptor function. J Physiol. 2003;546:641–646. doi: 10.1113/jphysiol.2002.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnir B, Everitt AB, Lim MS, Gage PW. Spontaneously opening GABA(A) channels in CA1 pyramidal neurones of rat hippocampus. J Membr Biol. 2000;174:21–29. doi: 10.1007/s002320001028. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. Adv Pharmacol. 2006;54:171–203. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Storustovu SI, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of {gamma} -aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2005 doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage- independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You T, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol. 2005;87:3–16. doi: 10.1016/j.pbiomolbio.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano JJ, Gibbs TT, Farb DH. Ethanol potentiates. Brain Res. 1988;455:377–380. doi: 10.1016/0006-8993(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette L, Tate DL, Wilson A. Alcohol consumption and menstrual distress in women at higher and lower risk for alcoholism. Alcohol Clin Exp Res. 1990;14:152–157. doi: 10.1111/j.1530-0277.1990.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO. GABA-level increasing and anticonvulsant effects of three different GABA uptake inhibitors. Neuropharmacology. 2000;39:2399–2407. doi: 10.1016/s0028-3908(00)00075-7. [DOI] [PubMed] [Google Scholar]
- Davidoff RA. Alcohol and presynaptic inhibition in an isolated spinal cord preparation. Arch Neurol. 1973;28:60–63. doi: 10.1001/archneur.1973.00490190078011. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice. J Neurophysiol. 2006a;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I.2006b. The source of GABA mediating tonic inhibition: A new measurement method to examine correlations between tonic and phasic inhibitory activity
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Hevers W, Lüddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA Transporter-1 (GAT1) Deficient Mice: Differential Tonic Activation of GABAA versus GABAB Receptors in the Hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol (Lond) 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kuntz A, Clement HW, Lehnert W, van CD, Hennighausen K, Gerlach M, Schulz E. Effects of secretin on extracellular amino acid concentrations in rat hippocampus. J Neural Transm. 2004;111:931–939. doi: 10.1007/s00702-003-0082-y. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol. 2006;91:655–660. doi: 10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol. 2006 doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Foster GV, Hoehn-Saric R, Svikis DS, Hipsley PA. Family history of alcoholism in women with generalized anxiety disorder who have premenstrual syndrome: patient reports of premenstrual alcohol consumption and symptoms of anxiety. Alcohol Clin Exp Res. 1994;18:664–670. doi: 10.1111/j.1530-0277.1994.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Mereu G, Gessa GL. Low doses of ethanol inhibit the firing of neurons in the substantia nigra, pars reticulata: a GABAergic effect? Brain Res. 1985;360:325–330. doi: 10.1016/0006-8993(85)91249-1. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic {alpha}{beta} subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Nestoros JN. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science. 1980;209:708–710. doi: 10.1126/science.7394531. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyitrai G, Kekesi KA, Juhasz G. Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr Top Med Chem. 2006;6:935–940. doi: 10.2174/156802606777323674. [DOI] [PubMed] [Google Scholar]
- Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Perry BL, Miles D, Burruss K, Svikis DS. Premenstrual symptomatology and alcohol consumption in college women. J Stud Alcohol. 2004;65:464–468. doi: 10.15288/jsa.2004.65.464. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J Neurophysiol. 1996;75:1573–1588. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAA receptor alpha6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the alpha6 gene. J Neurosci. 2006;26:3357–3364. doi: 10.1523/JNEUROSCI.4799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: Role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA(A) receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Pittman QJ, French ED. Effects of ethanol on CA1 and CA3 pyramidal cells in the hippocampal slice preparation: an intracellular study. Brain Res. 1987;414:22–34. doi: 10.1016/0006-8993(87)91323-0. [DOI] [PubMed] [Google Scholar]
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology (Berl) 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci U S A. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK. Ethanol and the benzodiazepine-GABA receptor-ionophore complex. Experientia. 1989;45:413–418. doi: 10.1007/BF01952022. [DOI] [PubMed] [Google Scholar]
- Tobin MB, Schmidt PJ, Rubinow DR. Reported alcohol use in women with premenstrual syndrome. Am J Psychiatry. 1994;151:1503–1504. doi: 10.1176/ajp.151.10.1503. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Mike A. Nonsynaptic receptors for GABA and glutamate. Curr Top Med Chem. 2006;6:941–948. doi: 10.2174/156802606777323782. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol--a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ GABAA receptors at low concentrations known to have effects in humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Werner DF, Blednov YA, Ariwodola OJ, Silberman Y, Logan E, Berry RB, Borghese CM, Matthews DB, Weiner JL, Harrison NL, Harris RA, Homanics GE. Knockin mice with ethanol-insensitive alpha1-containing gamma-aminobutyric acid type A receptors display selective alterations in behavioral responses to ethanol. J Pharmacol Exp Ther. 2006;319:219–227. doi: 10.1124/jpet.106.106161. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol. 2006;96:2425–2436. doi: 10.1152/jn.00545.2006. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J Pharmacol Exp Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]


