Abstract
Ion channels are effector proteins that mediate uterine excitability throughout gestation. This review will focus primarily on the role of potassium channels in regulating myometrial tone in pregnancy and labor contractions. During gestation, potassium channels maintain the uterus in a state of quiescence by contributing to the resting membrane potential and counteracting contractile stimuli. This review summarizes the current knowledge about the significance of the potassium channels in maintaining a normal gestational period and initiating labor contractions at term.
Keywords: Myometrium, Potassium channel, Pregnancy, Labor
1. Introduction
Potassium efflux from myometrial cells results in membrane repolarization and this efflux is the primary ionic current responsible for maintaining the resting membrane potential. Potassium channels comprise one group of proteins that contribute significantly to uterine quiescence during pregnancy. In myometrial smooth muscle cells (MSCs), changes in the expression or activity of K+ channels can translate into an inadequate repolarization leading to aberrant uterine activity. Thus, K+ channel alterations may contribute to certain pathophysiological conditions such as dystocia, preterm labor and post-term labor. These proteins are the focus of extensive research given their role in maintaining uterine quiescence throughout pregnancy and their potential role in the induction of myometrial contractions during labor.
In myometrial cells, K+ channels are an underlying component of the mechanism that allows for adaptation of the gravid uterus to increases in stretch and intrauterine pressure. To date, several types of K+ channels have been identified in the myometrium. The most abundant and well studied include the large-conductance calcium- and voltage-sensitive K+ channel (BKCa channel), the ATP-sensitive K+ channel (KATP), the Shaker-like voltage-gated potassium channels (Kv), and small-conductance calcium-sensitive potassium channels (SK). The variety of K+ channels in this tissue reflects the multiplicity and complexity of the mechanisms involved in the regulation of uterine tonus. Because K+ channels are widely represented in the myometrium, molecular mechanisms that regulate potassium channels in pregnancy will be the main focus of this review; however other channels will be described briefly.
2. Potassium channel regulation and function
2.1 BKCa channels
The BKCa channels (also known as maxi-K and slo) are large-conductance, voltage- and calcium-sensitive K+ channels. They are one of the most extensively studied ion channels in uterine smooth muscle due to their abundance and significant repolarizing current. Relatively few BKCa channels need to be activated to produce uterine relaxation; thus these channels can have profound effects on myometrial activity. Multiple cellular regulators with uterotonic actions modulate this channel, suggesting that the channel’s major physiological role is to induce smooth muscle relaxation in response to depolarization and to mediate the action of uterotonic substances.
The contribution of the BKCa channel to the total cell K+ current varies depending upon gestational stage. In rat non-pregnant myocytes, BKCa channels contribute approximately 35% to whole cell repolarizing K+ current; however by late gestation, electrophysiological measurements show that loss of BKCa-generated currents is concomitant with an increased contribution of other K+ channel types to maintain uterine quiescence [1]. Contractile studies in mid- and late pregnant rats show that the BKCa opener NS1619 and inhibitor iberiotoxin (IbTX) do not alter spontaneous contractile activity induced by prostaglandins [2]. Complementary studies have demonstrated that BKCa has a more pronounced relaxant effect in mid-gestation versus late gestation after activation with adenylate cyclase [3]. These data all indicate an attenuated role for these channels in maintaining uterine quiescence in term gravid uterus from rats. Similar to rat myometrium, BKCa current is suppressed in late-pregnant mice compared to non-pregnant mice, however BKCa transcript and protein expression was enhanced suggesting that multiple mechanisms regulate BKCa channel function [4].
The modulation of BKCa current during pregnancy in many of these studies may result from altered sensitivity to cell signaling molecules known to activate channel activity. BKCa channels mediate uterine relaxation in response to adenylyl cyclase (AC) activation, which was present only in midterm but not late pregnancy or labor [3]. Thus, differential expression of AC in gestation (see Lopez Bernal 2007, this issue), or altered sensitivity of the BKCa channel to downstream signaling of AC, may explain differential activity of BKCa during uterine quiescence in the rat model of pregnancy. Nitric oxide (NO) also mediates uterine relaxation in pregnancy via activation of BKCa channels, and this effect depends on gestational stage. Nitric oxide was less effective in inducing uterine relaxation in late compared to mid-gestation rats [5]. In human pregnant myocytes, the open state probability of the BKCa increases in response to an NO donor [6]. Currently, it is not known whether NO activation of myometrial BKCa channels is by direct interaction and/or indirect activation via cyclic cGMP dependent pathways [6,7]; however it is apparent that BKCa channels are altered during pregnancy to hone their response to signaling molecules.
Hormonal regulation of myometrial tone in gestation depends, in part, on modulation of activity and/or expression of ion channels. Specifically, various hormones modulate BKCa channels to aid in the maintenance of pregnancy. Sex hormones and hormones of the hypothalamus-pituitary-adrenal axis affect uterine tone in part by regulating expression of BKCa gene products [8]. Carvajal et al [9] observed that chorion exerted a relaxant effect in pregnant myometrium pre-contracted with oxytocin. This relaxation depended on activation of BKCa channels, suggesting that relaxin generated by the feto-placental unit and ovaries during gestation activates BKCa channels. This result was verified in cultured human myometrial smooth muscle cells where relaxin increased open state probability of BKCa channels via a PKA-mediated pathway [10].
Molecular and cellular studies have elucidated that differential regulation of BKCa channels during pregnancy results from various mechanisms including alternative splicing, association with accessory ß-subunits, post-translational modification, and association with proteins that regulate channel expression and current phenotype. The BKCa channel is encoded by a single gene, KCNMA1 (also referred to as slowpoke) and derives its diversity by alternative splicing of its pre-mRNA. Splicing of the BKCa transcript results in functionally distinct variants (Figure 1A), which differ in their calcium and voltage sensitivity [11], hormonal sensitivity [12, 13], ability to be phoshorylated [14] and trafficking [15].
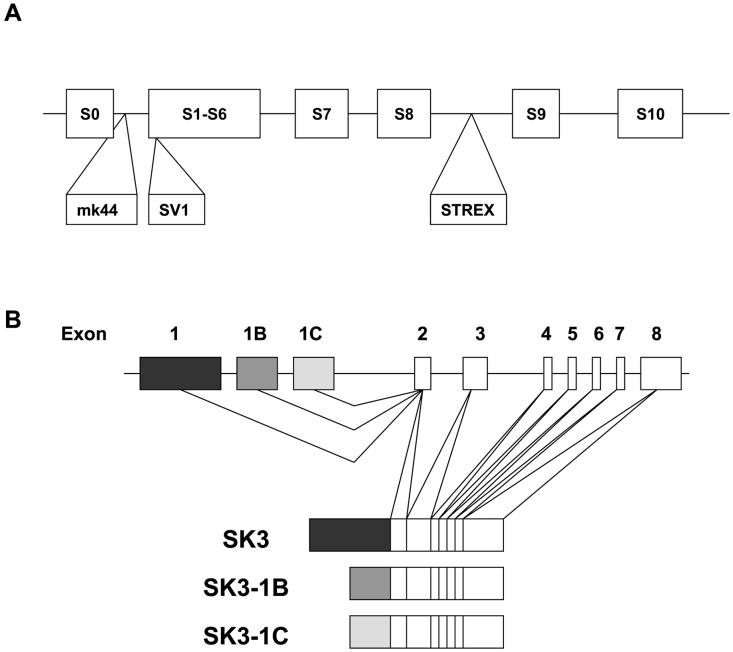
Figure 1.
(A) Isoforms of the BKCa channel, mK44, SV1, and STREX are the protein products resulting from alternative splicing of the KCNMA gene. S0-S6 represents hydrophobic transmembrane spanning domains, while S7-S10 represents intracellular hydrophobic domains. (B) Isoforms of the SK3 channel derived from alternative start codons (adapted from 51and 52).
Alternative splicing of the BKCa channel may be a molecular mechanism by which this channel is able to respond to a wide variety of regulatory substances in a tissue specific manner. Multiple splice variants have been identified; however few studies have focused on their function in myometrium during pregnancy. Starting at mid-gestation, BKCa isoforms with decreased sensitivity to Ca2+ increase in mouse myometrium [4]. While human BKCa isoforms with decreased sensitivity do not show a higher level of expression until the onset of labor, they allow the uterus to be in a more excitable state during labor [11, 16]. Recent studies indicate that specific BKCa isoforms may function as myometrial-specific proteins. In humans, the mK44 isoform contains 44 additional amino acids in the first intracellular loop and is less sensitive to Ca2+ and voltage compared to BKCa channels lacking these 44 amino acids [11]. Although the levels of BKCa channel transcript decrease towards term in humans [17], the proportion that mK44 transcript comprises is up-regulated at late-term, suggesting that this isoform may modulate uterine activity near labor [16]. Recent studies in human myometrial cells have shown that mK44 is proteolytically cleaved, resulting in membrane localization of the N-terminus with retention of the C-terminus in the endoplasmic reticulum [18]. In response to an excitatory Ca2+ signal, the C-terminus translocates to the plasma membrane and reconstitutes with the N-terminus to produce a repolarizing current that induces uterine relaxation, underscoring the potential for myometrial specific regulation of BKCa channels [18].
Other splice variants have been identified that are not myometrial specific but could play an important role in myometrial excitability during gestation. The STREX isoform introduces 59 amino acids into the linker between S8 and S9 [13] and is regulated during pregnancy [19] by ACTH, 17ß-estradiol, and progesterone [12],[8, 20]. This variant introduces a second consensus PKA phosphorylation motif, which allows PKA phosphorylation to inhibit the activity of the channel [21]. Although alternative splicing is a way to gain variability from single gene products, it may also regulate protein expression. A splice variant termed SV1, in which 33-amino acids are inserted in the S1 transmembrane domain, caused retention of the channel in the ER, acting as a dominant-negative isoform [15]. Expression of BKCa isoforms with lower sensitivity to Ca2+ would be another explanation for Ca2+-insensitive BKCa currents in laboring myometrium [19].
Dynamic association of the BKCa channel with an accessory beta subunit (ß-subunit) is another molecular mechanism that provides diversity of BKCa function in MSCs. Four distinct genes, KCNMB1-4, encode the BKCa channel beta subunits with the ß1 subunit being the predominant isoform present in myometrium [22]. The ß1 subunit of the BKCa channel increases the voltage and calcium sensitivity of the channel [23] resulting in enhanced activation of BKCa channels to dampen uterine excitability and attenuate contractions. In addition, the ß1 subunit is regulated by 17ß-estradiol [4] and facilitates binding of 17ß-estradiol to the BKCa channel, but the exact mechanism remains controversial [24]. In mouse myometrium, the ß1 subunit transcript and protein are up-regulated at mid-gestation with post-partum expression returning to non-pregnant levels [4]. In human gravid uterus, expression of the ß1 subunit was lower during term and preterm labor compared to a non-laboring state [17]. This protein may play a role in maintaining uterine quiescence in mid- and late pregnancy when intrauterine pressure and myometrial stretch gradually reach maximum. Uncoupling of BKCa channels from their ß1 subunits would generate channels with low sensitivity to Ca2+. During human term and pre-term labor, however, a decrease in expression of both proteins, but not uncoupling, appears to be one mechanism to induce contractions [17].
Variability in channel behavior among distinct BKCa isoforms can also be in part due to disparate post-translational modification, which may underlie changes in uterine relaxant activity discussed earlier in this chapter. Enhanced activity of BKCa channels in pregnancy is often due to differential phosphorylation rather than an increase in BKCa expression levels. The membrane-bound PKG activates BKCa channels in human non-laboring myometrium thus contributing to myometrial quiescence during gestation [25]. Similarly, in rats, BKCa isoforms inhibited by PKA phosphorylation decreased throughout pregnancy whereas expression of isoforms activated by PKA peaked at mid-gestation [20].
Association of BKCa channels with membrane and intracellular proteins is currently emerging as a novel mechanism to regulate uterine relaxation. Beta-receptor agonists are commonly used in clinical practice to postpone pre-term labor. In myometrium, ß2- and ß3-receptors have been reported to form a functional complex with BKCa channels to induce channel activation and uterine relaxation in response to activation of beta-receptors [26,27]. Inhibition of BKCa channels with the specific blocker iberiotoxin (IbTX) abolished the effects of the ß3-receptor activation in both myometrial cells and uterine tissue [26]. Immunoprecipitations suggest a physical association of receptors between BKCa channels and ß2-receptors, however how this alters BKCa current directly is not known [27]. Both studies implicate an important role for this channel in tocolytic therapy with ß-adrenergic agents. Also, myometrial BKCa channels are present in caveolae and clustering of receptors and channels within these signaling structures may be one mechanism for receptor-channel interactions in the absence of direct association [28].
Another explanation for diminished BKCa current at late gestation is attenuated membrane channel expression [29]; however this appears to be species-specific. In pregnant rats, unlike in mice, inhibition of BKCa current was due to down-regulation of channel transcript and protein expression [29]. In humans, increased levels of BKCa channels in late pregnant non-laboring were observed yet decreased BKCa channel expression was seen in pre-term and term laboring uterus indicating that these channels may be a part of the mechanism to induce spontaneous labor contractions [17,30]. However, channel expression and intracellular regulation are not mutually exclusive. Myocytes isolated from human laboring myometrium express BKCa channels with low sensitivity to Ca2+ and inhibitors [31]. Recent studies have discovered that the current phenotype measured in laboring tissue can be mimicked by measuring BKCa current in the presence of actin depolymerizing agents [28]. The recent linkage of myometrial BKCa channels activity with the cytoskeletal components may elucidate other mechanisms to regulate uterine contraction [28,31].
Alternative splicing, ß-subunit expression, post-translational modifications and differential expression on the cell surface are only some of the mechanisms identified that regulate BKCa channel function in MSCs. Association with certain membrane and intracellular structures, such as caveolae and interaction with receptors are also mechanisms that will need to be further explored to understand the complex nature of BKCa channel regulation in uterine function.
KATP channels
Functional studies indicate that the ATP-sensitive inward rectifying potassium channels (KATP) have an important role in regulation of myometrial quiescence during pregnancy. The inward rectifier K+ channel family Kir6 comprises the potassium channel component of the KATP, while the sulfonylurea receptor (SUR) is responsible for the ATP sensitivity, pharmacological properties, and trafficking of this channel [32-34] (Figure 2). The predominant myometrial isoform is Kir6.1/SUR2B, although Kir6.2/SUR1 has been detected at the transcript level [35, 36]. KATP in smooth muscle maintains basal membrane potential despite a relatively low open state probability [37]. Studies have also shown that Kir6.1/SUR2B is down regulated late in pregnancy, which could facilitate enhanced myometrial activity necessary for labor contractions [36]. This channel is inhibited by intracellular ATP and stimulated by MgADP, thus coupling metabolic state to changes in cellular excitability [38]. In other smooth muscle types, these channels have also been linked to smooth muscle relaxation in hypoxia due to a precipitous drop in ATP [39], however whether this occurs in myometrium is not known.
Figure 2.
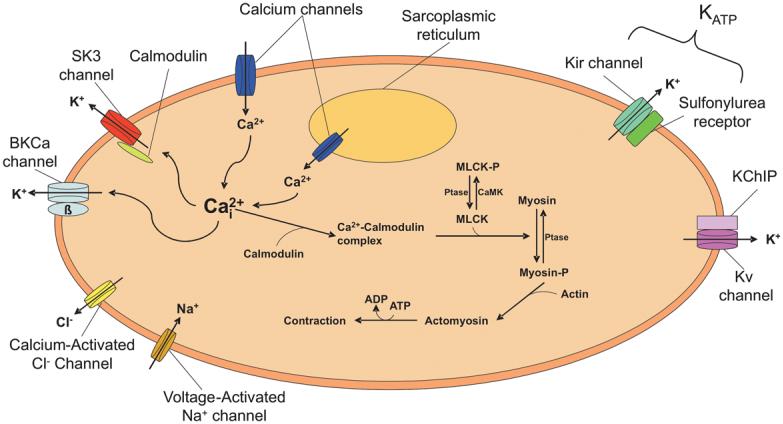
A schematic figure of a myometrial smooth muscle cell showing a variety of ion channels that are involved in regulation of membrane potential and cell excitability. Protein phosphatase (Ptase), myosin light chain kinase (MLCK), phosphorylated myosin light chain kinase (MLCK-P), calcium calmodulin-dependent protein kinase (CamK), Kv channel interacting protein (KChIP).
Over-expression of KATP subunits Kir.6.1 and SUR2B contribute to an inhibition of oxytocin-induced contractions in late pregnant but not term-pregnant rats [40]. Similarly, in the human uterus, KATP channels comprised primarily of Kir6.1 and SUR2B subunits, are expressed at higher levels in non-pregnant myometrium compared to late gestation [36]. These findings may indicate that in humans, down-regulation of KATP channels may contribute to increased uterine excitability and induction of labor contractions in term pregnancy. In human myometrium, the expression of KATP depends on both the gestational stage and the presence of labor contractions [41]. Pinacidil, a KATP opener, inhibits oxytocin-induced contractions in late pregnant non-laboring myometrium [41]. This effect was attenuated in laboring myometrium from term and preterm uteri. Between the two latter groups, inhibition was more pronounced in term labor uterine strips. The differential effects of pinacidil may reflect altered activity and/or expression levels of KATP channels in gestation and labor [41].
The regulation of KATP channels in the myometrium may also differ in spontaneous versus agonist-induced contractions. Kafali et al [42] found that in late pregnant rats, KATP channels may mediate the relaxation phase of spontaneously induced contractions. In mid- and late pregnant rat myometrium neither the KATP opener levcromakalim or KATP inhibitor glibenclamide had an effect on PGF E2 and PGF I2-induced contractions [2]. These authors concluded that in mid- and late pregnant rats, KATP are not a part of the mechanism to regulate protstaglandin-induced uterine contractions. KATP channel activity during pregnancy also may depend on second-messengers. In late pregnant rat myometrium KATP channels mediate uterine relaxation in response to activation of adenylyl cyclase (AC) and guanylyl cyclase (GC) [3]. GC was expressed at all stages of gestation with AC present only in midterm but not during late pregnancy or labor. Thus, differential regulation of AC and GC in gestation may explain the regulation in uterine quiescence by KATP channels in a rat model of pregnancy. KATP activation resulted in equally potent relaxation in midterm, late term or laboring uterus in rats [3]. This finding suggests that, unlike in humans, KATP is not down-regulated during labor in rats. Inhibition of contractions by KATP depended both on AC and GC. Further molecular and cellular characterization of this channel in combination with functional experiments will aid in understanding the regulation of this channel during pregnancy.
Small conductance Ca2+ sensitive potassium channels
Small conductance Ca2+ sensitive and voltage insensitive potassium channels (SK) generate a hyperpolarizating current in excitable cells following action potential generation, and thus may induce relaxation of smooth muscle. SK channels belong to the KCNN family of potassium channels and derive their molecular diversity from both being encoded by multiple genes and utilizing alternative start codons within each gene [43] (Figure 1B). SK channels constitutively associate with calmodulin, which mediates gating of the channels through binding of calcium [44]. Even though SK channels do not possess a consensus calmodulin-binding motif, a calmodulin binding site has been mapped to a conserved stretch of amino acids in the cytoplasmic C-termini [45]. In addition to gating, calmodulin is also involved in the trafficking of SK channels to the plasma membrane [46].
SK channels recently emerged as critical regulators of myometrial contractility during gestation and labor. Transgenic mice over-expressing isoform 3 of SK channels (SK3) have a distinct phenotype of compromised labor possibly due to inefficient labor contractions [47]. In human non-pregnant and pregnant myometrium, apamin, an inhibitor of SK channels, attenuated relaxation induced by NO [48, 49]. Recent studies have demonstrated that SK3 expression depresses phasic contractions in mouse uterus by limiting the influx of Ca2+ [50]. In PC12 cell line, the SK3-1B splice variant of the SK3 channel silences endogenous SK current by acting in a dominant-negative fashion [51]. Another splice variant of the SK3 channel, SK3-1C, suppressed the membrane expression of SK1, SK2, SK3 and intermediate conductance Ca2+-activated K+ channels by sequestering the protein intracellularly [52]. Modulation of SK channels expression and function during pregnancy has not been studied to date. Thus, further investigation into the function of SK channels in modulating uterine function and parturition will be intriguing.
Voltage-gated potassium channels (Kv channels)
Voltage-dependent K+ channels (Kv) are widely expressed in uterine smooth muscle. In response to depolarization, efflux of K+ through these channels induces repolarization of the MSCs to resting membrane potential. Voltage-gated potassium channels contribute to resting membrane potential in myocytes and may play a role in maintaining uterine quiescence before labor [53]. In MSCs, small depolarizations open L-type Ca2+ channels in MSCs resulting in enhanced Kv channel activity, which is important for da mpening cell excitation and maintaining basal uterine tone. Phenotypically, there are several types of voltage-gated K+ currents, which differ in their kinetics and pharmacological sensitivity. In human myometrium, two main types of Kv currents have been identified using electrophysiological methods, delayed rectifiers and rapidly inactivating currents [1,54,55].
The Kv channel subfamily that appears to play the most predominant role in the myometrium during pregnancy has been the Kv4 subfamily. Electrophysiological studies have described rapidly inactivating K+ current in rat pregnant myometrium generated by the Kv4 family of K+ channels [56]. Of this channel subfamily, Kv4.2 and their accessory subunits increased before parturition, whereas Kv4.1 and Kv4.3 expression declined during gestation suggesting that soluble factors, possibly hormones, regulate Kv4 channels in pregnancy [56]. This was further supported by research showing that in ovariectomized rats, estrogen down-regulated membrane localization, protein and current expression of Kv4.3 channels [57]. These findings suggested that prior to parturition elevated estrogen levels increase myometrial contractility by inhibiting function of Kv4.3 channels. Similarly, in cultured human myometrial cells, 17ß-estradiol, but not progesterone, inhibited currents generated by Kv channels thus increasing excitability of the MSC membranes [55].
Kv4 channels associate with many different subunits, most of which have been studied in tissues other than the myometrium [58-60]. One of these subunits is the Kv channel interacting proteins (KChIPs), which are calcium-binding proteins [61]. Two isoforms, KChIP2 and 4 have been found in the myometrium [56]. In rats, KChIP2 is upregulated in late gestation, and enhances Kv4 channels cell membrane density by trafficking channels from the endoplasmic reticulum to the cell membrane. KChIP also alters channel kinetics to allow greater Kv4 channel current [61,62]. The KChIP proteins may play a significant role in reducing myometrial excitability and potentially dampening uterine contractions.
Two pore channels
The role of other potassium channels in regulation of myometrial contractility in gestation and parturition remains unresolved. Two pore domain channels (2P) are inward rectifying K+ channels sensitive to pH, hypoxia and stretch. Similar to KATP and Kv channels, they may set a resting membrane potential in myometrial smooth muscle cells. The isoforms 1, 4 and 5 of TWIK-related acid-sensitive K+ channels (TASK) and isoform 1 of TWIK-related K+ channels (TREK) have been identified in human pregnant myometrium [63], however their role during pregnancy needs further elucidation.
3. Regulation of Na+ and Cl- channels present in the myometrial smooth muscle
Other channels discovered in pregnant myometrium are chloride (Cl-), sodium (Na+), non-specific cation, and calcium (Ca2+) channels, the latter being covered by other reviews in this journal. Functionally, Na+ channels generate fast inward current resulting in membrane depolarization. The expression of the two voltage-gated Na+ channels (Nav2.1, Nav2.3) has been reported in human and mouse myometrium [64,65], however the role for Na+ channels in regulating uterine tone in pregnancy is not well understood since the majority of these channels are inactivated at the resting membrane potential. Several studies in rats have shown an increase in Na+ current during gestation. Martin C. et al [66] reported that both functional and non-functional Na+ channels are present in rat pregnant uterus based on identification of high and low affinity binding sites for the Na+ channel inhibitors, tetrodotoxin (TTX) and saxitoxin. However other studies using the same preparations of rat uterine cells, did not find Na+ channels with a low sensitivity to TTX [67, 68]. Electrophysiological studies detected both fast inactivating and TTX-sensitive Na+ currents in myocytes of pregnant rats that increased throughout gestation, and these studies also reported a subpopulation of myocytes lacking Na+ channels. These authors concluded that in gestation, Na+ current density increases towards term in part due to a higher number of cells expressing Na+ channels. They also suggest that, besides faster action potential propagation in labor, Na+ channels may augment uterine excitability through an elevation of intracellular Ca2+ due to activation of Na+/Ca2+ exchanger in response to an increase in transmembrane transport of Na+. These hypotheses remain untested.
Yoshino M. et al [69] have demonstrated the presence of Na+ channels in non-pregnant uterine myocytes following estrogen stimulation with augmentation and faster kinetics as pregnancy progressed. These currents peaked at term and were nearly undetectable postpartum. This study concluded that a combination of mechanisms, including increased Na+ current density and inhibition of expression of K+ channels result in a more excitable myometrium starting mid-gestation and promotes coordinated uterine contractions during labor. While there is disagreement about the existence of the subpopulation of uterine myocytes lacking Na+ channels, there is general consensus that high Na+ current density close to labor may contribute to generation of coordinated uterine contractions necessary for expulsion of the fetus. The distinct roles of Nav2.1 and Nav2.3 channels in gestation and labor is unclear since these channels have not been functionally characterized and the study of these channels in pregnancy remains an area for further investigation.
The contribution of chloride ions to the regulation of myometrial tone has been acknowledged in the past. The Ca2+ -dependent Cl- channels have been identified in human myometrial smooth muscle cells [70-72]. In response to an increase in intracellular Ca2+, Cl- channels generate an outward current, which results in depolarization of the cell membrane and increased excitability of the myometrium. These channels may regulate the pacemaker activity, thus consequently affecting spontaneous uterine contractions by increasing their amplitude and frequency [71,72]. However, a role for Cl- channels in regulation of uterine contractions remains unclear due to the absence of selective inhibitors/openers of Cl- channels, the lack of selectivity of the channels for Cl- ions, and the uncertainty concerning their molecular structure [73].
SUMMARY
Ion channels are a part of the molecular mechanism(s) regulating uterine smooth muscle excitability in gestation and labor (Figure 2). Potassium channels, in particular, are crucial in maintaining myometrial quiescence in pregnancy. Dysfunction or decreased expression of K+ channels would diminish MSC repolarizing current resulting in induction of premature uterine contractions and pre-term labor. Over-expression of K+ channels current late in pregnancy may suppress coordinated myometrial activity needed for term labor contractions. The voltage-sensitive Na+ and Cl- channels may contribute to spontaneous uterine contractions by supporting a pacemaker activity of uterine smooth muscle cells, and thus, also be important in sustaining labor contractions. Myometrial ion channels represent a promising subject for therapeutic manipulation to treat and/or prevent pre- and post-term labor. However, many other tissues, besides myometrium, express these channels. Therefore it is important to discover uterine-specific mechanisms that regulate K+, Na+ and Cl- channels in order to aid in the alleviation of conditions leading to uterine dysfunction.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (HD-037831 to S.K.E.) and the March of Dimes (#21-FY04-169 to S.K.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- [1].Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J Gen Physiol. 1998;112:737–56. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bailie CA, Vedernikov YP, Saade GR, Garfield RE. Prostaglandin-induced activation of uterine contractility in pregnant rats does not involve potassium channels. Am J Obstet Gynecol. 2002;186:453–7. doi: 10.1067/mob.2002.120484. [DOI] [PubMed] [Google Scholar]
- [3].Okawa T, Longo M, Vedernikov YP, Chwalisz K, Saade GR, Garfield RE. Role of nucleotide cyclases in the inhibition of pregnant rat uterine contractions by the openers of potassium channels. Am J Obstet Gynecol. 2000;182:913–8. doi: 10.1016/s0002-9378(00)70346-2. [DOI] [PubMed] [Google Scholar]
- [4].Benkusky NA, Korovkina VP, Brainard AM, England SK. Myometrial maxi-K channel beta1 subunit modulation during pregnancy and after 17beta-estradiol stimulation. FEBS Lett. 2002;524:97–102. doi: 10.1016/s0014-5793(02)03011-9. [DOI] [PubMed] [Google Scholar]
- [5].Okawa T, Vedernikov YP, Saade GR, Longo M, Olson GL, Chwalisz K, et al. Roles of potassium channels and nitric oxide in modulation of uterine contractions in rat pregnancy. Am J Obstet Gynecol. 1999;181:649–55. doi: 10.1016/s0002-9378(99)70508-9. [DOI] [PubMed] [Google Scholar]
- [6].Shimano M, Nakaya Y, Fukui R, Kamada M, Hamada Y, Maeda K, et al. Activation of Ca2+-activated K+ channels in human myometrium by nitric oxide. Gynecol Obstet Invest. 2000;49:249–54. doi: 10.1159/000010254. [DOI] [PubMed] [Google Scholar]
- [7].Schiemann WP, Westfall DP, Buxton IL. Smooth muscle adenosine A1 receptors couple to disparate effectors by distinct G proteins in pregnant myometrium. Am J Physiol. 1991;261:E141–50. doi: 10.1152/ajpendo.1991.261.1.E141. [DOI] [PubMed] [Google Scholar]
- [8].Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–6. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- [9].Carvajal JA, Thompson LP, Weiner CP. Chorion-induced myometrial relaxation is mediated by large-conductance Ca2+-activated K+ channel opening in the guinea pig. Am J Obstet Gynecol. 2003;188:84–91. doi: 10.1067/mob.2003.102. [DOI] [PubMed] [Google Scholar]
- [10].Meera P, Anwer K, Monga M, Oberti C, Stefani E, Toro L, et al. Relaxin stimulates myometrial calcium-activated potassium channel activity via protein kinase A. Am J Physiol. 1995;269:C312–7. doi: 10.1152/ajpcell.1995.269.2.C312. [DOI] [PubMed] [Google Scholar]
- [11].Korovkina VP, Fergus DJ, Holdiman AJ, England SK. Characterization of a novel 132-bp exon of the human maxi-K channel. Am J Physiol Cell Physiol. 2001;281:C361–7. doi: 10.1152/ajpcell.2001.281.1.C361. [DOI] [PubMed] [Google Scholar]
- [12].Holdiman AJ, Fergus DJ, England SK. 17beta-Estradiol upregulates distinct maxi-K channel transcripts in mouse uterus. Mol Cell Endocrinol. 2002;192:1–6. doi: 10.1016/s0303-7207(02)00136-3. [DOI] [PubMed] [Google Scholar]
- [13].Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J Biol Chem. 1997;272:11710–7. doi: 10.1074/jbc.272.18.11710. [DOI] [PubMed] [Google Scholar]
- [14].Perez G, Toro L. Differential modulation of large-conductance KCa channels by PKA in pregnant and nonpregnant myometrium. Am J Physiol. 1994;266:C1459–63. doi: 10.1152/ajpcell.1994.266.5.C1459. [DOI] [PubMed] [Google Scholar]
- [15].Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276:16232–9. doi: 10.1074/jbc.M008852200. [DOI] [PubMed] [Google Scholar]
- [16].Curley M, Morrison JJ, Smith TJ. Analysis of Maxi-K alpha subunit splice variants in human myometrium. Reprod Biol Endocrinol. 2004;2:67. doi: 10.1186/1477-7827-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matharoo-Ball B, Ashford ML, Arulkumaran S, Khan RN. Down-regulation of the alpha- and beta-subunits of the calcium-activated potassium channel in human myometrium with parturition. Biol Reprod. 2003;68:2135–41. doi: 10.1095/biolreprod.102.010454. [DOI] [PubMed] [Google Scholar]
- [18].Korovkina VP, Brainard AM, England SK. Translocation of an endoproteolytically cleaved maxi-K channel isoform: mechanisms to induce human myometrial cell repolarization. J Physiol. 2006;573:329–41. doi: 10.1113/jphysiol.2006.106922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benkusky NA, Fergus DJ, Zucchero TM, England SK. Regulation of the Ca2+-sensitive domains of the maxi-K channel in the mouse myometrium during gestation. J Biol Chem. 2000;275:27712–9. doi: 10.1074/jbc.M000974200. [DOI] [PubMed] [Google Scholar]
- [20].Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 2005;579:4856–60. doi: 10.1016/j.febslet.2005.07.069. [DOI] [PubMed] [Google Scholar]
- [21].Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, et al. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–20. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- [22].Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- [23].McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–50. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- [24].Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–31. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- [25].Zhou XB, Wang GX, Ruth P, Huneke B, Korth M. BK(Ca) channel activation by membrane-associated cGMP kinase may contribute to uterine quiescence in pregnancy. Am J Physiol Cell Physiol. 2000;279:C1751–9. doi: 10.1152/ajpcell.2000.279.6.C1751. [DOI] [PubMed] [Google Scholar]
- [26].Doheny HC, Lynch CM, Smith TJ, Morrison JJ. Functional coupling of beta3-adrenoceptors and large conductance calcium-activated potassium channels in human uterine myocytes. J Clin Endocrinol Metab. 2005;90:5786–96. doi: 10.1210/jc.2005-0574. [DOI] [PubMed] [Google Scholar]
- [27].Chanrachakul B, Pipkin FB, Khan RN. Contribution of coupling between human myometrial beta2-adrenoreceptor and the BK(Ca) channel to uterine quiescence. Am J Physiol Cell Physiol. 2004;287:C1747–52. doi: 10.1152/ajpcell.00236.2004. [DOI] [PubMed] [Google Scholar]
- [28].Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289:C49–57. doi: 10.1152/ajpcell.00399.2004. [DOI] [PubMed] [Google Scholar]
- [29].Eghbali M, Toro L, Stefani E. Diminished surface clustering and increased perinuclear accumulation of large conductance Ca2+-activated K+ channel in mouse myometrium with pregnancy. J Biol Chem. 2003;278:45311–7. doi: 10.1074/jbc.M306564200. [DOI] [PubMed] [Google Scholar]
- [30].Chanrachakul B, Matharoo-Ball B, Turner A, Robinson G, Broughton-Pipkin F, Arulkumaran S, et al. Immunolocalization and protein expression of the alpha subunit of the large-conductance calcium-activated potassium channel in human myometrium. Reproduction. 2003;126:43–8. doi: 10.1530/rep.0.1260043. [DOI] [PubMed] [Google Scholar]
- [31].Khan RN, Smith SK, Morrison JJ, Ashford ML. Ca2+ dependence and pharmacology of large-conductance K+ channels in nonlabor and labor human uterine myocytes. Am J Physiol. 1997;273:C1721–31. doi: 10.1152/ajpcell.1997.273.5.C1721. [DOI] [PubMed] [Google Scholar]
- [32].Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–7. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- [33].Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Sulfonylurea receptors set the maximal open probability, ATP sensitivity and plasma membrane density of KATP channels. FEBS Lett. 1999;445:131–6. doi: 10.1016/s0014-5793(99)00102-7. [DOI] [PubMed] [Google Scholar]
- [34].Clement JPt, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, et al. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–38. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- [35].Chien EK, Zhang Y, Furuta H, Hara M. Expression of adenosine triphosphate-sensitive potassium channel subunits in female rat reproductive tissues: overlapping distribution of messenger ribonucleic acid for weak inwardly rectifying potassium channel subunit 6.1 and sulfonylurea-binding regulatory subunit 2. Am J Obstet Gynecol. 1999;180:1121–6. doi: 10.1016/s0002-9378(99)70604-6. [DOI] [PubMed] [Google Scholar]
- [36].Curley M, Cairns MT, Friel AM, McMeel OM, Morrison JJ, Smith TJ. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol Hum Reprod. 2002;8:941–5. doi: 10.1093/molehr/8.10.941. [DOI] [PubMed] [Google Scholar]
- [37].Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617–24. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dunne MJ, Petersen OH. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986;208:59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- [39].Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–4. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- [40].Sawada K, Morishige K, Hashimoto K, Tasaka K, Kurachi H, Murata Y, et al. Gestational change of K+ channel opener effect is correlated with the expression of uterine KATP channel subunits. Eur J Obstet Gynecol Reprod Biol. 2005;122:49–56. doi: 10.1016/j.ejogrb.2004.11.026. [DOI] [PubMed] [Google Scholar]
- [41].Longo M, Jain V, Vedernikov YP, Hankins GD, Garfield RE, Saade GR. Effects of L-type Ca(2+)-channel blockade, K(+)(ATP)-channel opening and nitric oxide on human uterine contractility in relation to gestational age and labour. Mol Hum Reprod. 2003;9:159–64. doi: 10.1093/molehr/gag023. [DOI] [PubMed] [Google Scholar]
- [42].Kafali H, Kaya T, Gursoy S, Bagcivan I, Karadas B, Sarioglu Y.The role of K(+) channels on the inhibitor effect of sevoflurane in pregnant rat myometrium Anesth Analg 200294174–8. table of contents [DOI] [PubMed] [Google Scholar]
- [43].Shmukler BE, Bond CT, Wilhelm S, Bruening-Wright A, Maylie J, Adelman JP, et al. Structure and complex transcription pattern of the mouse SK1 K(Ca) channel gene, KCNN1. Biochim Biophys Acta. 2001;1518:36–46. doi: 10.1016/s0167-4781(01)00166-x. [DOI] [PubMed] [Google Scholar]
- [44].Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–7. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- [45].Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, et al. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J Neurosci. 1999;19:8830–8. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee WS, Ngo-Anh TJ, Bruening-Wright A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin: cell surface expression and gating. J Biol Chem. 2003;278:25940–6. doi: 10.1074/jbc.M302091200. [DOI] [PubMed] [Google Scholar]
- [47].Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–6. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- [48].Modzelewska B, Kostrzewska A, Sipowicz M, Kleszczewski T, Batra S. Apamin inhibits NO-induced relaxation of the spontaneous contractile activity of the myometrium from non-pregnant women. Reprod Biol Endocrinol. 2003;1:8. doi: 10.1186/1477-7827-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Modzelewska B, Kleszczewski T, Kostrzewska A. The effect of a selective inhibition of potassium channels on the relaxation induced by nitric oxide in the human pregnant myometrium. Cell Mol Biol Lett. 2003;8:69–75. [PubMed] [Google Scholar]
- [50].Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, et al. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol. 2007;292:C832–40. doi: 10.1152/ajpcell.00268.2006. [DOI] [PubMed] [Google Scholar]
- [51].Tomita H, Shakkottai VG, Gutman GA, Sun G, Bunney WE, Cahalan MD, et al. Novel truncated isoform of SK3 potassium channel is a potent dominant-negative regulator of SK currents: implications in schizophrenia Mol Psychiatry 20038524–35. 460 [DOI] [PubMed] [Google Scholar]
- [52].Kolski-Andreaco A, Tomita H, Shakkottai VG, Gutman GA, Cahalan MD, Gargus JJ, et al. SK3-1C, a dominant-negative suppressor of SKCa and IKCa channels. J Biol Chem. 2004;279:6893–904. doi: 10.1074/jbc.M311725200. [DOI] [PubMed] [Google Scholar]
- [53].Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med. 1997;216:57–64. doi: 10.3181/00379727-216-44156. [DOI] [PubMed] [Google Scholar]
- [54].Knock GA, Smirnov SV, Aaronson PI. Voltage-gated K+ currents in freshly isolated myocytes of the pregnant human myometrium. J Physiol. 1999;518(Pt 3):769–81. doi: 10.1111/j.1469-7793.1999.0769p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Knock GA, Tribe RM, Hassoni AA, Aaronson PI. Modulation of potassium current characteristics in human myometrial smooth muscle by 17beta-estradiol and progesterone. Biol Reprod. 2001;64:1526–34. doi: 10.1095/biolreprod64.5.1526. [DOI] [PubMed] [Google Scholar]
- [56].Suzuki T, Takimoto K. Differential expression of Kv4 pore-forming and KChIP auxiliary subunits in rat uterus during pregnancy. Am J Physiol Endocrinol Metab. 2005;288:E335–41. doi: 10.1152/ajpendo.00250.2004. [DOI] [PubMed] [Google Scholar]
- [57].Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, et al. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–90. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- [58].Yang EK, Alvira MR, Levitan ES, Takimoto K. Kvbeta subunits increase expression of Kv4.3 channels by interacting with their C termini. J Biol Chem. 2001;276:4839–44. doi: 10.1074/jbc.M004768200. [DOI] [PubMed] [Google Scholar]
- [59].Lundby A, Olesen SP. KCNE3 is an inhibitory subunit of the Kv4.3 potassium channel. Biochem Biophys Res Commun. 2006;346:958–67. doi: 10.1016/j.bbrc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [60].Beck EJ, Bowlby M, An WF, Rhodes KJ, Covarrubias M. Remodelling inactivation gating of Kv4 channels by KChIP1, a small-molecular-weight calcium-binding protein. J Physiol. 2002;538:691–706. doi: 10.1113/jphysiol.2001.013127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–6. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- [62].Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, et al. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–54. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- [63].Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, et al. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–30. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- [64].George AL, Jr., Knittle TJ, Tamkun MM. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus: evidence for a distinct gene family. Proc Natl Acad Sci U S A. 1992;89:4893–7. doi: 10.1073/pnas.89.11.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Felipe A, Knittle TJ, Doyle KL, Tamkun MM. Primary structure and differential expression during development and pregnancy of a novel voltage-gated sodium channel in the mouse. J Biol Chem. 1994;269:30125–31. [PubMed] [Google Scholar]
- [66].Martin C, Arnaudeau S, Jmari K, Rakotoarisoa L, Sayet I, Dacquet C, et al. Identification and properties of voltage-sensitive sodium channels in smooth muscle cells from pregnant rat myometrium. Mol Pharmacol. 1990;38:667–73. [PubMed] [Google Scholar]
- [67].Ohya Y, Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am J Physiol. 1989;257:C408–12. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- [68].Inoue Y, Sperelakis N. Gestational change in Na+ and Ca2+ channel current densities in rat myometrial smooth muscle cells. Am J Physiol. 1991;260:C658–63. doi: 10.1152/ajpcell.1991.260.3.C658. [DOI] [PubMed] [Google Scholar]
- [69].Yoshino M, Wang SY, Kao CY. Sodium and calcium inward currents in freshly dissociated smooth myocytes of rat uterus. J Gen Physiol. 1997;110:565–77. doi: 10.1085/jgp.110.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kaya T, Guvenal T, Karadas B, Cetin A, Soydan AS. Effects of 5-nitro-2-(3-phenylpropylamino) benzoic acid, anthracene-9-carboxylate, and zaprinast on endothelin-1-induced contractions of pregnant rat myometrium. Eur J Obstet Gynecol Reprod Biol. 2002;105:114–9. doi: 10.1016/s0301-2115(02)00149-5. [DOI] [PubMed] [Google Scholar]
- [71].Jones K, Shmygol A, Kupittayanant S, Wray S. Electrophysiological characterization and functional importance of calcium-activated chloride channel in rat uterine myocytes. Pflugers Arch. 2004;448:36–43. doi: 10.1007/s00424-003-1224-7. [DOI] [PubMed] [Google Scholar]
- [72].Yarar Y, Cetin A, Kaya T. Chloride channel blockers 5-nitro-2-(3-phenylpropylamino) benzoic acid and anthracene-9-carboxylic acid inhibit contractions of pregnant rat myometrium in vitro. J Soc Gynecol Investig. 2001;8:206–9. doi: 10.1016/s1071-5576(01)00113-7. [DOI] [PubMed] [Google Scholar]
- [73].Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–58. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]