Abstract
Follicle stimulating hormone (FSH) is secreted from the pituitary gland to regulate reproduction in vertebrates. FSH signals through a G-protein coupled receptor (FSHR) on the target cell surface. We describe here the strategy to produce a soluble FSH-FSHR complex that involves the co-secretion of a truncated FSHR ectodomain (FSHRHB) and a covalently-linked FSHαβ heterodimer from baculovirus-infected insect cells. FSH binds to FSHRHB with a high affinity comparable to that for the full-length receptor. The crystal structure of the FSH-FSHRHB complex provides explanations for the high affinity and specificity of FSH interaction with FSHR, and it shows an unexpected dimerization of these complexes. Here we also compare the crystal structure with theoretical models of the FSH-FSHR binding mode. We conclude that the FSH-FSHRHB structure gives an authentic representation of FSH binding to intact FSHR.
Keywords: Glycoprotein hormones, Gonadotropin receptors, Insect cell expression, Crystal structure
1. Introduction
Follicle stimulating hormone (FSH) is essential for the reproduction of vertebrates. Mutations in the hormone and its receptor cause failure to develop ovarian follicles in females as well as impaired spermatogenesis in males, both of which could lead to infertility (Simoni et al., 1997; Themmen and Huhtaniemi, 2000). Together with the luteinizing hormone (LH), chorionic gonadotropin (CG) and thyroid stimulating hormone (TSH), FSH belongs to the family of glycoprotein hormones (Pierce and Parsons, 1981). These hormones are heterodimeric molecules comprising α- and β-subunits (Pierce and Parsons, 1981). The glycoprotein hormones exert their actions on target cells by binding to specific G-protein coupled receptors (GPCR), which are characterized by seven transmembrane helices (Dias et al., 2002; Ascoli et al., 2002; Szkudlinski et al., 2002). Unlike most GPCRs, glycoprotein hormone receptors have large extracellular domains containing leucine-rich repeats (LRRs) (Dias et al., 2002; Ascoli et al., 2002; Szkudlinski et al., 2002). Interaction between a glycoprotein hormone and the ectodomain of its receptor leads to activation of the transmembrane domain for signal transduction (Dias et al., 2002; Ascoli et al., 2002; Szkudlinski et al., 2002). Although the crystal structures of human CG (Wu et al., 1994; Lapthorn et al., 1994) and FSH (Fox et al., 2001) have both been solved previously, structural information for any of the glycoprotein hormone receptors has until now defied experimental analysis.
We have assembled a complex of human FSH and the extracellular hormone-binding domain of its receptor (FSHRHB) by co-expressing the hormone and receptor. The stable complex formed by FSH and FSHRHB reflects the high affinity and specificity of the interactions between FSH and FSHR on cell surfaces. The structure of the FSH-FSHRHB complex has been determined at 2.9 Å resolution, which provides a molecular understanding of the receptor-hormone interface and a suggested mode of dimerization (Fan and Hendrickson, 2005a). Here we also compare the crystal structure with theoretical models of glycoprotein hormone-receptor binding.
2. Methods
2.1. Construction of transfer vectors for expression in insect cells
The entire FSHβ chain including its signal sequence was amplified from its cDNA clone using the forward primer 5’-TAGGGCGAATTCACCAGGATGAAGACA CTCCAGTTTTTCTTCCTTTTCTGTTGC-3’ and the reverse primer 5’-GCTAGAGGA TCCACCACCAGAACCACCACCTTCTTTCATTTCACCAAAGGAGCAGTAGCT GGG-3’. The sequence encoding the mature protein of FSHα was amplified with the forward primer 5’-GCTAGAGGATCCGGTGGTGGTTCTGGTGGTGGTGCTCCT GATGTGCAGGATTGCCCAGAATGC-3’ and the reverse primer 5’-CCTAGGGCG GCCGCTTATTAAGATTTGTGATAATAACAAGTACTGCAGTG-3’. The PCR product containing the FSHβ sequence was digested with EcoRI and BamHI, and the PCR product of mature FSHα sequence was digested with BamHI and NotI. These digested PCR fragments were ligated together into an EcoRI-NotI digested pFastBac Dual plasmid. This joining thus encoded a single-chain FSH whereby the C-terminus of FSHβ is connected to the N-terminus of FSHα by a 15-residue Gly-Ser rich linker dictated by the primer sequences.
FSHRHB (residues 1–268) was amplified from its cDNA clone using the forward primer 5’-GATCCGAGCTCGAGATAATTATGGCCCTGCTCCTGGTCTCTTTGCTG GCA-3’ and the reverse primer 5’-GAGCGGGGTACCTTATTACTTATCATCATC ATCCTTGTAATCGCTGGCTTCCATGAGGGCGACAAGCTTTTCCAG-3’. A FLAG tag was engineered at the C-terminus of the FSHR through the reverse primer. Receptor constructs with alternative C-terminal truncations of the FSHR ectodomain were amplified similarly. In each case, the PCR product was digested with XhoI and KpnI and then ligated into the pFastBac Dual plasmid which already contained the single-chain FSH. The genes for single-chain FSH and for FSHRHB were placed under the control of the polyhedrin and p10 promoters, respectively. The sequences of both the receptor and hormone constructs were verified by DNA sequencing.
2.2. Insect cell culture and protein purification
Trichoplusia ni (High Five) cells were cultured in monolayer or suspension at 27°C in Grace medium (Life Technologies, Inc.) supplemented with 10% fetal bovine serum (Life Technologies, Inc.). The cells were adapted into serum-free medium (SF900II SFM from Life Technologies, Inc.) for protein expression.
Recombinant baculoviruses encoding both single-chain FSH and truncated ectodomain constructs of FSHR were produced in High Five cells using bacmid DNA transposed with the transfer vectors. High Five cells grown to 2.0 x 106 cells/ml were infected with each baculovirus carrying the FSH and FSHR genes. The cell culture supernatant was harvested 72 hours after infection and applied to an anti-FLAG antibody (M2) affinity column. The FSH-FSHR complex eluted from the antibody column using FLAG peptide was purified further by gel filtration column chromatography (Superdex 200, Pharmacia) in 20 mM Tris, pH 8.0 and 150 mM NaCl.
2.3. Enzymatic deglycosylation
The FSH-FSHRHB complex was partially deglycosylated using endoglycosidase F1, F2 and F3, either alone or in combination with one another. The deglycosylation reactions were carried out at 20°C for 5 days in 50 mM sodium acetate, pH 4.5 or 50 mM sodium citrate, pH 5.5. The FSH-FSHRHB protein was separated from deglycosylation enzymes by ion exchange chromatography (MonoQ, Pharmacia) in 20 mM Tris, pH 8.0 using a salt gradient from 0 to 500 mM NaCl. Partially deglycosylated FSH-FSHRHB complex was concentrated to 9.8 mg/ml in 20 mM Tris, pH8.0 for crystallization.
2.4. Production of free hormones
Recombinant human FSH, CG and LH were produced in insect cells using the baculovirus expression systems. In each case, the genes encoding the hormone α- and β-subunits were subcloned into a pFastBac Dual plasmid (Invitrogen) under separate promoters. The α-construct comprises its native signal sequence and entire mature sequence (residues 1–92). The β-construct comprises the native signal sequence and entire mature sequence of FSH (residues 1–111), CG (1–145) or LH (1–121). A FLAG tag is also engineered at the C-terminal end of every hormone β-chain to facilitate purification by antibody affinity chromatography. Each hormone is further purified by gel filtration chromatography. Recombinant hCG expressed in CHO cells was obtained from Serono Inc. through Joyce Lustbader.
2.5. Competition binding assays
The FSH-FSHRHB complex and excess amount of free FSH, free CG or free LH were incubated in a reaction buffer containing 20 mM Tris, pH 8.0 and 150 mM NaCl for 30 min at room temperature. The reaction mixture was then assayed on a 8–25% native gel.
2.6. Estimation of the dissociation constant (Kd) of soluble FSH-FSHRHB complex
We estimated the Kd of the soluble FSH-FSHRHB complex based on its concentration in cell culture.
where [H], [R] and [HR] represent the concentrations of free hormone, free receptor and their complex, respectively. The yield of purified FSH-FSHRHB protein is about 0.1 mg per liter of insect cell culture, which corresponds to 1.3 nM of the complex. When expressed alone, the production level of free FSH is about 1.6 mg per liter of insect cell culture. Given that the estimated molecular mass of free FSH is about 40 kD based on its mobility on SDS page, the concentration of free hormone in the cultured medium is about 40 nM. We do not have any direct measure of the amount of free receptor in the medium. Since a FLAG tag is engineered at the receptor C-terminus, all expressed receptor protein, whether it is in hormone-bound or free form, would be purified from the medium by the antibody affinity column. Upon gel filtration chromatography of this protein mixture, there is no detectable amount of any properly folded FSHRHB, indicating either that there is very little free receptor or that most of it has precipitated or formed soluble aggregates. This result has been confirmed by expressing FSHRHB alone in insect cells. If we assume the concentration of free receptor in the cell culture is at most 1/10 that of the complex, the Kd of the FSH-FSHRHB complex would be equal to or lower than approximately 4 nM.
2.7. Structural superposition and illustrations
The program LSQMAN (Kleywegt, 1996) was used to determine the optimal structural alignment between the FSH molecule in the FSH-FSHRHB complex structure (1XWD) and hCG in the theoretical hCG-LHR model (1XUL). The hormone molecules were then superimposed in O (Jones et al., 1991) using the transformation matrix that related their best aligned segments.
Figures were generated using RIBBONS (Carson, 1997), Adobe Illustrator and Adobe Photoshop.
3. Results and Discussion
3.1. Assembly of a soluble FSH-FSHRHB complex
In order to assemble a stable FSH-FSHR complex, we co-expressed the receptor and hormone using the baculovirus expression system (Fig. 1A). In addition, the α- and β- subunits of the heterodimeric hormone were covalently stabilized with a Gly-Ser rich linker (GGGSGGGSGGGSGGG) from the C-terminus of FSHβ to the N-terminus of FSHα (Fig. 1A). Single-chain FSH and CG constructs with different linkers have previously been shown to retain receptor-binding activities (Sugahara et al., 1995; Narayan et al., 1995; Schmidt et al., 2001). Genes encoding the extracellular domain of FSHR and the single-chain FSH were inserted into the same vector for expression. A FLAG tag was engineered at the C-terminus of the receptor to facilitate purification of the complex by affinity column chromatography.
Fig. 1.
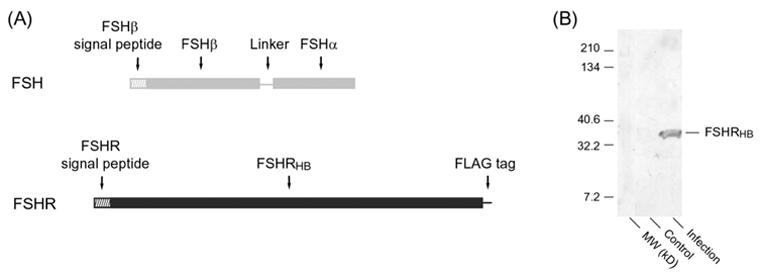
(A) Schematic diagram of the baculovirus expression constructs for human FSHRHB and the covalently linked human FSHα and FSHβ subunits.
(B) Western blot of the culture medium of insect cells that were infected with the recombinant virus for FSHRHB and single-chain FSH. Culture samples were concentrated 20-fold and run on a 4-15% SDS gel in the presence of 100 mM dithiothreitol (DTT). The blot was developed using the M2 anti-FLAG antibody as the primary antibody.
To dissect the ligand-binding region of FSHR, we tested constructs with different C-terminal truncations of the FSHR ectodomain. The ectodomains of all glycoprotein hormone receptors consist of a series of leucine-rich repeats (LRRs) flanked at each end by cysteine clusters. We discovered early in our studies that it was important to retain the N-terminal cluster, but the C-terminal cysteine-rich region is not required for hormone binding. We found that the hormone binding domain of the receptor, FSHRHB, contains the N-terminal cysteine cluster and the LRRs (residues 1–268). Expression of the truncated receptor ectodomain could be detected by western blot using an anti-FLAG antibody (Fig. 1B). A slightly shorter construct of FSHR containing residues 1–247 was also secreted as properly folded protein in complex with single-chain FSH. We were able to obtain crystals of the FSH-FSHR (1–247) complex that diffracted to 9 Å. FSHR constructs that were shortened further were hardly secreted even in the presence of FSH. On the other hand, longer constructs that additionally included part of the C-terminal cysteine-rich region were secreted as soluble aggregates. A construct that encoded the entire ectodomain of FSHR was hardly secreted with FSH.
FSHRHB forms a stable complex with FSH when secreted from insect cells. Fig. 2A shows the gel filtration chromatogram of co-secreted FSH and FSHRHB. The correctly folded protein peak contains both the receptor and hormone according to N-terminal sequencing of the two bands from SDS-PAGE electrophoresis (Fig. 2B) as well as the band from a native gel (Fig. 2C). The retention time for the folded complex by gel filtration chromatography corresponds to a 72 kD protein, which is close to the exact molecular mass of the glycosylated FSH-FSHRHB complex determined by analytical ultracentrifugation (78 kD) (Fan and Hendrickson, 2005a). The molecular mass of the FSH-FSHRHB complex is consistent with a 1:1 binding stoichiometry between the receptor and hormone, with 32% of the mass being contributed by carbohydrates.
Fig. 2.
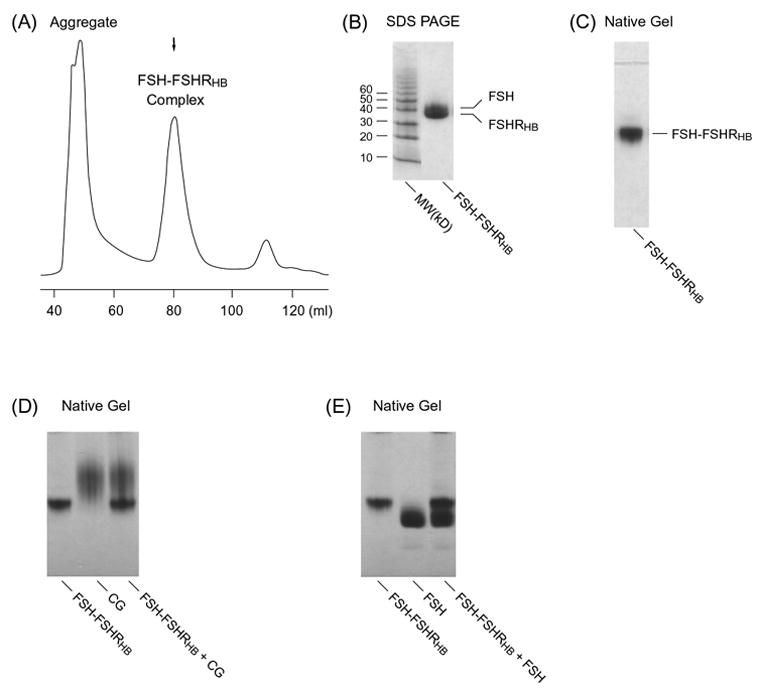
(A) Gel filtration column chromatography of the FSH-FSHRHB complex. Samples of the indicated peak fraction were assayed on (B) 8–25% SDS gel and (C) 8–25% native gel. Samples of purified FSH-FSHRHB (5 μg) and free hormone ligands were also analyzed by 8–25% native gel electrophoresis for possible additional interactions upon mixture: (D) for 5 μg of free CG, and (E) for 7.5 μg of free FSH.
Human FSH binds to cell surface FSHR with high affinity and a dissociation constant (Kd) in the nanomolar (nM) range (Simoni et al., 1997; Ascoli et al., 2002). We have estimated the Kd of the soluble FSH-FSHRHB complex based on its concentration in cell culture. The yield of purified FSH-FSHRHB complex was used to calculate the concentration of the complex in the medium, and the concentration of free hormone was estimated from the yield of free FSH when expressed alone. Since FSHRHB was secreted as soluble aggregates, we assumed that the concentration of free receptor in the medium could not possibly exceed 1/10 that of the complex. We have then obtained an upper limit of 4 nM for the Kd for our recombinant FSH-FSHRHB complex, which is comparable to the affinity observed for FSH binding to membrane-bound FSHR on cell surfaces. This is in keeping with measurements of the affinity of similar ectodomain constructs with their cognate hormones, which typically even exceed the tight binding of hormone to the intact receptor on membranes (Simoni et al., 1997). Moreover, the stability of the FSH-FSHRHB complex through immunoaffinity and gel filtration chromatography, as well during crystallization, favors a high affinity complex, or at least one with an extremely low rate constant of dissociation, consistent with studies of others (Simoni et al., 1997; Ascoli et al., 2002).
The interaction between the co-secreted FSH and FSHRHB is highly specific as demonstrated by competition binding assays. First, in accordance with the preference of FSHR for FSH, the soluble FSH-FSHRHB complex could not be dissociated by excess amount of free hCG or LH hormone. As shown in Fig. 2D for hCG, addition of free hCG did not change the mobility of hCG or FSH-FSHRHB complex on native gel, indicating that hCG could not displace FSH in binding to FSHRHB. Second, in the presence of excess amount of free FSH, there was no additional binding observed between the free FSH and the FSH-FSHRHB complex. Incubation of the FSH-FSHRHB complex with excess amount of free FSH did not produce any novel band that would correspond to a second binding event (Fig. 2E). This indicates that the hormone-binding site on FSHRHB had already been occupied by the single-chain FSH that was co-secreted with FSHRHB. These observations confirm that the tight association between co-expressed FSH and FSHRHB is not due to non-specific binding.
3.2. Partial deglycosylation and crystallization of the FSH-FSHRHB complex
The fully glycosylated FSH-FSHRHB (1–268) complex could be crystallized in several different forms, one of which is shown in Fig. 3C. The best of these crystals only diffracted to 9 Å spacings. In order to improve the resolution limits of these crystals, we carried out enzymatic deglycosylation of the FSH-FSHRHB complex. Partial deglycosylation was observed using endoglycosidase F1, F2 and F3, either alone or in combination with one another. We chose to use a combination of endoglycosidase F2 and F3 because the cleaved product had a relatively tighter band on native gel when compared with the fully glycosylated control (Fig. 3B), indicating a more homogeneous sample. SDS gels showed that about 20% of the carbohydrate was removed, and that sugars were mainly being taken from FSH, which was the top band in the control sample (Fig. 3A). As a result of deglycosylation, the hormone and receptor condensed into one band due to their similar molecular weight (Fig. 3A). Removal of carbohydrate reduced the solubility of the FSH-FSHRHB complex, causing about 50% of the protein to precipitate out of solution. There was an overall loss of 80% from the deglycosylation procedure. Nevertheless, partial deglycosylation did not disrupt the interaction between FSH and FSHRHB; the complex stayed together on gel filtration and ion-exchange chromatorgraphy after the endoglycosidase treatment.
Fig. 3.
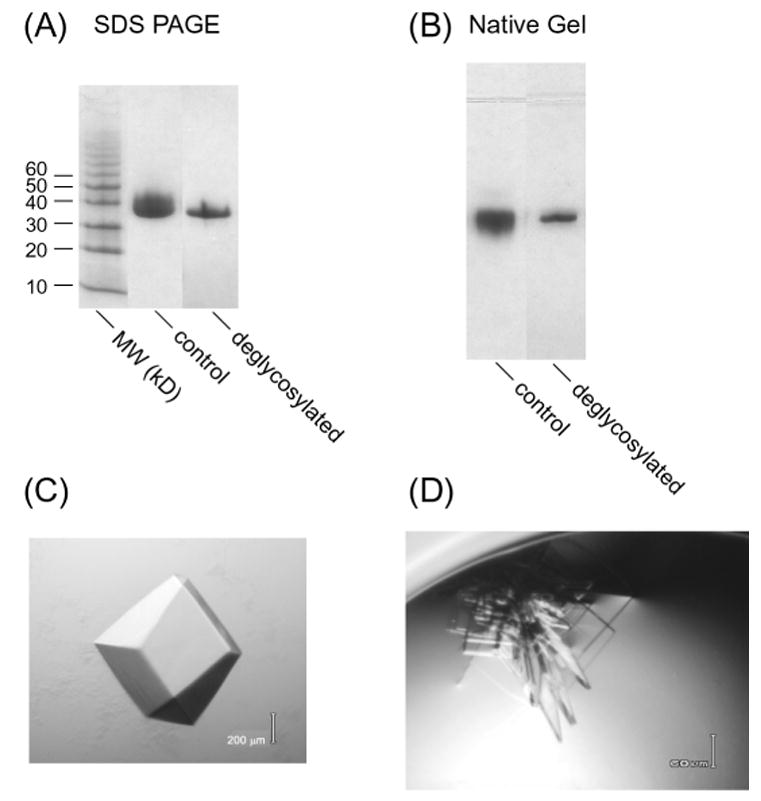
Fully glycosylated (control) and partially deglycosylated FSH-FSHRHB complex were assayed on (A) 8–25% SDS gel and (B) 8–25% native gel.
(C) A crystal of fully glycosylated FSH-FSHRHB complex grown at 4°C from 16% tert-butanol and 0.1 M sodium citrate, pH 6.0.
(D) Thin plate crystals of partially deglycosylated FSH-FSHRHB complex grown at 20°C from 10% PEG 3350 and 0.1 M Li2SO4.
The partially deglycosylated FSH-FSHRHB complex crystallized in a new crystal form (Fig. 3D). These crystals grew as clusters of plates which were 5–10 μm thick. Single crystals were broken off the cluster for measurement of x-ray diffraction data. The best of these crystals diffracted to a limit corresponding to 2.9 Å resolution. The structure of the FSH-FSHRHB complex was determined by molecular replacement using the known structure of FSH as the search model (Fan and Hendrickson, 2005a).
3.3. Structure of the FSH-FSHRHB complex
The structure of the FSH-FSHRHB complex shows the hormone bound in a hand-clasp fashion to the concave face of a curved receptor (Fan and Hendrickson, 2005a) (Fig. 4). The structure of FSHR consists of ten highly irregular repeats, which vary both in length and overall conformation. The hormone binding site on FSHR includes all ten parallel β-strands and loops just C-terminal to these strands. The receptor binding site on FSH is formed by the C-terminal segments of both FSHα- and β-subunits as well as the
 and
and
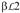 loops. None of the carbohydrate moieties on the hormone or on the receptor is involved in the interaction interface.
loops. None of the carbohydrate moieties on the hormone or on the receptor is involved in the interaction interface.
Fig. 4.
Ribbon diagram of the crystal structure of human FSH bound to FSHRHB. FSH α- and β-chains are in green and cyan, respectively. FSHRHB is in red. The observed N-linked carbohydrates at Asn52, Asn78 of FSHα , Asn7 and Asn24 of FSHβ , and Asn191 of FSHRHB are in yellow. Disulfide bonds are in black. Adapted with permission from Nature (Fan and Hendrickson, 2005a).
The linker peptide used to stabilize the FSHα β heterodimer does not appear to affect the association between FSH α- and β-subunits or the receptor binding mode of FSH. First of all, the entire linker together with four C-terminal residues of FSHβ and two N-terminal residues of FSHα (five in the second protomer) are not visible in the electron density map, indicating that they are disordered and highly flexible. From the location of these termini and as designed, the covalent linker connecting the C-terminus of FSHβ and N-terminus of FSHα must span a region on the FSH surface opposite to the receptor-binding site, and therefore would not interfere with receptor binding.
The interactions between FSH and FSHRHB suggest that the binding mode is universal among all glycoprotein hormones and receptors. First, the structures of the hormone and the hormone-binding domain of the receptor are expected to be very similar among their family members because of high sequence similarity. Second, the structural elements found at the receptor-hormone interface of FSH-FSHRHB complex have also been implicated in the interactions of other receptor-hormone pairs. Finally, many residues of FSHRHB involved in contacting the common α-subunit of hormone are conserved among receptor homologues, consistent with a universal binding mode. The FSH-FSHRHB interface has a high buried charge density, and many residues with complementary charges contribute to the universal interactions.
The structure of FSH-FSHRHB complex also demonstrates that receptor specificity is jointly mediated by the hormone α- and β-subunits. There are four predominant specificity-determining sites on the surface of the receptor at residues L55, K179, I222 and the structurally adjacent E76 and R101. Interactions between receptor residues 55 and 179 and the hormone seat-belt region of FSHβ determine the selection between FSH and TSH versus LH/CG, consistent with mutational analyses (Dias et al., 1994; Smits et al., 2003; Vischer et al., 2003a). The sites at residues 76, 101 and 222 distinguish between FSH and TSH.
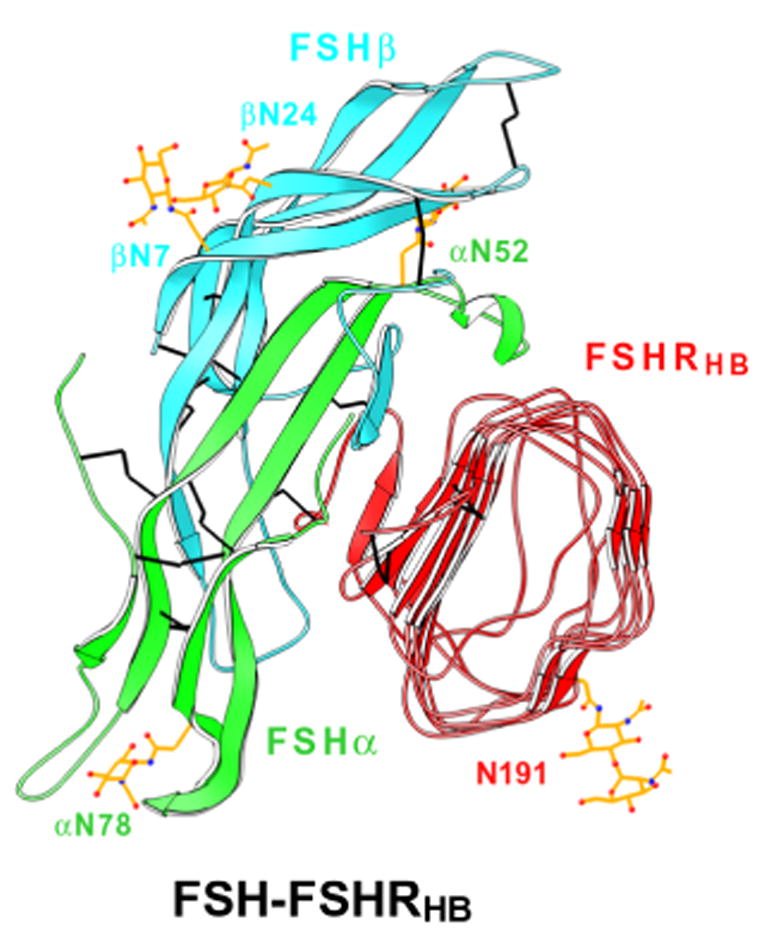
3.4. Implications of dimeric associations in the crystal
There are two FSH-FSHRHB complexes in the asymmetric unit of the crystal, and these associate as a biologically plausible dimer. This observation provoked us to perform experiments in solution where we found dimers characterized by a dissociation constant of Kd ~ 400μM (Fan and Hendrickson, 2005a). The associated complexes in the crystallographic dimer are related by approximate two-fold symmetry (rotation χ= 175.2°, screw translation tχ= −1.2 Å) with a dimer interface that involves only the outer surface of the two receptor molecules (Fig. 5A). The direct contacts are mainly hydrophobic and the highly conserved Y110 residue predominates in the interaction. The weak intrinsic affinity of the FSHRHB dimer is expected to be enhanced when FSHRHB is tethered to the cell membrane. The mode of interaction in this principal dimer is plausible for all glycoprotein hormones, and could exist in uncomplexed receptors.
Fig. 5.
Dimeric associations. (A–C) Ribbon diagrams of quasi-symmetric crystallographic dimers of FSH-FSHRHB complexes, colored as in Fig. 4. The view is parallel to the symmetry axis, as if looking at the membrane. (D–E) Schematic illustrations of dimeric receptors on the cell membrane. Projected outlines from the complexes shown above and from rhodopsin are shown for the hormone (cyan), hormone-binding domain (orange), linker and 7TM transmembrane domain (black dotted trace). The view is perpendicular to figures directly above, parallel with the membrane plane. (A) Principal crystallographic dimer. (B) Principal dimer with outline of an offset 7TM dimer disposed for interaction with one of the bound FSH molecules. (C) Alternative crystallographic dimer. (D) Symmetric principal dimer with both 7TM domains engaged. (E) Asymmetric principal dimer with only one 7TM domain engaged. Note that dimer axes for the two components are not aligned. (F) Symmetric alternative dimer with both 7TM domains engaged.
The crystal packing is such that an alternative dimeric association of FSH-FSHRHB complexes also exists (suggested to us by Xuliang Jiang). It has three separate areas of contact: each hormone of the dimer contacts the N-terminus of the receptor from the neighboring complex, and the two receptors also interact with each other through their N-terminal cystein-rich regions (Fig. 5C). Because the N-terminal segment is highly variable among the different glycoprotein hormone receptors, this mode of dimerization is unlikely to occur for LHR or TSHR. Neither would it be expected to exist in absence of the FSH ligand. This alternative dimer is also much less symmetric, with a substantial screw translation (rotation χ=175.2°, screw translation tχ= −6.6 Å).
Compelling evidence has been presented recently for functional dimerization of TSHR through the seven-transmembrane (7TM) helical portion of the receptor and for an asymmetry in hormone binding, with negative cooperativity between two binding sites (Urizar et al., 2005). How might ectodomain dimers be related to 7TM dimers, and what are the implications for signal transduction? We previously noted the puzzling separation of the FSHRHB dimers in light of a prospective association of 7TM domains into dimers (Fan and Hendrickson, 2005a). The C-termini of FSHRHB domains in the principal dimer are 74Ǻ apart, and the centers of their respective 7TM domains would be separated by ~90Ǻ if each were equivalently engaged with
 tips of FSH in the complex (Fig. 5D). These separations are 88Ǻ and ~65Ǻ, respectively, in the alternative dimer. Even here, however, the
tips of FSH in the complex (Fig. 5D). These separations are 88Ǻ and ~65Ǻ, respectively, in the alternative dimer. Even here, however, the
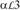 tips would be too far apart for simultaneous engagement with a 7TM dimer (Fig. 5F). Actual structures of 7TM dimers are not known, but a model of the rhodopin dimer has been fitted to images of retinal disc membranes made by atomic force microscopy (Fotiadis et al., 2004). The distance between 11-cis retinal sites in this model is ~31Ǻ, far less than hormone separations in the ectodomain dimers.
tips would be too far apart for simultaneous engagement with a 7TM dimer (Fig. 5F). Actual structures of 7TM dimers are not known, but a model of the rhodopin dimer has been fitted to images of retinal disc membranes made by atomic force microscopy (Fotiadis et al., 2004). The distance between 11-cis retinal sites in this model is ~31Ǻ, far less than hormone separations in the ectodomain dimers.
Asymmetry in the dimeric signaling complex provides for a possible resolution of the apparent dimer mismatch. The distance between FSHR residues 360 at the start of helices TM1 of the rhodopsin-based 7TM dimer model for FSHR is 78Ǻ, which is very similar to the distance between FSHRHB C-termini in principal dimers and, incidentally, also similar to the separation of C-termini in dimers of glutamate-receptor ectodomains (Kunishima et al., 2000). The linker segment that connects FHSRHB to its 7TM domain comprises 98 residues (269–366), including three disulfide bonds, and it is straightforward to picture a symmetric empty receptor with aligned two-fold axes for its ectodomain and 7TM-domain dimers. This would have 7TM sites occluded from possible interaction with the hormone, but the linker segment may afford sufficient flexibility to permit a relative displacement and possible tilting. Upon hormone binding, the ligand at one site could engage one 7TM domain while that bound at the second site would be remote from the 7TM dimer (Fig. 5B and 5E). The linkers are each 42Ǻ long, but they are differently disposed in this asymmetric model. Such physical asymmetry may relate to the observed negative cooperativity (Urizar et al., 2005) and it has significant implications for the mechanism of G-protein stimulation of GPCRs.
3.5. Comparison with theoretical models of the complex
Based on the first structure of a LRR-containing protein, that of porcine ribonuclease inhibitor (Kobe and Deisenhofer, 1993), several models have been predicted for the extracellular domain of glycoprotein hormone receptors (Jiang et al., 1995; Kajava et al., 1995; Bhowmick et al., 1996; Kleinau et al., 2004). Secondary structure prediction suggested the presence of alternating β-strands and α-helices in the ectodomain of these receptors (Jiang et al., 1995). This had led to the initial prediction that the ectodomain structure of glycoprotein hormone receptors would also feature β α conformations as in the structure of RI, albeit with less developed helical structure (Jiang et al., 1995; Kajava et al., 1995; Bhowmick et al., 1996) (Fig. 6). Recently, homology modeling of the TSHR ectodomain based on the NgR structure suggested that it adopts a LRR fold with extended conformations on the convex side (Kleinau et al., 2004). The crystal structure of FSHRHB is devoid of any helices despite of the fact that some residues on the outer circumference have (φ,ψ ) angles in the left-handed helical region. The interstrand segments mostly adopt extended conformations with the presence of several short β-strands. The structure of FSHRHB also exhibits different curvatures for its two parts, which was not anticipated in any of the theoretical models.
Fig. 6.
Ribbon diagrams comparing the predicted hCG-LHRHB interaction model (hCG in green; LHRHB model in gray) with the crystal structure of human FSH-FSHRHB complex (FSH in blue; FSHRHB in red). The structure of hCG in the predicted model was superimposed onto the structure of receptor-bound FSH. (A) Top view. (B) Side view.
Different models have also been proposed for the interaction between glycoprotein hormones and the ectodomain of their receptors based on mutagenesis data, peptide binding studies and antibody blocking assays (Jiang et al., 1995; Moyle et al., 1995; Dias and Van Roey, 2001; Sohn et al., 2003; Moyle et al., 2004; Kleinau et al., 2004). Most of the theoretical models predicted that the hormone would bind to the concave surface of the receptor solenoid structure, but the relative orientation of the hormone long axis and the receptor solenoid varied from being parallel to perpendicular (Jiang et al., 1995; Dias and Van Roey, 2001; Sohn et al., 2003; Kleinau et al., 2004).
The model by Jiang et al. 1995 of the complex between human CG (hCG) and the LHR ectodomain, as deposited into the Protein Data Bank (accession code 1XUL), roughly predicted the binding mode observed in the crystal structure of the FSH-FSHRHB complex. Notably, both have the long axis of the hormone perpendicular to the receptor tube (Fig. 6). Superposition of the CG-LHR interaction model with the crystal structure of the FSH-FSHRHB complex shows, however, that the receptor was predicted to bind to a wrong surface of the hormone (Fig. 6). Moreover, the modeling was not decisive on the orientation of the hormone long axis with respect the direction of the parallel β-strands of the receptor molecule (Jiang et al., 1995), although the deposited complex did have this correct orientation. This receptor model, based exclusively on RI, also left residual helical character in backside strands, which would have been removed based on later templates (Kleinau et al., 2004). This example illustrates some of the current limitations of modeling in predicting complex protein-protein interactions.
We are not able to perform a direct superposition between the FSH-FSHRHB crystal structure and any other of the theoretical models because no coordinates have been deposited for these models. Unlike most theoretical models, which correctly predicted the hormone-binding site to involve the parallel β-sheet on the concave surface of the receptor structure, models by Moyle and coworkers suggested that hormone would bind to the upper rim of the LRR-containing structure, contacting only the loops that follow the parallel β-strands (Moyle et al., 1995; Moyle et al., 2004). Moyle et al. (2004) proposed instead that a module formed by the cysteine-rich cluster at the C-terminal end of the receptor ectodomain would contact the parallel β-sheet of the receptor. The Moyle models missed essentially all of the structural elements of the receptor that are involved in hormone-binding in the observed complex (Fan and Hendrickson, 2005a).
3.6. The experimental structure in light of theoretical challenges
Moyle has tried to explain the disagreement between the crystal structure and his theoretical model by impugning the validity of the experimental result. At the recent ICGR conference, he suggested in his presentation and in open discussion that the binding mode observed in the crystal structure is due to non-specific binding of FSH to a truncated FSHR ectodomain at the low expression level of the receptor-hormone complex in cell culture and that the receptor structure in the crystal as truncated may be distorted from naturally greater intrinsic curvature. More recently, Moyle et al. (2005) have proposed that the binding mode observed in the structure of the FSH-FSHRHB complex may represent the way the hormone would bind to an alternatively spliced variant of the receptor, which lacks the cysteine-cluster domain that links the LRR domain to the transmembrane helical domain, but does not reflect authentic binding to the full-length receptor. Moyle has also questioned the relevance of some mutational tests in mapping the hormone-receptor interface (Moyle et al., 2005).
Here we provide additional experimental detail to validate the authenticity of the recombinant human FSH-FSHRHB complex that we produced from insect cell culture. We have demonstrated that this soluble complex was secreted into the culture medium as a homogeneous species. Based on its discrete mobility on native gels, the complex remained the same species over a wide range of concentrations, from about 0.1 mg/ml in diluted fractions off the gel filtration column to 10 mg/ml in crystallization samples. The interaction between the co-secreted FSH and FSHRHB had native-like affinity (Kd ≤ 4nM) and was highly specific. The FSH-FSHRHB complex remained intact in the presence of excess hCG or LH. Moreover, it did not bind additional free FSH, indicating that the bound FSH already occupied the high-affinity hormone-binding site on FSHRHB. The proposition that truncation or the crystal lattice has distorted the structure of FSHRHB is implausible in light of its similarity of FSHRHB to other LRR structures, none of which show shape changes in different lattices or upon ligand binding (Kobe and Deisenhofer, 1993; Kobe and Deisenhofer, 1995; Huizinga et al., 2002; Uff et al., 2002; Dumas et al., 2004; Schubert et al., 2002). We conclude that the binding mode observed in the crystal structure of FSH-FSHRHB complex does authentically represent the specific interaction between FSH and its cognate receptor under native conditions.
The hormone-receptor interaction observed in the crystal structure is consistent with extensive mutagenesis studies, which have identified the parallel β-sheet of the receptor as its hormone-binding site (Bhowmick et al., 1996; Bhowmick et al., 1999; Schmidt et al., 2001; Song et al., 2001; Smits et al., 2002; Smits et al., 2003; Vischer et al., 2003a; Vischer et al., 2003b). The structure is also consistent with studies implicating specific hormone segments in receptor binding. Namely, the common C-terminus of the α-chain (Chen et al., 1992; Yoo et al., 1993; Grossmann et al., 1995; Arnold et al., 1998), the discriminating seat-belt segment of the β-chain (Keutmann et al., 1989; Santa Coloma and Reichert, 1990; Campbell et al., 1991; Dias et al., 1994; Moyle et al., 1994; Grossmann et al., 1997), the
 loop (Erickson et al., 1990; Leinung et al., 1991; Liu and Dias, 1996) and the
loop (Erickson et al., 1990; Leinung et al., 1991; Liu and Dias, 1996) and the
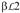 loop (Keutmann et al., 1987). The crystal structure also provides an explanation for many aspects of specificity, again consistent with mutational analyses (Fan and Hendrickson, 2005a; Fan and Hendrickson, 2005b). In our reading of Moyle’s model, wherein the hormone only makes contacts with the rim of the LRR structure and not with the β-sheet surface (Moyle et al., 1995; Moyle et al., 2004), this theoretical alternative is at odds with the body of mutational analysis. Incidentally, the concordance between mutagenesis results and the crystal structure does not simply reflect a mode of binding restricted to FSHRHB and alternatively spliced receptor variants (Moyle et al., 2005). Most of the mutagenesis experiments were performed using full-length receptors on cell surfaces (Bhowmick et al., 1996; Bhowmick et al., 1999; Song et al., 2001; Smits et al., 2002; Smits et al., 2003; Vischer et al., 2003a; Vischer et al., 2003b).
loop (Keutmann et al., 1987). The crystal structure also provides an explanation for many aspects of specificity, again consistent with mutational analyses (Fan and Hendrickson, 2005a; Fan and Hendrickson, 2005b). In our reading of Moyle’s model, wherein the hormone only makes contacts with the rim of the LRR structure and not with the β-sheet surface (Moyle et al., 1995; Moyle et al., 2004), this theoretical alternative is at odds with the body of mutational analysis. Incidentally, the concordance between mutagenesis results and the crystal structure does not simply reflect a mode of binding restricted to FSHRHB and alternatively spliced receptor variants (Moyle et al., 2005). Most of the mutagenesis experiments were performed using full-length receptors on cell surfaces (Bhowmick et al., 1996; Bhowmick et al., 1999; Song et al., 2001; Smits et al., 2002; Smits et al., 2003; Vischer et al., 2003a; Vischer et al., 2003b).
Probes of protein-protein interfaces, such as made by mutations or by challenges with peptides or antibodies, are oftentimes not completely incisive. For examples, disruptions may be due to misfolding rather than intended local perturbation, and chimeras may retain functions of the host protein as well as giving gain-of-function to that of the substitution. It is only prudent to be cautious in interpreting such experiments, but equally it is foolish to be dismissive of sound evidence. Moyle argues that experiments implicating involvement of the FSHα C-terminus in receptor interactions are likely due to disturbance of the interface between FSHα and FSHβ , and thereby dismisses this as a corroboration of the crystal structure (Moyle et al., 2005). In fact, however, the crystal structures of free hCG (Wu et al., 1994; Lapthorn et al., 1994), free FSH (Fox et al., 2001) and receptor-complexed FSH (Fan and Hendrickson, 2005a) have widely different FSHα C-terminal conformations but virtually identical heterodimeric associations. Moreover, this FSHα : FSHβ interface has exceptionally large buried surface (4080Ǻ2), exceptionally low buried charge density (0.37 charges/nm2), and high shape complementary (0.74) with 35 inter-chain hydrogen bonds. There is nothing from the structures to support the assertion of heterodimer perturbabtion from C-terminal modification. Moyle also dismisses incisive experiments that implicate 88DSDS91 of FSHβ (Dias et al., 1994) with K179 of FSHR (Smits et al., 2003; Vischer et al., 2003a) as evidence, consistent with their match in the crystal structure, for specificity determination in a universal mode of binding. Instead, mistakenly we think, he uses the relaxed specificity of the resulting chimeras as evidence for distinctive modes of interaction for the different gonadotropins (Moyle et al., 2005).
To be sure, the crystal structure of the FSH-FSHRHB complex leaves many unanswered questions about signal transduction through gonadotropin receptors. We feel that additional experimental structures have a better chance at further illuminating the process than additional theoretical modeling.
4. Conclusions
Human FSH forms a homogeneous complex with FSHRHB when co-expressed in insect cells from a baculovirus construct. The two components are specifically associated with one another, interacting with an affinity comparable to that of the hormone for the intact receptor on cell surfaces. The purified complex separates sharply and symmetrically on gel filtration columns and in native gel electrophoresis, and it crystallizes readily. The crystal structure was refined at 2.9Ǻ resolution with excellent stereochemical ideality and agreement with the diffraction data.
Like other LRR proteins, FSHRHB is a curved solenoidal tube characterized by a tightly packed, continuous hydrophobic core and a flat, parallel β-sheet. This is a robust structural scaffold that is unlikely to be perturbed in overall shape by ligand binding or lattice interactions. FSH binds to the slightly concave, β-sheet surface of the receptor where it interacts with all ten parallel β-strands of FSHRHB. The linker peptide that fuses FSHβ to FSHα is disordered but positioned away from the contact surface. The hormone-receptor interface is large and intimately complementary, consistent with the observed high affinity of association. This interface has features compatible with a universal but specific mode for glycoprotein-hormone binding to their respective receptors, consistent with many mutational tests. Specific interactions observed at the hormone-receptor interface in the FSH-FSHRHB crystal structure agree well with structural elements implicated in binding by mutagenesis studies of hormone binding to intact receptors on cell membranes.
We conclude that the crystal structure of FSH complexed with FSHRHB provides an authentic representation of the binding of FSH to intact FSHR. This is not to say that there are no other interactions between hormone and receptor; indeed, the hormone does most likely interact with transmembrane helical and/or cysteine-cluster linker domains of the receptor in the course of receptor activation. But just as hormone binding and receptor activation have long been known to be largely separable (Simoni et al., 1997; Dias et al., 2002; Ascoli et al., 2002; Szkudlinski et al., 2002), so too are the structural features that underlie these phenomena. Concerns raised about the authenticity of the FSH-FSHRHB structure are unfounded.
Acknowledgments
We thank the organizers of the International Conference on Gonadotropins and Receptors, Drs. Steven Birken and David Puett, for the invitation to speak, Drs. James Dias, Gilbert Vassart, Deborah Segaloff, Mario Ascoli, Marco Muda, Xuliang Jiang, Axel Themmen, Galina Kovalevskaya, Jan Bogerd, Sabine Costagliola, Tae Ji, Prema Narayan, George Bousfield, Alfredo Ulloa-Aguirre, Irving Boime, David Ben-Menahem, Yongsheng Li and many other participants at the conference for helpful discussion. We also thank the referees for helpful suggestions and Xuliang Jiang for discussions about dimers. This work was supported in part by NIH grant GM68671. Q. R. F. was an Agouron Institute fellow of the Jane Coffin Childs Memorial Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold CJ, Liu C, Lindau-Shepard B, Losavio ML, Patrascu MT, Dias JA. The human follitropin α-subunit C terminus collaborates with a β-subunit cystine noose and an α-subunit loop to assemble a receptor-binding domain competent for signal transduction. Biochemistry. 1998;37:1762–1768. doi: 10.1021/bi971816o. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Bhowmick N, Huang J, Puett D, Isaacs NW, Lapthorn AJ. Determination of residues important in hormone binding to the extracellular domain of the luteinizing hormone/chorionic gonadotropin receptor by site-directed mutagenesis and modeling. Mol Endocrinol. 1996;10:1147–1159. doi: 10.1210/mend.10.9.8885249. [DOI] [PubMed] [Google Scholar]
- Bhowmick N, Narayan P, Puett D. Identification of ionizable amino acid residues on the extracellular domain of the lutropin receptor involved in ligand binding. Endocrinology. 1999;140:4558–4563. doi: 10.1210/endo.140.10.7077. [DOI] [PubMed] [Google Scholar]
- Campbell RK, Dean-Emig DM, Moyle WR. Conversion of human choriogonadotropin into follitropin by protein chemistry. Proc Natl Acad Sci U S A. 1991;88:760–764. doi: 10.1073/pnas.88.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M. Ribbons. Methods Enzymol. 1997;277:493–505. [PubMed] [Google Scholar]
- Chen F, Wang Y, Puett D. The carboxy-terminal region of the glycoprotein hormone α-subunit: contributions to receptor binding and signaling in human chorionic gonadotropin. Mol Endocrinol. 1992;6:914–919. doi: 10.1210/mend.6.6.1379673. [DOI] [PubMed] [Google Scholar]
- Dias JA, Zhang Y, Liu X. Receptor binding and functional properties of chimeric human follitropin prepared by an exchange between a small hydrophilic intercysteine loop of human follitropin and human lutropin. J Biol Chem. 1994;269:25289–25294. [PubMed] [Google Scholar]
- Dias JA, Van Roey P. Structural biology of human follitropin and its receptor. Arch Med Res. 2001;32:510–519. doi: 10.1016/s0188-4409(01)00333-2. [DOI] [PubMed] [Google Scholar]
- Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vitam Horm. 2002;64:249–322. doi: 10.1016/s0083-6729(02)64008-7. [DOI] [PubMed] [Google Scholar]
- Dumas JJ, Kumar R, McDonagh T, Sullivan F, Stahl ML, Somers WS, Mosyak L. Crystal structure of the wild-type von Willebrand factor A1-glycoprotein Ibα complex reveals conformation differences with a complex bearing von Willebrand disease mutations. J Biol Chem. 2004;279:23327–23334. doi: 10.1074/jbc.M401659200. [DOI] [PubMed] [Google Scholar]
- Erickson LD, Rizza SA, Bergert ER, Charlesworth MC, McCormick DJ, Ryan RJ. Synthetic alpha-subunit peptides stimulate testosterone production in vitro by rat Leydig cells. Endocrinology. 1990;126:2555–2560. doi: 10.1210/endo-126-5-2555. [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005a;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structural biology of glycoprotein hormones and their receptors. Endocrine. 2005b;26:179–188. doi: 10.1385/endo:26:3:179. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389. doi: 10.1210/mend.15.3.0603. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Szkudlinski MW, Zeng H, Kraiem Z, Ji I, Tropea JE, Ji TH, Weintraub BD. Role of the carboxy-terminal residues of the alpha-subunit in the expression and bioactivity of human thyroid-stimulating hormone. Mol Endocrinol. 1995;9:948–958. doi: 10.1210/mend.9.8.7476992. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Szkudlinski MW, Wong R, Dias JA, Ji TH, Weintraub BD. Substitution of the seat-belt region of the thyroid-stimulating hormone (TSH) β-subunit with the corresponding regions of choriogonandotropin or follitropin confers luteotropic but not follitropic activity to chimeric TSH. J Biol Chem. 1997;272:15532–15540. doi: 10.1074/jbc.272.24.15532. [DOI] [PubMed] [Google Scholar]
- Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- Jiang X, Dreano M, Buckler DR, Cheng S, Ythier A, Wu H, Hendrickson WA, Tayar NE. Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone-receptor interactions. Structure. 1995;3:1341–1353. doi: 10.1016/s0969-2126(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in the models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kajava AV, Vassart G, Wodak SJ. Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure. 1995;3:867–877. doi: 10.1016/S0969-2126(01)00222-2. [DOI] [PubMed] [Google Scholar]
- Keutmann HT, Charlesworth MC, Mason KA, Ostrea T, Johnson L, Ryan RJ. A receptor-binding region in human choriogonadotropin/lutropin beta subunit. Proceedings of National Academy of Science USA. 1987;84:2038–2042. doi: 10.1073/pnas.84.7.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keutmann HT, Mason KA, Kitzmann K, Ryan RJ. Role of the β 93-100 determinant loop sequence in receptor binding and biological activity of human leutinizing hormone and chorionic gonadotropin. Mol Endocrinol. 1989;3:526–531. doi: 10.1210/mend-3-3-526. [DOI] [PubMed] [Google Scholar]
- Kleinau G, Jäschke H, Neumann S, Lättig J, Paschke R, Krause G. Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem. 2004;279:51590–51600. doi: 10.1074/jbc.M404748200. [DOI] [PubMed] [Google Scholar]
- Kleywegt GJ. Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr D. 1996;52:842–857. doi: 10.1107/S0907444995016477. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature. 1993;366:751–756. doi: 10.1038/366751a0. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374:183–186. doi: 10.1038/374183a0. [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, Morgan FJ, Isaacs NW. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Leinung MC, Reed DK, McCormick DJ, Ryan RJ, Morris JC. Further characterization of the receptor-binding region of the thyroid-stimulating hormone α subunit: a comprehensive synthetic peptide study of the α-subunit 26-46 sequence. Proc Natl Acad Sci U S A. 1991;88:9707–9711. doi: 10.1073/pnas.88.21.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Dias JA. Long loop residues 33–58 in the human glycoprotein hormone common α subunit contain structural components for subunit heterodimerization and human follitropin-receptor binding. Arch Biochem Biophys. 1996;329:127–135. doi: 10.1006/abbi.1996.0200. [DOI] [PubMed] [Google Scholar]
- Moyle WR, Campbell RK, Myers RV, Bernard MP, Han Y, Wang X. Co-evolution of ligand-receptor pairs. Nature. 1994;368:251–255. doi: 10.1038/368251a0. [DOI] [PubMed] [Google Scholar]
- Moyle WR, Campbell RK, Rao SNV, Ayad NG, Bernard MP, Han Y, Wang Y. Model of human chorionic gonadotropin and lutropin receptor interaction that explains signal transduction of the glycoprotein hormones. J Biol Chem. 1995;270:20020–20031. doi: 10.1074/jbc.270.34.20020. [DOI] [PubMed] [Google Scholar]
- Moyle WR, Xing Y, Lin W, Cao D, Myers RV, Kerrigan JE, Bernard MP. Model of glycoprotein hormone receptor ligand binding and signaling. J Biol Chem. 2004;279:44442–44459. doi: 10.1074/jbc.M406948200. [DOI] [PubMed] [Google Scholar]
- Moyle WR, Lin W, Myers RV, Cao D, Kerrigan JE, Bernard MP. Models of glycoprotein hormone receptor interaction. Endocrine. 2005;26:189–205. doi: 10.1385/ENDO:26:3:189. [DOI] [PubMed] [Google Scholar]
- Narayan P, Wu C, Puett D. Functional expression of yoked human chorionic gonadotropin in baculovirus-infected insect cells. Mol Endocrinol. 1995;9:1720–1726. doi: 10.1210/mend.9.12.8614408. [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Santa Coloma TA, Reichert LEJ. Identification of a follicle-stimulating hormone receptor-binding region in hFSH-β-(81–95) using synthetic peptides. J Biol Chem. 1990;265:5037–5042. [PubMed] [Google Scholar]
- Schmidt A, MacColl R, Lindau-Shepard B, Buckler DR, Dias JA. Hormone-induced conformational change of the purified soluble hormone binding domain of follitropin receptor complexed with single chain follitropin. J Biol Chem. 2001;276:23373–23381. doi: 10.1074/jbc.M100057200. [DOI] [PubMed] [Google Scholar]
- Schubert WD, Urbanke C, Ziehm T, Beier V, Machner MP, Domann E, Wehland J, Chakraborty T, Heinz DW. Structure of Internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Smits G, Govaerts C, Nubourgh I, Pardo L, Vassart G, Costagliola S. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol. 2002;16:722–735. doi: 10.1210/mend.16.4.0815. [DOI] [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22:2692–2703. doi: 10.1093/emboj/cdg260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Youn H, Jeoung M, Koo Y, Yi C, Ji I, Ji TH. Orientation of follicle-stimulating hormone (FSH) subunits complexed with the FSH receptor. J Biol Chem. 2003;278:47868–47876. doi: 10.1074/jbc.M307751200. [DOI] [PubMed] [Google Scholar]
- Song YS, Ji I, Beauchamp J, Isaacs NW, Ji TH. Hormone interactions to leu-rich repeats in the gonadotropin receptors. J Biol Chem. 2001;276:3426–3435. doi: 10.1074/jbc.M003772200. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Pixley MR, Minami S, Perlas E, Ben-Menahem D, Hsueh AJ, Boime I. Biosynthesis of a biologically active single peptide chain containing the human common α and chorionic gonadotropin β subunits in tandem. Proc Natl Acad Sci U S A. 1995;92:2041–2045. doi: 10.1073/pnas.92.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- Uff S, Clemetson JM, Harrison T, Clemetson KJ, Emsley J. Crystal structure of the platelet glycoprotein Ibα N-terminal domain reveals an unmasking mechanism for receptor activation. J Biol Chem. 2002;277:35657–35663. doi: 10.1074/jbc.M205271200. [DOI] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer HF, Granneman JCM, Bogerd J. Opposite contribution of two ligand-selective determinants in the N-terminal hormone-binding exodomain of human gonadotropin receptors. Mol Endocrinol. 2003a;17:1972–1981. doi: 10.1210/me.2003-0172. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Granneman JCM, Noordam MJ, Mosselman S, Bogerd J. Ligand selectivity of gonadotropin receptors. J Biol Chem. 2003b;278:15505–15513. doi: 10.1074/jbc.M300634200. [DOI] [PubMed] [Google Scholar]
- Wu H, Lustbader JW, Liu Y, Canfield RE, Hendrickson WA. Structure of human chorionic gonadotropin at 2.6 Å resolution from MAD analysis of the selenomethionyl protein. Structure. 1994;2:545–558. doi: 10.1016/s0969-2126(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Yoo J, Zeng H, Ji I, Murdoch WJ, Ji TH. COOH-terminal amino acids of the α subunit play common and different roles in human choriogonadotropin and follitropin. J Biol Chem. 1993;268:13034–13042. [PubMed] [Google Scholar]


