Abstract
Background and purpose:
The pharmacological properties of compounds NCX 1512 and NCX 1514, synthesized by linking the histamine H1-receptor antagonist cetirizine to NO-releasing spacer groups, are reported. The aim was to establish if the compounds retained the antihistamine action of the parent compound, to assess their efficacy as NO donors and to test if they had broader antiallergic activity than cetirizine in the lung.
Experimental approach:
Antihistamine activity of NCX 1512 and NCX 1514 was investigated in vitro in the guinea pig ileum, in tracheal rings (GPTR) and lung parenchymal strips (GPLP) of the guinea-pig. The NO-releasing capacity was investigated in vascular preparations; the isolated rabbit and guinea-pig aorta and guinea-pig pulmonary artery. Kinetics of NO release were assessed in a rat whole blood assay.
Key results:
Both NCX 1512 and NCX 1514 retained activity as H1-receptor antagonists in the guinea pig ileum and airway preparations. The NO-releasing NCX compounds relaxed the rabbit aorta, an action prevented by the guanylyl cyclase inhibitor ODQ (10 μM). NCX 1512 and NCX 1514 did not relax the antigen (ovalbumin) pre-contracted GPTR, whereas the NO donors NCX 2057 and DEA-NONOate relaxed guinea-pig pre-contracted vascular and tracheal preparations. Cetirizine (1–100 μM) and NCX 1512 (1–100 μM) reduced the cumulative (0.01–100 μg ml−1) ovalbumin-induced constriction in GPTR, but had no significant effect in GPLP.
Conclusions and implications:
NCX 1512 and NCX 1514 act as antihistamines and NO donors. However, there was no improved effect compared to cetirizine on antigen-induced constriction of the central and peripheral lung.
Keywords: NO donors, antihistamine, cetirizine, smooth muscle, airways, vessel, ovalbumin
Introduction
Nitric oxide (NO) is a free radical gas long recognized as an air pollutant. More recently, following the structural elucidation of endothelial-derived relaxant factor (EDRF) (Furchgott and Zawadzki, 1980; Palmer et al., 1987), NO has been characterized as a biological signalling molecule regulating a wide range of important cellular functions (Moncada et al., 1991; Coleman, 2001). In the vessel wall, basal NO production by endothelial cells represents a primary mechanism regulating resting tone and causes vasodilatation when synthesized in response to different stimuli, such as shear stress or vasoactive agents. Moreover, endogenous NO can inhibit inflammatory cell activation and different chemical mediators of the inflammatory process (Coleman, 2001, Larsson et al., 2005).
Administration of exogenous NO has also received considerable attention, mainly due to its therapeutic ability to exert profound haemodynamic effects (Macrae et al., 2004). Nitro vasodilators relax arteries and veins, and are established drugs for the treatment of angina pectoris. Exogenous NO is also known to relax central airways (Gustafsson, 1993), and there are implications that exogenous NO exerts anti-inflammatory cellular effects (Wenk et al., 2002, 2004).
In recent years, several NO-releasing non-steroidal anti-inflammatory drugs (NO-NSAIDs) have been synthesized by ester linkage of a NO-releasing spacer group to conventional NSAID (Fiorucci et al., 2001; Bolla et al., 2006). In experimental models, these compounds retain the anti-inflammatory and antipyretic activity of the parent NSAID. Although exhibiting a reduced gastrointestinal toxicity. More recently, the strategy of linking NO-releasing functional groups to a parent molecule has been extended to compounds that are chemically and pharmacologically unrelated to NSAIDs. Examples include NO-prednisolone and NO-mesalamine. These compounds have the potential to have either enhanced therapeutic efficacy or improved side effect profile (Paul-Clark et al., 2000; Fiorucci et al., 2001; Mallei et al., 2005).
The mast cell is a key effector cell in allergic inflammation, and the release of histamine from activated mast cells contributes significantly to the symptoms of allergic rhinitis, conjunctivitis, urticaria and other allergic reactions. Anti histamines that block histamine H1 receptors therefore have a central role in the treatment of hay fever (Simons, 2004) and data from allergen bronchoprovocations of asthmatic subjects suggest that antihistamines may have beneficial effects as part of combination therapy in the treatment of asthma (Roquet et al., 1997; Reinartz et al., 2005).
In view of the anti-inflammatory properties exhibited by NO donors in several experimental models (Lagente et al., 2004), a new class of antihistamines coupled to NO donors has been developed with the hypothesis that such compounds may have antiallergic and anti-inflammatory properties beyond those of the parent antihistamine.
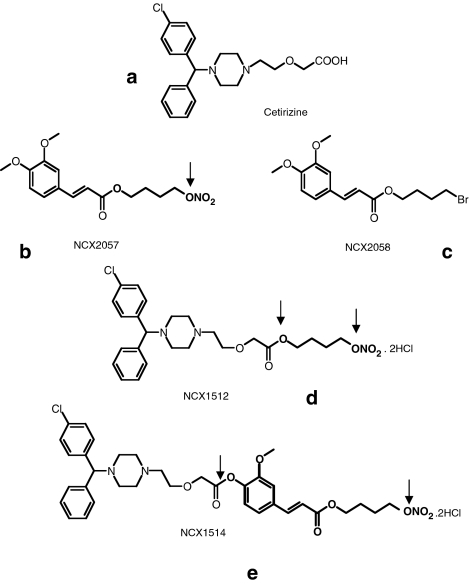
The aim of this study was threefold. First, to establish if the new compounds retained the antihistamine action of the parent compound. Second, to assess the efficacy of the new compounds as NO donors and compare their capacity with a known NO-donor. The third objective was to test whether or not the new compounds had broader antiallergic activity than cetirizine in in vitro models of acute antigen-induced airway constriction representing central airways and peripheral lung. The two compounds [2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]aceticacid 4(nitroxy)butyl ester dihydrochloride (NCX 1512) and ferulic acid nitroxybutyl ester: 3-[4-[2[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetyloxy]3-methoxyphenyl]2-propenoic acid 4-(nitroxy)butyl ester dihydrochloride (NCX 1514) have been synthesized by linking the histamine H1-receptor antagonist cetirizine to a NO-donating spacer group (Figure 1). For investigations of the mode of action of the two compounds, their actions were compared with those of the NO donor 3-(4-hydroxy-3 methoxyphenyl)-2-propenoic acid 4-(nitroxy) butyl ester (NCX 2057) (spacer group of NCX 1514) and its bromine analogue 3-(4-hydroxy-3 methoxyphenyl)-2-propenoic acid 4-bromo butyl ester (NCX 2058) (Figure 1). Compound NCX 2057 was also compared with the NO donor nitric oxide (NO)- and nitroxyl (HNO)-generating diazenium diolates (NONOate). The capacity as NO donors was tested in rabbit aorta and guinea-pig vascular and tracheal preparations, whereas the efficacy as H1 antagonists was tested in guinea-pig ileum, tracheal rings (GPTR) and lung parenchyma (GPLP), where GPTR represented the central airways and GPLP, the peripheral lung. The present study represents the first report on the pharmacologic properties of NCX 1512 and NCX 1514 acting both as antihistamines and NO donors.
Figure 1.
Structure of the NO-releasing derivatives of cetirizine, the NO-donor NCX 2057, the bromine derivative NCX 2058 and cetirizine. (a) Cetirizine. (b) NCX 2057. (c) NCX 2058. (d) NCX 1512. (e) NCX 1514. Arrows are indicating cleavage of the linkers.
Methods
Ethical approval
The guinea-pig ileum preparation and rabbit aorta preparation were approved by the local Institutional Animal Care and Use Committee. Animal housing at the Department of Pharmacological Sciences complies with the Italian law, N. 116, January 27 1992, recipient of the EU directive 86/609 for the safety and protection of experimental animals. The guinea-pig studies of tracheal and vascular rings and lung parenchyma were approved by the Regional Committee of Animal Experimentation Ethics (N14/02, N127/04) in Stockholm, Sweden.
Rabbit aorta
White male New Zealand rabbits (Harlan Italy – S Pietro al Natisone, UD, Italy) (1.8–2.4 kg bw) were anaesthetized with xylazine (5 mg kg−1) and ketamine (5 mg kg−1). Aortas were gently removed to avoid damage to the endothelial lining, cleared of fat and connective tissue, and cut into 2–3 mm wide rings. Three rings were connected by surgical silk to form a chain and mounted under a resting tension of 20 mN (2 g) in a glass organ bath filled with Krebs' Henseleit bicarbonate solution (NaCl 118.90 mM, KCl 4.66 mM, KH2PO4 1.18 mM, MgSO4 1.10 mM, CaCl2 2.52 mM, glucose 5.55 mM, NaHCO3 25 mM, ascorbic acid 0.35 mM, pH 7.4), bubbled with carbogen gas (5.0% CO2 in O2) and with a temperature of 37°C. After an equilibration period of 60 min, a cumulative concentration–response curve of noradrenaline (NA) (0.1–3 μM) was obtained. At the plateau, acetylcholine (ACh) (3 μM) was added to evoke endothelium-dependent relaxation. After washing, NA was again added and, upon reaching a stable plateau, the drugs under study were added to evaluate their vasorelaxant effect. A submaximal concentration of NA (0.3 μM) allowed maintenance of a constant contractile plateau over a prolonged period of time, and was therefore selected for assessment of the relaxant efficacy of the NCX compounds. The compounds were added to the preparations so as to produce final bath concentrations of dimethylsulphoxide (DMSO) of less than 0.7% (v/v). This concentration of DMSO was established not to affect the NA precontracted vascular preparation. The maintained reactivity of the preparations was tested again by NA (0.3 μM) at the end of the experiment. Changes in isometric contractions were monitored by a force transducer connected to a ‘Power MacLab' Bridge Amplifier. In selected experiments, the endothelial lining was damaged by gentle rubbing of the intimal surface. Maintenance of the contractile response was confirmed by NA (0.3 μM), whereas lack of endothelial NO release was indicated by the loss of the ACh-dependent vasodilation.
Guinea-pig ileum
Male guinea-pigs (Harlan Italy – S Pietro al Natisone, UD, Italy) (250–300 g body weight (bw)) were anaesthetized with sodium pentothal (20 mg kg−1). The terminal ileum was removed, a strip of approximate length 2–3 cm was dissected and suspended in a glass organ bath containing Tyrode's solution (NaCl 140 mM, KCl 3 mM, MgCl2 6H2O 0.05 mM, CaCl2 1 mM, glucose 8.4 mM, NaHCO3 12 mM, NaH2PO4 H2O 0.5 mM), bubbled with carbogen gas (5.0% CO2 in O2) and with a temperature of 37°C. After an equilibration period of 1 h, under a basal tension of 20 mN (2 g), changes in isometric contraction were monitored by a force transducer connected to a ‘Power MacLab' Bridge Amplifier. Histamine (1 μM) was added and the contractile response to histamine was proved reproducible upon successive administrations. Preparations producing a constant, contractile plateau of at least 1 g tension above basal tone were kept for assessment of the compounds efficacy against histamine. Cetirizine, NCX 1512, NCX 1514, NCX 2057 and NCX 2058 (0.01–1.0 μM) were administered according to a ‘therapeutic' protocol where their capacity to reverse an ongoing histamine-dependent tone was investigated. The final concentration of DMSO in the bath never exceeded 0.5% (v/v), and control preparations were given DMSO alone. The maintained reactivity of the preparations was determined with histamine (1 μM) at the end of each experiment.
Ovalbumin sensitization
For studies of antigen-induced contractions, male Dunkin Hartley guinea-pigs (300–350 g bw) were sensitized to chicken egg albumin (ovalbumin) at least 4 weeks before experiment. A stock solution of ovalbumin was prepared by dissolving 500 mg ovalbumin in 10 ml 0.9% NaCl and 10 ml 2% aluminium hydroxide gel and shaken for 1 h. The guinea-pigs were given a subcutaneous injection of 0.4 ml ovalbumin (10 mg) in the neck and an intraperitoneal injection of 0.4 ml ovalbumin (10 mg).
Lung parenchymal strips, tracheal and vascular rings
Guinea-pigs (500–900 g bw) were killed by an overdose of inhaled CO2 and the heart–lungs were quickly removed en bloc and placed in ice-cold Tyrode's solution (prepared each day, containing NaCl 149.2 mM, KCl 2.7 mM, NaHCO3 11.9 mM, glucose 5.5 mM, CaCl2 1.8 mM, MgCl2 0.5 mM and NaH2PO4 0.4 mM). The lung parenchyma was cut parallel to the peripheral margins, yielding four to eight strips, each having a size of 2 × 2 × 20 mm. The trachea was cut into eight rings.
The lung parenchymal strips were set up at a resting tension of 2.5 mN (0.25 g) and the tracheal rings at a resting tension of 30 mN (3 g) in 5 ml organ baths filled with Tyrode's solution, bubbled with carbogen gas (5% CO2 in O2) to keep a pH of 7.4, and the temperature was kept at 37°C. Changes in smooth muscle tension, that is, contractions and relaxations, were recorded via isometric force–displacement transducers connected to a Grass polygraph, and responses were displayed via a chart-recorder or by using the IOX data acquisition system (EMKA, Paris, France). Data were analysed either manually from charts or by the software program Dataanalyst (EMKA, Paris, France). After an equilibration period of 90 min and washes each 15 min, histamine (1–30 μM) was added cumulatively to establish control reactivity of the GPLP and Guinea-pig trachea (GPT) preparations. Another wash and equilibration period between histamine and treatment period was performed. Histamine and ovalbumin were added as cumulative challenge of increasing concentrations. For study of NO donors, the GPTR (from senstitized animals) was precontracted with ovalbumin (100 μg ml−1). Maximum contractions of the preparation were determined with histamine (1 mM), ACh (1 mM) and KCl (40 mM) at the end of each experiment and other responses were expressed as percent of maximum contractions. Fluid was collected after 15, 30, 60, 90 and 120 min from the GPTR organ baths after addition of 1 μM of the NO donors NCX 1512, NCX 1514, SNP, DEA-NONOate, NCX 2057 and NCX 2058 to assess the formation of nitrite over time. Nitrite was analysed with a chemiluminescence method as described previously (Lundberg and Govoni, 2004).
Guinea-pig pulmonary artery (GPPA) and aorta (GPA) were prepared as rings with the endothelium gently removed and then mounted in the organ baths under a resting tension of 15 mN (1.5 g). After an equilibration period of 60 min and washes each 10 min, NA (10 μM) was added and drugs were given at the plateau to study relaxations.
Electron paramagnetic resonance (EPR) analysis
The kinetics of release of NO was studied for compounds NCX 2057, NCX 1512 and SNAP using HbFe(II)NO formation in rat whole blood, measured by EPR spectroscopy as previously described (Govoni et al., 2006). The concentration of HbFe(II)NO in the incubation mixture reflects the quantity of nitric oxide accumulated at different times.
Data analysis and statistical procedures
All data are presented as mean±standard error of the mean (s.e.m.). Statistical analysis for concentration–response curves for the ileum, aorta and airway preparations were made by analysis of variances (ANOVA) followed by the post hoc tests, Holm Sidak's t-test or Bonferroni's t-test. A P-value of less than 0.05 was considered significant. EC50 values represent the drug concentration required to produce 50% relaxation in a NA or histamine precontracted strip (mean±s.e.m.). RM% represents the peak relaxation observed at the maximal drug concentration used.
Drugs
Cetirizine-HCl, NCX 1512, NCX 1514, NCX 2057 and NCX 2058 have been synthesized at NicOx Laboratories in Milano and made available from NicOx Research Institute. The NO-releasing cetirizine derivatives were synthesized starting from the parent compound according to a synthetic process used for other NO-releasing agents (Burgaud et al., 2002). The substance obtained was the NCX 1512 (Figure 1). It was isolated as dihydrochloride salt to improve solubility. Its molecular weight is 578.93. The NO donating spacer of NCX-1512 could not be studied as a NO-donor alone, as it was not chemically stable when dissociated from cetirizine. The other substance, we report here is the NO-cetirizine compound having as linker the NCX 1514 (Figure 1). Its molecular weight is 755.10. NCX 2057 is the linker–NO complex that is present in the NCX 1514 moiety. Compound NCX 2057 is a synthesized organic nitrate (3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid 4-(nitroxy) butyl ester) that is metabolized to its parent compound ferulic acid and a NO-donating substance (Govoni et al., 2006). Molecular weight is 311.29. Compound NCX 2058 is the control substance to NCX 2057, with a bromine derivative instead of a NO-releasing spacer group. Molecular weight is 329.19. All compounds were supplied as powder and kept at 4°C.
NaCl, KCl, CaCl2, MgSO4, NaHCO3, KH2PO4 and glucose were obtained from VWR International (West Chester, PA, USA). DEA-NONOate was purchased from Calbiochem (San Diego, CA, USA). Histamine dihydrochloride, acetylcholine, noradrenaline, ovalbumin (chicken egg albumin, grade II), ODQ (1H(1,2,4) oxadiazolo (4,3,−a) quinoxalin-1-one), nitrite and DMSO were purchased from Sigma-Aldrich (St Louis, MO, USA).
Ovalbumin was dissolved in 0.9% NaCl. The other drugs were dissolved and diluted in DMSO, Tyrode's solution or Millipore water. Dilutions of drugs were freshly made from the stocks for each experiment. The drugs were given 15 min before the challenges and remained present in the organ bath fluid during the remaining experiments. Ovalbumin was added as cumulative challenge of increasing concentrations (1–100 000 ng ml−1) every 10 min without changing bath fluid. Additions of test compounds were assessed only upon attainment of a well-sustained plateau and the time elapsed between two successive administrations never exceeded 15 min. Concentrations indicated refer to the final drug concentration in the organ baths.
Results
Kinetics of nitric oxide release
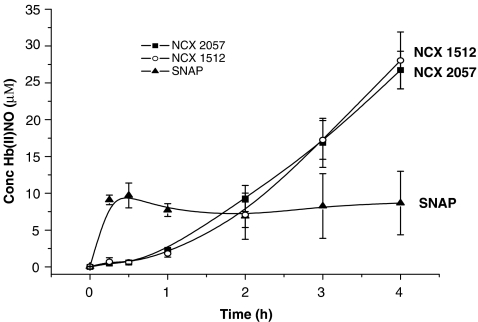
In the EPR assay, compounds NCX 1512 and NCX 2057 displayed a slow and progressive release of NO during 4 h whereas SNAP caused a release that was maximal within 30 min (Figure 2). In the organ bath experiments, NO release was monitored by measurements of nitrite. The accumulation of nitrite increased concentration dependently after exposure of GPTR to NCX 1512, NCX 1514 and NCX 2057 with threshold of around 1 μM and highest values obtained at 100 μM (n=3 for each drug, data not shown).
Figure 2.
Time course of HbFe(II)NO EPR spectra generated after incubation of NCX 1512 (100 μM), NCX 2057 (100 μM) and SNAP (100 μM) in whole deoxygenated rat blood. All values have been corrected for any effect of the vehicle. Data are presented as means±s.e.m. of three replications.
NO-dependent vasodilation in strips of rabbit aorta
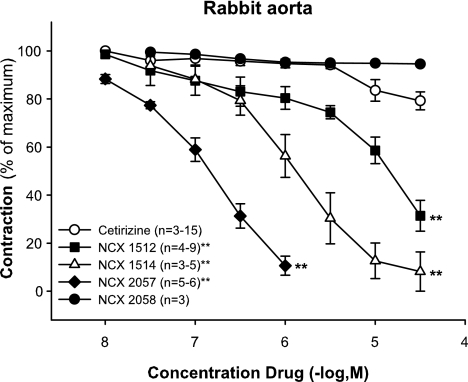
NA (0.1–3.0 μM) caused a concentration-dependent increase in vascular tone that was reversible following wash out. The tested NO-releasing derivatives of cetirizine and the NO-donor NCX 2057 relaxed the NA pre-contracted preparations (Figure 3). In contrast, cetirizine (0.01–100 μM) displayed only a negligible relaxant effect at the highest concentrations (10–100 μM) (Figure 3), whereas the control substance of NCX 2057, the bromine derivative NCX 2058 was inactive (Figure 3). The NO-releasing compounds exhibited the same maximal relaxation (same RM%), with complete reversal of the NA-induced contraction being obtained at the highest concentrations tested for each of the NO-releasing substance (Figure 3). The rank order of potency and EC50 values for the tested compounds were: NCX 2057 (0.4±0.25 μM)>NCX 1514 (1.24±0.7 μM)>NCX 1512 (15±8 μM).
Figure 3.
Effect of NCX 1512 (0.01–30 μM; n=4–9), NCX 1514 (0.03–30 μM; n=3–5), NCX 2057 (0.01–1 μM; n=5–6), NCX 2058 (0.03–30 μM; n=3) compared to cetirizine (0.01–30 μM; n=3–15) on contractions induced by 0.3 μM of NA on rabbit aorta. Data are expressed as mean±s.e.m.; statistical analysis was performed by two-way ANOVA. **P<0.01.
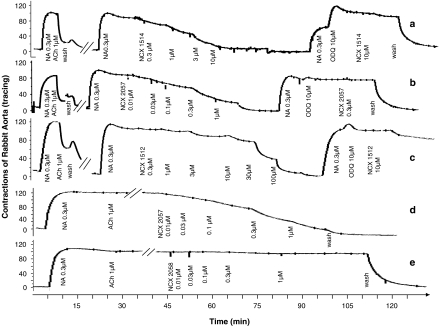
Pretreatment of the aortic rings with the guanylate cyclase inhibitor, ODQ; 10 μM, prevented vasodilation induced by NCX 1512, NCX 1514 and NCX 2057 (Figure 4a–c). The dependency of the vasodilation on the supply of exogenous NO was confirmed as the efficacy and potency of compound NCX 2057 was fully maintained in endothelium-denuded preparations (Figure 4d). The compound NCX 2058 was inactive also in the denuded preparation (Figure 4e).
Figure 4.
Effect of NO-releasing derivatives of cetirizine, NCX 1514 (0.3–10 μM; a) and NCX 1512 (0.3–100 μM; c), and of the NO-donor NCX 2057 (0.01–1 μM; b) in the absence and presence of the guanylate cyclase inhibitor, ODQ (10 μM) on precontracted rabbit aorta; effect of NCX 2057 (0.01–1 μM; d) and NCX 2058 (0.01–1 μM; e) on endothelium-denuded pre-contracted aorta. Representative tracings of three to five experiments per treatment.
The time course of the vasodilation induced by compound NCX 1514 was prolonged; administration of a single concentration of NCX 1514, equal to its EC50 value (1.2 μM), caused a vascular relaxation that was slow in onset and sustained during at least the observation period of 1 h (data not shown).
Histamine antagonism in guinea-pig ileum
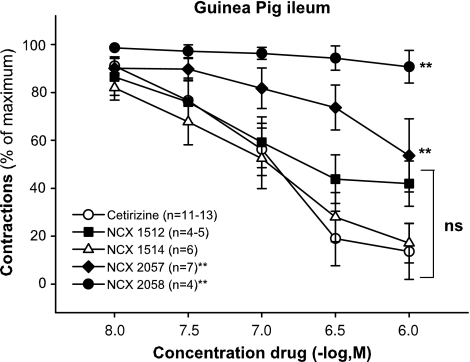
Histamine (1 μM) caused a contractile response in guinea-pig ileum that was fast in onset and readily reversible upon washout of the tissue bath. Cetirizine caused a complete reversal of the histamine-dependent response. Compound NCX 1514 was equi-efficaceous as cetirizine (same RM%). Compound NCX 1512 antagonized the histamine-induced response in a similar concentration range as NCX 1514 and cetirizine (Figure 5). The rank order of potency (and EC50 values) for the tested compounds were NCX 1512 (0.05±0.01 μM)>cetirizine (0.10±0.02 μM)⩾NCX 1514 (0.14±0.05 μM). The NO donor NCX 2057 also relaxed histamine precontracted preparations but was less potent and less efficacious than the antihistamines, whereas NCX 2058 was inactive.
Figure 5.
Effect of NO derivatives of cetirizine NCX 1512 (0.01–1 μM; n=4–5) and NCX 1514 (0.01–1 μM; n=6) and the NO donor NCX 2057 (0.01–1 μM; n=7) and NCX 2058 (0.01–1 μM; n=4) compared to cetirizine (0.01–1 μM; n=11–13) on contractions induced by histamine 1 μM on guinea-pig ileum. Data are expressed as mean±s.e.m.; statistical analysis was performed by two-way ANOVA. **P<0.01; ns, not significant (P>0.05).
Histamine contractions in airway preparations
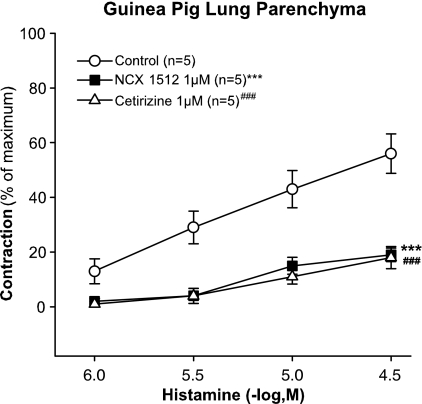
In the strips of GPLP, cetirizine and NCX 1512 (1 μM) almost totally abolished the cumulative concentration response to histamine (1–30 μM) (Figure 6). Similar results were obtained in the GPT with equal antagonism of histamine by cetirizine and NCX 1512 when tested at the concentration of 1 μM (data not shown).
Figure 6.
Effect of pretreatment with NCX 1512 (1 μM, n=5) and cetirizine (1 μM, n=5) compared to control (n=5) on concentration–response curves to histamine (1–30 μM) in GPLP. Data are expressed as mean±s.e.m.; statistical analysis was performed by two-way ANOVA. ***P<0.001; ###P<0.001.
Antigen-induced contractions in airway preparations
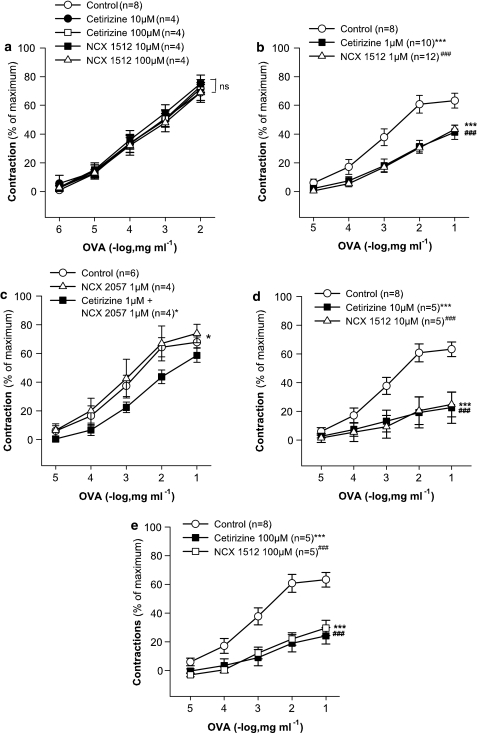
In strips of GPLP from sensitized animals, where the response to antigen is resistant to antihistamines alone (Jonsson and Dahlén, 1994), neither cetirizine (1–100 μM) nor NCX 1512 (1–100 μM) had any significant effect on the cumulative concentration–response to antigen (ovalbumin, 1–10 000 ng ml−1) (Figure 7a shows data for 10 and 100 μM cetirizine and NCX 1512). By contrast, in the GPT, the cumulative concentration–response to ovalbumin (10–100 000 ng ml−1) was significantly displaced to the right by cetirizine and NCX 1512 at 1 μM concentration of both drugs (Figure 7b). There was, however, no difference between the effects of cetirizine and NCX 1512 in this respect. Co-administration of cetirizine (1 μM) and the NO-donor NCX 2057 (1 μM) (Figure 7c) caused inhibition of the antigen response in the guinea-pig trachea similar to that observed with NCX 1512 (Figure 7b). When given alone, NCX 2057 (1 μM) had no significant effect on the ovalbumin-induced contractions (Figure 7c). Higher concentrations of compound NCX 1512 (10 and 100 μM) were no more effective than cetirizine (10 and 100 μM) alone (Figure 7d and e).
Figure 7.
Drug effects on the concentration–response curve to ovalbumin (1–100 000 ng ml−1) in sensitized guinea-pig airway preparations. (a) Effect of pretreatment with cetirizine (10 and 100 μM, n=5), NCX 1512 (10 and 100 μM, n=5) compared to control (n=8) in GPLP. (b) Effect of pretreatment with cetirizine (1 μM, n=10), NCX 1512 (1 μM, n=12) compared to control (n=8) tracheal rings. (c) Effect of pretreatment with NCX 2057 (1 μM, n=4) and combined treatment with NCX 2057 (1 μM) and cetirizine (1 μM, n=4) compared to control (n=6) in tracheal rings. (d) Effect of pretreatment with cetirizine (10 μM, n=5) and NCX 1512 (10 μM, n=5) compared to control (n=8) in tracheal rings. (e) Effect of pretreatment with cetirizine (100 μM, n=5) and NCX 1512 (100 μM, n=5) compared to control (n=8) in tracheal rings. Data are expressed as mean±s.e.m.; statistical analysis was performed by two-way ANOVA. ns, not significant (P>0.05); *P<0.05; ***P<0.001; ###P<0.001.
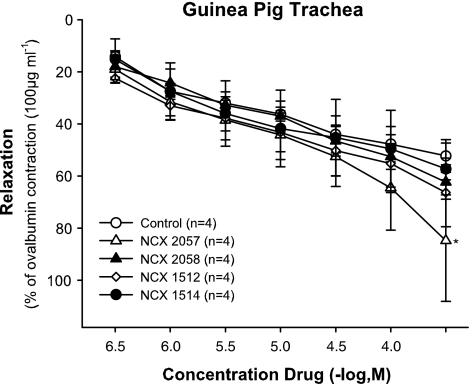
Finally, as the compounds that were NO donors were unable to affect the responses to antigen over and above the effects of cetirizine when administered before the antigen challenge, their effects on ongoing contractions were assessed. Thus, in drug untreated control tissues, the NO releasing derivatives of cetirizine, as well as NCX 2057 and NCX 2058 were administered cumulatively (0.3–300 μM) at the peak of the antigen response (Figure 8). In contrast to the potent relaxant effects displayed by the compounds in the vascular tissues (Figures 3 and 4), all compounds had very weak effects in the ovalbumin precontracted preparations. In fact, it was only NCX 2057 at concentrations above 30 μM that produced relaxations that were significantly different from the spontaneous decline in tone of preparations given solvent alone.
Figure 8.
Effect of 0.3–100 μM of NCX 1512 (n=4), NCX 1514 (n=4), NCX 2057 (n=4) and NCX 2058 (n=4) on 100 μg ml−1 ovalbumin precontracted sensitized GPTR compared to control (n=4). Data are expressed as mean±s.e.m.; statistical analysis was performed by two-way ANOVA. ns, not significant (P>0.05); *P<0.05.
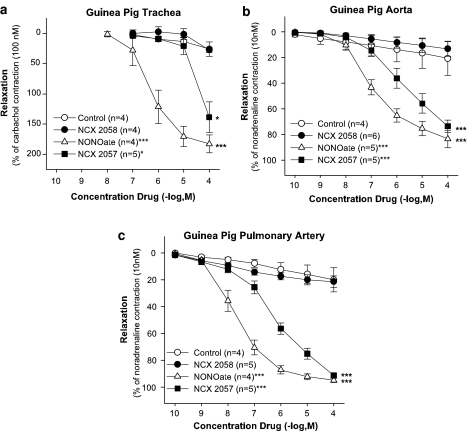
Effect of NCX 2057 and NONOate in tracheal and vascular preparations
The effect of NCX 2057 was also assessed in carbachol (0.1 μM). Precontracted tracheal rings and the relaxant capacity was compared with the NO-donor NONOate in the GPTR ring and in preparations of GPA and GPPA (Figure 9a, b and c). Both NONOate (0.0001–100 μM) and NCX 2057 (0.0001–100 μM) dose dependently relaxed the NA precontracted aorta and pulmonary artery whereas compound NCX 2058 was ineffective. NONOate was more potent than NCX 2057 and this difference in potency was also seen in carbachol precontracted GPTR where NONOate (0.01–100 μM) dose dependently relaxed the airway smooth muscle, in contrast to NCX 2057 (0.1–100 μM) that only at high concentrations, above 10 μM, relaxed the GPTR.
Figure 9.
Effect of the NO donors NCX 2057 (0.0001–100 μM) and NONOate (0.0001–100 μM) on precontracted non-sensitized GPTR, GPA and GPPA. (a) Effect of cumulative concentrations of NCX 2057 (0.01–100 μM) and NONOate (0.01–100 μM) (n=5) on 10 nM LTD4 precontracted GPTR compared to control (n=6) and NCX 2058. (b) Effect of cumulative concentrations of NCX 2057 (0.001–100 μM) and NONOate (0.0001–100 μM) (n=4) on 10 μM NA precontracted GPA compared to control (n=4). (c) Effect of cumulative concentrations of NCX 2057 (0.001–100 μM) and NONOate (0.0001–100 μM) (n=4) on 10 μM NA precontracted GPPA compared to control (n=4). Data are expressed as mean±s.e.m.; statistical analysis was performed by two way ANOVA. *P>0.05; ***P<0.001.
Discussion and conclusions
This investigation demonstrated that the new NO-releasing derivatives of cetirizine retained their antihistamine properties while acquiring the added function to release NO in concentrations that cause profound relaxations of the rabbit aorta. As the original discovery of EDRF, strips of isolated rabbit aorta have been considered as a sensitive and reliable bioassay preparation for NO detection. The capacity of the new derivatives of cetirizine, NCX 1512 and NCX 1514, to release NO was thus demonstrated in the aortic ring experiments and also supported by measurements in rat whole blood and the tissue baths.
The linker–NO complex that was available in the absence of the antihistamine moiety, that is, compound NCX 2057, was also a powerful NO releaser and its concentration–response curve was similar to compound NCX 1514 in the rabbit aorta. Moreover, the EC50 values of NCX 2057 and NCX 1514 were in a comparable micromolar range, indicating that the NO-releasing spacer group, NCX 2057, is functioning as a NO donor in the linker–NO complex of the NCX 1514 moiety. Chemical modifications such as NO-donor group substitution with a bromine group (NCX 2058) or pharmacological interventions with the guanylate cyclase inhibitor ODQ, aimed at preventing the action of NO, invariably led to the loss of the vasodilation induced by the NO-releasing series of compounds. The compound NCX 2057 relaxed in similar way both intact and denuded aorta preparations, indicating that NO was cleaved from the compound and became bioavailable in the vascular tissue. An interesting feature of the new NO-releasing derivatives of cetirizine is represented by the different kinetics of NO release. In this respect NCX 1514 is particularly interesting as it seems to provide NO over a prolonged period of time, making it a very useful pharmacological tool.
Cetirizine is a selective H1-receptor antagonist. The affinity of cetirizine to bind to H1 receptors is more than 500 times higher than its affinity for H2 and H3 receptors (Gillard et al., 2003). The results of this study clearly indicate that the new NO-releasing derivatives of cetirizine fully retain their pharmacological activity as H1-receptor antagonists. This is supported by data obtained in the longitudinal smooth muscle of the guinea-pig ileum, as an example of the presence of H1 receptors mediating a rapid and promptly reversible contraction. This experimental model is widely utilized for the evaluation of H1 antagonists. The guinea-pig ileum demonstrated, by the largely superimposable EC50 values reported for cetirizine and the NO-releasing derivatives of cetirizine NCX 1512 and 1514, that the potencies of all three drugs as antihistamines were equal in this tissue.
The guinea-pig airway preparations we selected to use in this study, GPLP and GPTR, have a pharmacology that is closely similar to that of human airways. Both preparations are in vitro models for acute, mast cell-driven, antigen-induced, contractions and the mediators of these contractile responses (Jonsson and Dahlén, 1994) are those established for the anaphylactic contraction of human airways in vitro and in vivo, namely histamine and leukotrienes (Björck and Dahlén, 1993; Roquet et al., 1997). In the trachea, the histamine component of the antigen-induced contraction dominates (Lindstrom and Andersson, 1997), whereas in the GPLP, antihistamines alone have no inhibitory effect on the antigen-induced contraction (Jonsson and Dahlén, 1994). It was hypothesized that interactions between a H1 histamine receptor antagonist and a NO-donor thus effectively could be evaluated in the antihistamine-sensitive GPT. Likewise, it was considered possible to isolate the effects of the NO-donor component of the compounds in the antihistamine-resistant preparations of GPLP.
First, in the preparations of the central and peripheral lung, both cetirizine and NCX 1512 equally inhibited the contractions to cumulative concentrations of histamine, indicating that the novel compound NCX 1512 also preserved its pharmacological activity as a H1-receptor antagonist in the respiratory system. Second, there was, however, not an improved antiallergic effect of NCX 1512 compared to cetirizine, as cetirizine and NCX 1512 had similar pronounced inhibitory effects on antigen-induced contractions in the trachea. The same major degree of inhibition remained when higher concentrations of NCX 1512 were tested (10–100 μM) to promote higher levels of NO release. Furthermore, in the peripheral lung model, it was confirmed that cetirizine by itself had no significant effect on the antigen-induced contraction. Once again NCX 1512 did not show any improved inhibitory effect, despite increasing the concentrations of NCX 1512 to very high levels in this preparation. The lack of inhibitory effect of NO on the antigen response was also suggested by the inability of the NO-releasing compound NCX 2057 to affect the antigen-induced responses in the GPTR.
It is noteworthy that in comparison with vessel preparations, the NO-releasing derivatives of cetirizine, NCX 1512 and 1514, and the NO-donor NCX 2057 had comparatively weak relaxant effects in the tracheal preparation. For example, NCX 2057 had an EC50 of 0.4 μM in the rabbit aortic rings whereas in GPTR the EC50 was 30 μM. Also another potent NO-donor, DEA-NONOate, dose-dependently relaxed GPA, GPPA and GPTR, but consistently required higher concentrations in the trachea. It is therefore clear that airway smooth muscle appears much less sensitive to the relaxant effects of NO than blood vessels. It has in fact been reported that the peripheral lung is insensitive to exogenous NO, as nitroglycerin did not relax bovine lung parenchyma (Gruetter and Lemke, 1984). More recently, it was found that the NO-donor NONOate had no effect on precontracted GPLP (Larsson et al., 2005), suggesting that relative or absolute lack of NO sensitivity is a general characteristic of airway smooth muscle. It seems that the function of NO in the peripheral lung in fact is to modulate mast cell activation rather than to cause bronchodilation (Larsson et al., 2005).
In conclusion, we have documented that the new cetirizine derivatives with a NO-releasing spacer group indeed retained their antihistamine properties while acquiring the added function to release NO. Although this dual mode of action conferred no added benefit in the currently tested models of the early allergic responses in guinea-pig airways, it should be of value in other settings of inflammation where delivery of NO may synergize therapeutically with antagonism of H1 receptors. In particular, the compound NCX 1514 and NCX 1512, that have slow release kinetic profiles, may be of value in other systems with on-going inflammation, such as upper airways and eyes in allergic rhinitis and conjunctivitis, respectively.
Acknowledgments
The study was conducted with support from the Swedish MRC (project 9071), Heart-Lung Foundation, Stockholm County Council Research Funds, Karolinska Institute, Biolipox AB and also partial support of the EU grant LSHM-CT-2004005033. This publication reflects only the views of the authors. The European Commission is not liable for any use that may be made of information herein.
Abbreviations
- GPLP
guinea-pig lung parenchyma
- GPTR
guinea-pig tracheal rings
- NCX 1512
[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid 4(nitroxy)butyl ester dihydrochloride
- NCX 1514
ferulic acid nitroxybutyl ester: 3-[4-[2[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetyloxy]3-methoxyphenyl]2-propenoic acid 4-(nitroxy)butyl ester dihydrochloride
- NCX 2057
3-(4-hydroxy-3 methoxyphenyl)-2-propenoic acid 4-(nitroxy) butyl ester
- NCX 2058
3-(4-hydroxy-3 methoxyphenyl)-2-propenoic acid 4-bromo butyl ester
- bromo derivative of NCX-2057;NONOate
nitric oxide (NO)- and nitroxyl (HNO)-generating diazenium diolates
- ODQ
(1H(1,2,4) oxadiazolo (4,3,−a) quinoxalin-1-one)
Conflict of interest
The authors state no conflict of interest.
References
- Björck T, Dahlén SE. Leukotrienes and histamine mediate IgE-dependent contractions of human bronchi: pharmacological evidence obtained with tissues from asthmatic and non-asthmatic subjects. Pulm Pharmacol. 1993;6:87–96. doi: 10.1006/pulp.1993.1012. [DOI] [PubMed] [Google Scholar]
- Bolla M, Momi S, Gresele P, Del Soldato P. Nitric oxide-donating aspirin (NCX 4016): an overview of its pharmacological properties and clinical perspectives. Eur J Clin Pharmacol. 2006;62 Suppl 13:145–154. [Google Scholar]
- Burgaud JL, Riffaud JP, Del Soldato P. Nitric-oxide releasing molecules: a new class of drugs with several major indications. Curr Pharm Des. 2002;8:201–213. doi: 10.2174/1381612023396357. [DOI] [PubMed] [Google Scholar]
- Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Burgaud JL, Morelli A. Nitric oxide-releasing NSAIDs: a review of their current status. Drug Saf. 2001;24:801–811. doi: 10.2165/00002018-200124110-00002. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillard M, Christophe B, Wels B, Peck M, Massingham R, Chatelain P. H1 antagonists: receptor affinity versus selectivity. Inflamm Res. 2003;52 Suppl 1:S49–S50. doi: 10.1007/s000110300050. [DOI] [PubMed] [Google Scholar]
- Govoni M, Casagrande S, Maucci R, Chiroli V, Tocchetti P. In vitro metabolism of (nitroxy)butyl ester nitric oxide-releasing compounds: comparison with glyceryl trinitrate. J Pharmacol Exp Ther. 2006;317:752–761. doi: 10.1124/jpet.105.097469. [DOI] [PubMed] [Google Scholar]
- Gruetter CA, Lemke SM. Parenchymal and vascular strips from bovine lung respond differently to vasodilators. Eur J Pharmacol. 1984;106:619–623. doi: 10.1016/0014-2999(84)90067-0. [DOI] [PubMed] [Google Scholar]
- Gustafsson LE. Experimental studies on nitric oxide. Scand J Work Environ Health. 1993;19 Suppl 2:44–49. [PubMed] [Google Scholar]
- Jonsson EW, Dahlén SE. Interactions between leukotrienes and histamine in the anaphylactic contraction of guinea pig lung parenchyma. J Pharmacol Exp Ther. 1994;271:615–623. [PubMed] [Google Scholar]
- Lagente V, Naline E, Guenon I, Corbel M, Boichot E, Burgaud JL, et al. A nitric oxide-releasing salbutamol elicits potent relaxant and anti-inflammatory activities. J Pharmacol Exp Ther. 2004;310:367–375. doi: 10.1124/jpet.103.061739. [DOI] [PubMed] [Google Scholar]
- Larsson AK, Back M, Hjoberg J, Dahlén SE. Inhibition of nitric-oxide synthase enhances antigen-induced contractions and increases release of cysteinyl-leukotrienes in guinea pig lung parenchyma: nitric oxide as a protective factor. J Pharmacol Exp Ther. 2005;315:458–465. doi: 10.1124/jpet.105.086694. [DOI] [PubMed] [Google Scholar]
- Lindstrom EG, Andersson RG. Neurokinin A-LI release after antigen challenge in guinea-pig bronchial tubes: influence of histamine and bradykinin. Br J Pharmacol. 1997;122:417–422. doi: 10.1038/sj.bjp.0701382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37 3:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Macrae DJ, Field D, Mercier JC, Moller J, Stiris T, Biban P, et al. Inhaled nitric oxide therapy in neonates and children: reaching a European consensus. Intensive Care Med. 2004;30:372–380. doi: 10.1007/s00134-003-2122-3. [DOI] [PubMed] [Google Scholar]
- Mallei A, Aden SA, Bachis A, Brandoli C, Ongini E, Mocchetti I. The nitrosteroid NCX 1015, a prednisolone derivative, improves recovery of function in rats after spinal cord injury. Brain Res. 2005;1062:16–25. doi: 10.1016/j.brainres.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Paul-Clark M, Del Soldato P, Fiorucci S, Flower RJ, Perretti M. 21-NO-prednisolone is a novel nitric oxide-releasing derivative of prednisolone with enhanced anti- inflammatory properties. Br J Pharmacol. 2000;131:1345–1354. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinartz SM, Overbeek SE, Kleinjan A, Drunen CM, Braunstahl GJ, Hoogsteden HC, et al. Desloratadine reduces systemic allergic inflammation following nasal provocation in allergic rhinitis and asthma patients. Allergy. 2005;60:1301–1307. doi: 10.1111/j.1398-9995.2005.00911.x. [DOI] [PubMed] [Google Scholar]
- Roquet A, Dahlén B, Kumlin M, Ihre E, Anstren G, Binks S, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med. 1997;155:1856–1863. doi: 10.1164/ajrccm.155.6.9196086. [DOI] [PubMed] [Google Scholar]
- Simons FE. Advances in H1-antihistamines. N Engl J Med. 2004;351:2203–2217. doi: 10.1056/NEJMra033121. [DOI] [PubMed] [Google Scholar]
- Wenk GL, McGann-Gramling K, Hauss-Wegrzyniak B, Ronchetti D, Maucci R, Rosi S, et al. Attenuation of chronic neuroinflammation by a nitric oxide-releasing derivative of the antioxidant ferulic acid. J Neurochem. 2004;89:484–493. doi: 10.1111/j.1471-4159.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Rosi S, McGann K, Hauss-Wegrzyniak B. A nitric oxide-donating flurbiprofen derivative reduces neuroinflammation without interacting with galantamine in the rat. Eur J Pharmacol. 2002;453:319–324. doi: 10.1016/s0014-2999(02)02387-7. [DOI] [PubMed] [Google Scholar]