Abstract
Background and Purpose:
Experiments were designed to assess whether or not the intracellular concentration of calcium and reactive oxygen species (ROS) increase in endothelial cells of the rat thoracic aorta in response to releasers of endothelium-derived contracting factor (EDCF) and if so, whether or not a difference exists between spontaneously hypertensive (SHR) and normotensive (WKY) rats.
Experimental approach:
Calcium and ROS were measured by confocal microscopy, using Fura-red in combination with Fluo-4 and dichlorodihydrofluorescein diacetate, respectively.
Key results:
Acetylcholine caused a rapid increase in cytosolic calcium concentration in endothelial cells of both SHR and WKY, which was significantly more pronounced in aortae of the former strain. This rise of calcium was not affected by indomethacin (an inhibitor of cyclooxygenase) or Tiron plus diethyldithiocarbamate acid (DETCA) (membrane permeable antioxidants). In the presence of a nitric oxide synthase blocker, acetylcholine also caused a rapid increase in ROS in endothelial cells of SHR but not in those of WKY. The burst of ROS was prevented by indomethacin or Tiron plus DETCA.
Conclusions and implications:
These experiments show that endothelial cells of SHR are more prone to calcium and ROS overload upon stimulation with acetylcholine. The abnormal accumulation of calcium is a prerequisite to initiate the release of EDCF and can be mimicked using the calcium ionophore A23187. The sequence of events occurring during endothelium-dependent contractions firstly requires the accumulation of calcium, which then activates cyclooxygenase and produces ROS along with EDCF that in turn stimulates TP-receptors, resulting in EDCF-mediated contractions.
Keywords: endothelium-derived contracting factors, spontaneously hypertensive rats, reactive oxygen species, calcium dysfunction
Introduction
Endothelial dysfunction is characterized by an imbalance between the release of endothelium-dependent relaxing (EDRF) and contracting (EDCF) factors. Since the initial discovery of the obligatory role of endothelial cells in the relaxation of isolated arteries (Furchgott and Zawadzki, 1980), most of the attention of vascular biologists has focused on EDRF. Much less is known about the ability of the endothelial cells to release EDCF. The production of EDCF is prominent in arteries of mature spontaneously hypertensive rats (SHR), but not in their age-matched normotensive counterpart, Wistar–Kyoto rats (WKY) (Lüscher and Vanhoutte, 1986; Vanhoutte et al., 2005). Endothelial dysfunction is precipitated by ageing, diet, obesity, diabetes and hypertension, and the occurrence of EDCF-mediated contractions can be observed in arteries from aging normotensive and diabetic rats, and in humans with essential hypertension, acute estrogen deprivation and/or heart failure (Cohen, 2002; Taddei et al., 2002).
In the aorta of the SHR, endothelium-dependent contractions are reduced by the acute exposure to the combination of Tiron (a superoxide scavenger) and diethyldithiocarbamate acid (DETCA; superoxide dismutase inhibitor) or by a chronic treatment with dimethylthiourea (an in vivo depletor of free radicals) (Yang et al., 2002). Under bioassay conditions, the transfer of EDCF from the donor tissue to the bioassay preparation is diminished by the combination of superoxide dismutase plus catalase (Yang et al., 2003). Taken in conjunction, these studies suggest that the production of oxygen-derived free radicals play a role in EDCF-mediated contractions. The potent contracting factor, endothelin, is unlikely to contribute to such responses in the aorta of SHR, as they are not inhibited by endothelin-receptor antagonists. Instead, EDCF-mediated contractions are fully prevented by cyclooxygenase inhibitors including indomethacin (a non-selective cyclooxygenase inhibitor) and valeryl salicylate (a preferential inhibitor of cyclooxygenase-1; COX-1), whereas NS-398 (a preferential inhibitor of cyclooxygenase-2; COX2) only reduces the contraction moderately (Ge et al., 1995). Endothelium-dependent contractions are observed in the aorta of wild-type and COX-2 knockout but not in that of COX-1−/− mice (Tang et al., 2005b). These studies demonstrate that cyclooxygenase is crucial for the production of EDCF and that COX-1 is the isoform responsible. Endoperoxides and prostacyclin derived from COX-1 are the likely candidates accounting for EDCF (Ge et al., 1995; Gluais et al., 2005). These prostanoids diffuse across the intercellular space to the vascular smooth muscle, where they activate TP receptors and cause contraction (Yang et al., 2003; Gluais et al., 2005). The calcium ionophore A23187, which permits the free entry of extracellular calcium into endothelial cells, causes endothelium-dependent contractions in SHR that exhibit similar properties as those produced by acetylcholine, in that the contraction requires an intact endothelial layer and is sensitive to COX-1 inhibitors and TP-receptor blockers (Yang et al., 2004a; Gluais et al., 2005). Preliminary studies in the laboratory show that EDCF-mediated responses to A23187 are also sensitive to the antioxidants, Tiron plus DETCA. Such studies indicate that an increase in intracellular calcium is necessary for the production of endothelium-dependent contractions.
The present study aims to define why endothelium-dependent contractions occur more readily in the aorta of the SHR compared with that of the WKY. To test the hypothesis that the abnormal release of intracellular calcium and reactive oxygen species (ROS) in endothelial cells of SHR facilitates the occurrence of EDCF, the effect of the releasers of EDCF, acetylcholine and the calcium ionophore A23187, on the intracellular concentration of calcium and ROS in aortic endothelial cells of both strains was compared. The second objective of this study was to define the sequence of events (increase in calcium, activation of cyclooxygenase and production of oxygen-derived free radicals) during the production of EDCF. Determining the sequence of events that facilitates the production of EDCF may permit a better understanding of the complex events leading to endothelial dysfunction, and therefore enable the design of novel therapeutic agents to specifically correct the imbalanced release of endothelium-derived vasoactive substances.
Methods
Tissue preparation
Studies were conducted in 35–38 weeks old, male WKY or SHR. At this age, endothelium-dependent contractions occur readily in the aorta of SHR (Yang et al., 2004b; Tang et al., 2005a). The animals were housed in a temperature-controlled room (21±1°C) with a 12-h light/dark cycle (0700 lights on, 1900 lights off). Animals had free access to chow (LabDiet 5010, USA) and water. Arterial blood pressure was measured from carotid artery. The mean blood pressure of SHR and WKY is 200.6±5.3 and 116.1±4.8 mm Hg, respectively, n=12, P<0.05. The rats were anesthetized with pentobarbital sodium (70 mg ml−1 kg−1, intraperitoneally) and their thoracic aorta was removed and placed immediately in Krebs–Ringer buffer of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 11.1. The thoracic aortae were cleaned by removing adhering fat and connective tissue and were then cut into rings of approximately 3 mm in length. The investigation was approved by the Animal Care and Use Committee of The University of Hong Kong.
Isometric force
Aortic rings from SHR or WKY were suspended in organ chambers, which contained control solution (37°C), aerated with 95% O2 and 5% CO2. They were connected to force transducers (FT03, Grass Instrument Co., Quincy, IL USA) for isometric force recording (Data Logger, Pico Technology Ltd, Cambridge, UK). The rings were allowed to equilibrate for an hour at their optimal resting tension of approximately 0.0245 N (Tang et al., 2005a). They were incubated with Nω-nitro-L-arginine methyl ester (L-NAME, a nitric oxide synthase inhibitor; 10−4 M) for 40 min and then exposed to progressively increasing concentrations of acetylcholine (10−8–10−4 M) or the calcium ionophore A23187 (10−8–3 × 10−6 M) to elicit endothelium-dependent contractions (Auch-Schwelk et al., 1992; Yang et al., 2004b). Contractions to 6 × 10−2 M KCl were obtained at the start of the experiment. Changes in isometric force are expressed as absolute values in Newtons (N). In some preparations, the endothelium was removed mechanically by inserting the tip of a syringe needle into the rings and rolling them back and forth in a sylgard-based container filled with control solution. Certain rings were incubated with indomethacin (10−5 M) or Tiron (10−2 M) plus DETCA (10−4 M) for 40 min before evoking endothelium-dependent contractions.
Cytosolic-free calcium
Cytosolic-free calcium was measured using the fluorescent acetyloxymethyl ester calcium indicator dyes, Fura-red and Fluo-4. The advantages of using dual dyes, fluo-4 and fura-red to study intracellular calcium dynamics are that it minimizes the contribution of artifactual changes in the fluorescence signal not related to changes of calcium, for example the tissue movement in response to contractile agonists, dye-bleaching and non-homogenous dye loading (Lipp and Niggli, 1993; Budel et al., 2001; Marie and Bény, 2002). Fluo-4 emits a fluorescence that is approximately twice as bright than Fura-red, therefore the concentration of Fluo-4 and Fura-red used to monitor calcium changes was chosen to be 2 × 10−5 and 4 × 10−5 M, respectively. The dyes were dissolved in Krebs buffer containing 0.02% (v/v) pluronic acid F-127. Aortic rings were incubated for 60 min with the dye solution. This period allowed adequate penetration of the indicator into the cells and sufficient time for the acetyloxymethyl ester to cleave. The concentration of dyes and incubation time were chosen precisely to achieve a low, but detectable level of basal fluorescence, which permitted the identification of endothelial cells. Endothelial cells were characterized as round-shaped, whereas smooth muscle cells were characterized (if detectable) as long-rod shaped.
After loading of the dyes, the aortic rings were cut open longitudinally to form a strip which was pinned down into a specially designed closed-system viewing chamber (Leung et al., 2006). The intimal side of the aorta faced downwards with a 1 mm gap left between the aortic endothelium and the glass cover slip to ensure homogenous superfusion of the endothelium. Oxygenated (95% O2, 5% CO2) buffer solution containing the appropriate drug(s) was maintained at 37°C during the experiment using a temperature bath and perfused at the rate of 2 ml min−1 using a peristaltic pump. Before starting the experimental protocol, loaded strips were continuously superfused with buffer to ensure the removal of free dye. After 3 min of equilibration, calcium changes within endothelial cells were monitored in response to acetylcholine (3 × 10−6 M) or the calcium ionophore A23187 (10−6 M) using a laser confocal microscope (see below). These agonist concentrations were chosen based on their ability to cause maximal endothelium-dependent contractions (Figure 1). Addition of acetylcholine or A23187 to aortic strips, especially those of SHR caused contraction. This movement was problematic, as this leads to changes of the focal plane photographed by the confocal microscope. To prevent this, all preparations were treated with 3-[(6-amino-(4-chlorobenzensulphonyl)-2-methl-5,6,7,8-tetrahydronapht)-1-yl] propionic acid (S18886, 10−7 M; a specific TP-receptor antagonist given 40 min before fluorescence measurements) (Yang et al., 2003). It was reasoned that blocking the terminal part of the cascade would not affect upstream events but effectively prevent activation of smooth muscle and thus movement artifacts. Nitric oxide exerts acute and chronic inhibitory effects on the production of endothelium-dependent contractions and thus justifies the pretreatment of strips with the nitric oxide synthase blocker, L-NAME to optimize the occurrence of endothelium-dependent contractions (Auch-Schwelk et al., 1992; Yang et al., 2004b; Tang et al., 2005a). Rings without L-NAME were studied in parallel to reveal whether calcium or ROS release are altered in the presence of a functional nitric oxide synthase. Consequently, there were a total of four treatment groups in this study: (a) S18886 control; (b) S18886+L-NAME (10−4 M; to mimic experimental conditions when endothelium-dependent contractions are recorded) (Auch-Schwelk et al., 1992; Tang et al., 2005a); (c) S18886+L-NAME+Indomethacin (10−5 M) and (d) S18886+L-NAME+Tiron (10−2 M)+DETCA (10−4 M). Fluorescence signals emitted from aortic endothelial cells were scanned using a time series protocol to monitor agonist-induced changes in cytosolic calcium concentration in the endothelial cells. A scan was performed every 3 s for 100 s and maximal changes in mean intensity were expressed as changes in ratio (by dividing Fluo-4/Fura-red emitted fluorescence) before and after the addition of drugs. Time controls with perfusion of buffer with no agonist were carried out to ensure that there were no flow-induced calcium changes or artificial fluorescence increase (e.g. fluorescence derived from uncleaved dyes).
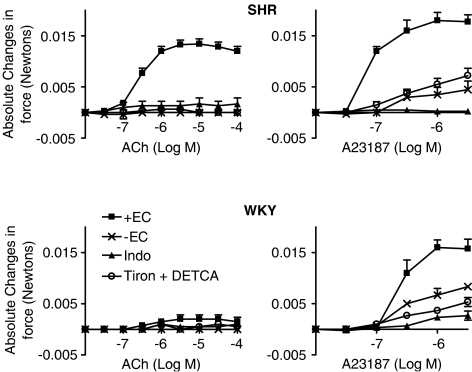
Figure 1.
Contractions to increasing concentrations of acetylcholine (left) or calcium ionophore A23187 (right) in rings of SHR or WKY aorta with (+EC) or without endothelium (−EC), in the absence or presence of either indomethacin (indo, 10−5 M) or Tiron (10−2 M) plus DETCA (10−4 M). Data are expressed as absolute changes in force (Newtons) and shown as means±s.e.m.; n=5.
Intracellular oxidative stress
Rings of aortae harvested from either SHR or WKY rats were incubated for 10 min at room temperature with 4 × 10−5 M dichlorodihydrofluorescein diacetate (DCF) in buffer with 0.02% (v/v) pluronic acid. DCF is a nonspecific ROS sensitive dye; it detects hydrogen peroxide, superoxide anions, hydroxyl radicals and peroxynitrite. The release of this assortment of oxidants is termed ‘oxidative stress'. The loaded aorta was cut open and placed on a glass slide and observed using the laser confocal microscope. Time series measurement of oxidative stress could not be performed on DCF-loaded aortic strips because DCF was not as resistant to excess laser exposure as the calcium-sensitive dyes, which produced intense background fluorescence. To prevent erroneous measurements, oxidative stress was quantified using single still images. The mean intensity of the fluorescence images was obtained 5 min after the application of acetylcholine (3 × 10−6 M) or the calcium ionophore A23187 (10−6 M). Relative fluorescence intensity was calculated using images obtained in the absence of stimuli but in the presence of corresponding inhibitors.
Laser-scanning confocal microscopy
Dye-loaded aortic endothelial cells were examined using an inverted Nikon Eclipse TE300 microscope with a × 10 objective, which was attached to the confocal argon ion Radiance-2100 laser scanning unit (Bio-Rad, Hertfordshire, England). To detect cytostolic calcium changes, the 488 nm argon ion laser line was directed to the sample to excite both Fura-red and Fluo-4 fluorescent probes. The emitted fluorescence was split via a dichroic beam splitter and further directed concomitantly onto two photomultiplier tubes. One tube was equipped with a band pass filter (510–525 nm) to record Fluo-4 emission. The second tube was equipped with a long pass filter (>590 nm) to record Fura-red emission.
An image field of 256 × 256 pixels was selected randomly on the aortic segment at the start of the experiment. The resulting fluorescence is a mean of all pixels in this fixed image size. Ratiometric images were obtained using a pixel-by-pixel division of the Fluo-4 fluorescence by the Fura-red fluorescence. The ratio values used for calculation correspond to the mean of the pixel ratios in the whole image. The LaserSharp 2000 program (Bio-Rad) was used to obtain mean intensity of all the scanned images for data calculation. The parameters of the confocal laser scanning were kept constant for all experiments, as follows: zoom factor =4, intensity =36.4%, pinhole size =1.2, gain =98%. To measure oxidative stress, the same setting was used except that the gain was reduced to 20% instead of 98% and only the first photomultiplier tube which measured emission at 510–525 nm was turned on.
Data analysis
Results are presented as mean±standard error of the mean (s.e.m.) with n= number of animals. Fitted curves were obtained to calculate Emax (maximum contraction) and EC50 (negative logarithm of the molar concentration of agonist inducing half of the maximum response). Statistical analysis was performed by Student's t-test for unpaired observations or by ANOVA analysis for multiple comparisons followed by Tukey's multiple comparison test, where appropriate. All curve fittings and statistical analyses were performed by using Prism version 3a (GraphPad Software, San Diego, CA, USA). A difference was accepted as statistically significant when probability (P) values were <0.05.
Drugs
Acetylcholine, calcium ionophore A23187, DETCA, DCF, L-NAME, pluronic acid F-127 and Tiron were purchased from Sigma Chemical Company (St Louis, MO, USA). Fluo-4 and Fura-red were purchased from Molecular Probes (Eugene, OR, USA). Dimethyl Sulphoxide (DMSO) was purchased from Merck (Darmstadt, Germany). S18886 was a kind gift from the Institut de Recherches Servier (Suresnes, France). All indicator dyes were first dissolved with pluronic acid F127 (0.02% v/v) and DMSO (0.1% v/v) and then further diluted using buffer solution. The calcium ionophore A23187 was dissolved with absolute DMSO and indomethacin was prepared in a sodium bicarbonate (5 × 10−3 M) solution. All other compounds were prepared in deionized water. Concentrations are expressed as final molar concentrations in the buffer.
Results
Isometric force
KCl (6 × 10−2 M) evoked contractions in aortic rings of SHR that were slightly but significantly smaller than those obtained in arteries of WKY (0.015±0.0003 N and 0.017±0.0005 N, respectively). Acetylcholine evoked concentration-dependent contractions in aortic rings of SHR with endothelium (Emax=0.013±0.002 N; EC50 =2.572 × 10−7 M (95% CI: 1.942–3.406 × 10−7 M)) but not in those of WKY. The calcium ionophore A23187 evoked concentration-dependent contractions in rings from both the SHR (Emax=0.018±0.003 N; EC50=7.892 × 10−8 M (95% CI: 0.539–1.156 × 10−7 M)) and the WKY (Emax=0.016±0.003 N; EC50=2.586 × 10−7 M (95% CI: 1.554–4.304 × 10−7 M)) but the contractions were more prominent in the hypertensive rats. The contractions were prevented when the endothelial layer was removed, or in rings treated with indomethacin or Tiron plus DETCA (Figure 1).
Basal calcium level
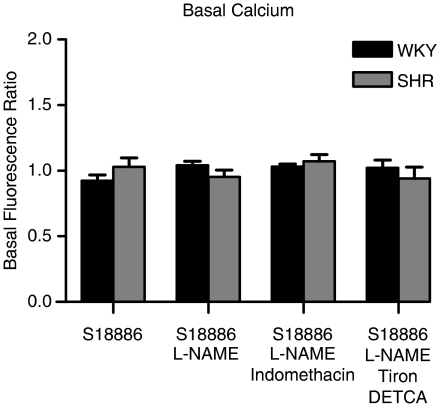
The basal cytosolic calcium level was comparable in endothelial cells of WKY and SHR. Basal calcium within endothelial cells was not significantly altered when the rings were incubated with L-NAME, indomethacin or Tiron plus DETCA for 40 min (Figure 2).
Figure 2.
Basal calcium concentration in endothelial cells of rat aorta expressed as fluorescence ratio. The fluorescence ratio is calculated by dividing the fluorescence emitted by the two calcium sensitive dyes, – Fluo-4/Fura-red. Data shown as means±s.e.m.; n=5.
Cytosolic calcium increase
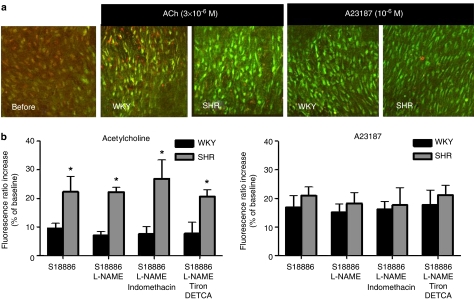
The addition of acetylcholine (3 × 10−6 M) to Fluo-4-Fura-red-loaded strips caused a rapid increase in intracellular calcium in aortic endothelial cells of both SHR and WKY, indicated by the immediate increase in Fluo-4 fluorescence intensity, together with the immediate decrease of Fura-red intensity (Figure 3). The fluorescence ratio increase caused by acetylcholine was significantly more pronounced in aortae of the hypertensive than the normotensive strain (Figure 4). This pattern of fluorescence increase was not altered whether or not the rings were treated with indomethacin or Tiron plus DETCA, or whether or not the nitric oxide synthase inhibitor, L-NAME was present (Figure 4).
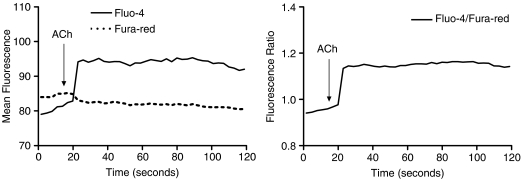
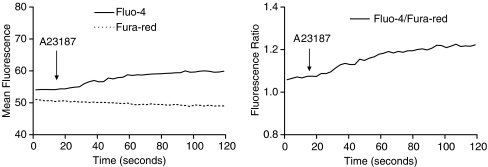
Figure 3.
The change in mean fluorescence intensity (left) and ratio between the fluorescence of fluo-4 and fura-red (right) against time upon infusion of acetylcholine (3 × 10−6 M) in aortic endothelial cells of an SHR strip.
Figure 4.
(a) Merged images taken from confocal microscopy showing responses to infusion of acetylcholine (3 × 10−6 M) or A23187 (10−6 M) on cytosolic calcium of aortic endothelial cells from WKY and SHR. All strips were treated with L-NAME (10−4 M) and S18886 (10−7 M). An increase in green fluorescence and a decrease in red fluorescence indicate calcium rise. (b) The increase in fluorescence ratio in endothelial cells from SHR and WKY in response to acetylcholine (3 × 10−6 M) (left) or A23187 (10−6 M) (right) is expressed in percentages of the baseline values. Ratio was obtained by dividing Fluo-4/Fura-red-emitted fluorescence. Data shown as means±s.e.m.; n=5. The asterisk indicates a statistically significant difference between the two rat strains (P<0.05).
The calcium ionophore A23187 (10−6 M) caused an increase in cytosolic calcium concentration in endothelial cells. There was no statistical significant difference between preparations from SHR and WKY (Figure 4). This rise of calcium by A23187 was not affected by indomethacin or Tiron plus DETCA treatment, or when L-NAME was omitted (Figure 4). The increase in cytosolic calcium level evoked by A23187 in WKY and SHR aortae was not significantly different from that in response to acetylcholine in SHR aortae.
The kinetics of calcium release by A23187 differed from that to acetylcholine. Acetylcholine caused an immediate increase in calcium, which rapidly reached a maximal level and remained at maximum within the 100 s observation period (Figure 3). When the receptor-independent agonist A23187 was used, the increase of calcium was progressive throughout the observation period (Figure 5). There was no difference in the speed of the calcium rise in response to acetylcholine or A23187 between WKY and SHR.
Figure 5.
The change in mean fluorescence intensity (left) and ratio of fluorescence of Fluo-4 and Fura-red (right) against time upon infusion of the calcium ionophore A23187 (10−6 M) in an SHR-loaded strip.
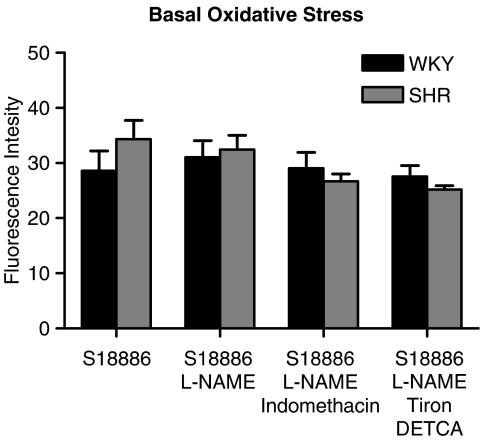
Basal oxidative stress
The basal level of oxidative stress in endothelial cells of WKY and SHR was not significantly different, whether the strips were treated or not with L-NAME, indomethacin or Tiron plus DETCA (Figure 6).
Figure 6.
Resting oxidative stress in aortic endothelial cells of SHR and WKY expressed as DCF fluorescence intensity. Data are shown as means±s.e.m.; n=5–6.
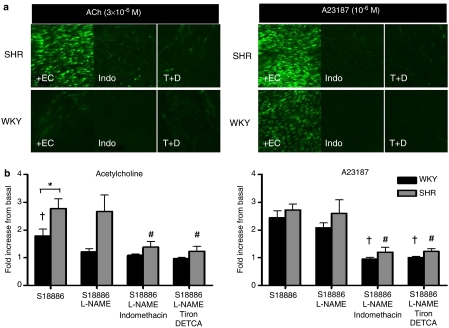
Intracellular oxidative stress increase
In the presence of L-NAME and in response in acetylcholine, the fluorescence intensity of DCF-loaded SHR aortic rings was increased compared to basal after 5 min of exposure to the muscarinic agonist, whereas there was no significant change in WKY aortae (Figure 7). This increase in oxidative stress in arteries of hypertensive rats was prevented when they were treated with indomethacin or Tiron plus DETCA and was not affected when L-NAME was omitted (Figure 7). Acetylcholine increased ROS significantly in WKY rings only in the absence of L-NAME. The amount of ROS released was significantly less than that produced in SHR rings. A23187 elicited a comparable increase in free radicals in aortae of WKY and SHR (Figure 7). The increase in oxidative stress by A23187 was prevented by indomethacin and Tiron plus DETCA and was not affected by the absence of L-NAME (Figure 7).
Figure 7.
(a) Images taken from confocal microscopy showing oxidative stress in response to acetylcholine (3 × 10−6 M) or A23187 (10−6 M) on aortic endothelial cells from SHR (top panel) and WKY (bottom panel). An increase in green fluorescence indicates oxidative stress increase. All rings shown were treated with L-NAME (10−4 M) and S18886 (10−7 M). +EC = with endothelium; indo = indomethacin; T+D = Tiron plus DETCA. (b) The increase in DCF fluorescence in endothelial cells from SHR and WKY in response to acetylcholine (3 × 10−6 M) (left) or A23187 (10−6 M) (right) was expressed as fold increase from baseline values; data shown as means±s.e.m.; n=5–6. The asterisk indicates a statistically significant difference between the two rat strains (P<0.05); The dagger indicates a statistically significant difference between WKY (S18886+L-NAME); The number sign indicates a statistically significant difference between SHR (S18886+L-NAME).
The occurrence of contractions, elevation of endothelial calcium and ROS in SHR and WKY aortic rings is summarized in Table 1.
Table 1.
The occurrence of contractions, intracellular calcium and ROS elevations in endothelial cells of rat aortic rings (in the presence of 10−4 M L-NAME and 10−7 M S18886)
| Contraction | Endothelial calcium | Endothelial ROS | |
|---|---|---|---|
| WKY | |||
| Ach | No | ↑ Not inhibited by indomethacin, Tiron+DETCA | No Change |
| A23187 | Yes; inhibited by indomethacin, Tiron+DETCA | ↑ Not inhibited by indomethacin, Tiron+DETCA | ↑ Inhibited by indomethacin, Tiron+DETCA |
| SHR | |||
| Ach | Yes; inhibited by indomethacin, Tiron+DETCA | ↑ Not inhibited by indomethacin, Tiron+DETCA (>WKY) | ↑ Inhibited by indomethacin, Tiron+DETCA (>WKY) |
| A23187 | Yes; inhibited by indomethacin, Tiron+DETCA | ↑ Not inhibited by indomethacin, Tiron+DETCA (=WKY) | ↑ Inhibited by indomethacin, Tiron+DETCA (=WKY) |
Abbreviations: DETCA, diethyldithiocarbamate; L-NAME, Nω-nitro-L-orginine methyl ester; ROS, reactive oxygen species; SHR, spontaneously hypertensive rats; WKY, Wistar–Kyoto rats.
Discussion
The study demonstrates that endothelium-dependent contractions in the WKY and SHR aortae are associated with an increase in endothelial intracellular calcium concentration and the endothelial generation of ROS. In cultured aortic endothelial cells, the resting cytosolic-free calcium concentration is significantly lower in preparations derived from SHR than from WKY rats (Wang et al., 1995). In the present study, where fresh endothelial cells were studied in situ, the basal resting cytosolic-free calcium concentration in endothelial cells of SHR and WKY was equivalent and treatment with indomethacin and antioxidants or removal of L-NAME did not modify these basal calcium levels.
Acetylcholine caused a rapid increase in cytosolic calcium concentrations in endothelial cells of both SHR and WKY. The increase of cytosolic calcium occurred in a matter of seconds and was maintained at its maximal level upon exposure to acetylcholine during the 100 s observation period. The speed of calcium increase caused by acetylcholine did not differ between SHR and WKY, whereas the magnitude of calcium release is significantly more pronounced in the aortic endothelial cells of hypertensive strain. Alternation in genes encoding major calcium transport pathways, including the sarcoplasmic endoplasmic reticulum calcium pumps and inositol 1,4,5-triphosphate receptors channels in aortic endothelial cells of hypertensive animals have been described (Mountian et al., 2001). These findings are in line with the current observation that there is a calcium handling dysfunction in the aortic endothelial cells of the SHR.
Calcium release by the muscarinic agonist was not affected whether or not cyclooxygenase was inhibited (by indomethacin) or ROS were removed (by the antioxidants Tiron plus DETCA) or if nitric oxide synthase remained operational (in the absence of L-NAME). These observations demonstrate that acetylcholine causes an increase in intracellular calcium within endothelial cells. This rise in calcium precedes the activation of cyclooxygenase, the production of ROS, the stimulation of TP receptors or the activation of nitric oxide synthase. The rapidity of the increase in cytosolic calcium strongly suggests that the calcium rise is the foremost step of the endothelium-dependent contraction cascade.
The calcium ionophore A23187 caused a comparable increase in cytosolic calcium in endothelial cells of WKY and SHR. The kinetic profile of calcium entry by A23187 was gradual and concurs with the properties of A23187 being a receptor-independent agonist which by opening pores in the cell membrane permits the free entry of extracellular calcium into the endothelial cells following its concentration gradient. This receptor-independent phenomenon explains why the same amount of calcium accumulation can be reached in endothelial cells of the WKY and the SHR. There was no difference in the speed of calcium elevation caused by A23187 between WKY and SHR. In both the SHR and WKY, increase in intracellular calcium caused by A23187 at 10−6 M is comparable to that elicited by 3 × 10−6 M acetylcholine in endothelial cells of the hypertensive strain. This indicates that if the abnormal accumulation of calcium can be mimicked within the endothelial cells and/or once the required critical threshold of calcium is reached, the production of EDCF can be triggered. High calcium levels are required to initiate the release of EDCF and this then explains why endothelium-dependent contractions can be produced by A23187 in aortas from both normotensive WKY and hypertensive SHR, whereas acetylcholine only evokes EDCF-mediated contractions mainly in the SHR (Yang et al., 2004a). The aorta of the SHR appeared far more susceptible to A23187-induced vasoconstriction as Emax is considerably higher and the EC50 value was significantly lower in these rats, despite the fact that A23187 induced similar level of calcium and ROS production in WKY and SHR. One possible explanation for this is, in addition to the requirement of abnormal calcium rise, changes in abundance or sensitivity of important components of the EDCF cascade also contribute to the level of endothelium-dependent contractions. Expressions of prostacyclin synthase (Numaguchi et al., 1999), COX-1 (Ge et al., 1995) and sensitivity to TP receptors located on the smooth muscle (Ge et al., 1995) are elevated in aortae of SHR as compared with WKY. All these modifications synergistically cause SHR to be more susceptible to A23187-induced endothelium-dependent contractions as compared with WKY.
The level of calcium increase owing to the calcium ionophore A23187 was not affected by indomethacin or Tiron plus DETCA or the absence of L-NAME, confirming that the activation of cyclooxygenase and the production of ROS or the stimulation of nitric oxide synthase occur downstream from the increase in intracellular calcium.
The basal level of oxidative stress is enhanced in the whole aorta and cultured smooth muscle cells of SHR compared to that of WKY (Wu et al., 2001). However, in the present study, the basal oxidative stress within endothelial cells as observed in situ in aortic segments taken from either SHR or WKY was similar. The present measurements were focused to evaluate oxidative stress within endothelial cells only and did not include vascular smooth muscle cells. Thus, the difference in oxidative stress of normotensive and hypertensive vascular beds as reported by others may be inherent to smooth muscle (Nabha et al., 2005; Wu et al., 2001, 2005). Treatment of the aortic strips with indomethacin, antioxidants or removal of nitric oxide synthase blocker did not affect the measurement of basal oxidative stress, suggesting that it is restricted and at a minimal level under resting conditions.
The present study reveals that EDCF-mediated responses are accompanied by a burst of ROS which is of endothelial origin. The burst of ROS must either accompany or be generated downstream of the activation of cyclooxygenase as blockade of this enzyme with indomethacin prevented the increase in oxidative stress. The free radicals presumably are derived directly from cyclooxygenase (Kontos et al., 1985; Kukreja et al., 1986) or cyclooxygnease-derived products may function as an autocrine stimulator of ROS. Antioxidant treatment with Tiron plus DETCA served as a negative control and prevented the stimulation of ROS, strengthening the interpretation that the intensity of fluorescence measured reflects the level of oxidative stress. The absence of ROS release in the WKY aorta (in the presence of L-NAME) is presumably due to the inability of acetylcholine to release enough calcium in the endothelial cells to trigger the initial step of the EDCF cascade. Thus, cyclooxygenase is not fully activated and consequently there is no production of ROS. The fact that acetylcholine fails to produce endothelium-dependent contractions in WKY is in line with the present data.
In the presence of a functional nitric oxide synthase, in other words when L-NAME was absent, acetylcholine increased oxidative stress in endothelial cells of both WKY and SHR, being significantly more prominent in the latter. DCF is a nonspecific ROS sensitive dye; it can detect a range of oxidants including superoxide anions, hydroxyl radicals, hydrogen peroxide and peroxynitrite (Gomes et al., 2005). The small amount of oxidative stress produced in the WKY is probably due to peroxynitrite formation derived from the reaction between nitric oxide and superoxide anions or enzymatic dismutation of superoxide anion from eNOS to hydrogen peroxide, as this modest oxidative stress discharge was prevented when either L-NAME or antioxidants were present.
When A23187 was used to mimic the augmented level of calcium within endothelial cells, it released ROS in both SHR and WKY to the same extent. These data support the concept that once a critical threshold of calcium is reached within the endothelial cells, it can trigger the activation of phospholipase A2 (and thus cyclooxygenase) and hence production of ROS in preparations from both normotensive and hypertensive rats. Indomethacin effectively prevented ROS production by A23187. This then again demonstrates that the activation of COX-1 is upstream of the production of ROS and not vice versa. The production of free radicals by A23187 logically was prevented by the combination of antioxidants, Tiron plus DETCA.
How ROS specifically contribute to endothelium-dependent contractions remains unknown. It is unlikely that ROS themselves are the sole factor accounting for EDCF, since endoperoxides, thromboxane A2 and prostacyclin all can contributes to EDCF-mediated responses, at least in the aorta of the SHR (Yang et al., 2002; Gluais et al., 2005; Vanhoutte et al., 2005). ROS may serve as an enhancer to augment endothelium-dependent contractions by catalyzing or favoring the breakdown of endoperoxides into constrictors rather than relaxing prostanoids. Furthermore, ROS formed by the endothelial cells may enhance smooth muscle contractility directly or augment TP-receptor sensitivity.
Laser-scanning confocal microscopy imaging allowed focusing on the endothelial cell layer and using the appropriate dyes permitted visualization of the rapid calcium and ROS fluctuations in endothelial cells. The present experiments provided direct quantitative measurements of calcium and ROS in endothelial cells of SHR and compared it with age-matched WKY. They showed that endothelial cells of SHR are more prone to calcium and ROS overload upon stimulation with acetylcholine. Abnormal accumulation of calcium is a prerequisite to initiate the release of EDCF and can be mimicked by using the calcium ionophore A23187. The precise sequence of events that occurs during endothelium-dependent contractions firstly requires accumulation of calcium, which then activates the cyclooxygenase pathway and produces ROS along with EDCF(s) that in turn stimulate TP receptors to cause the response of the vascular smooth muscle.
Abbreviations
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- DCF
dichlorodihydrofluorescein diacetate
- DETCA
diethyldithiocarbamate acid
- DMSO
dimethyl sulphoxide
- EDCF
endothelium-dependent contracting factors
- EDRF
endothelium-dependent relaxing factors
- Indo
indomethacin
- L-NAME
Nω-nitro-L-arginine methyl ester
- ROS
reactive oxygen species
- S18886
3-[(6-amino-(4-chlorobenzensulfonyl)-2-methl-5,6,7,8-tetrahydronapht)-1-yl] propionic acid∣SHR, spontaneously hypertensive rats
- T+D
Tiron+DETCA
- TP-receptor
thromboxane prostanoid receptor
- WKY
Wistar–Kyoto rats
Conflict of interest
The authors state no conflict of interest.
References
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;9:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Budel S, Schuster A, Stergiopoulos N, Meister JJ, Beny JL. Role of smooth muscle cells on endothelial cell cytosolic free calcium in porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2001;28:H1156–H1162. doi: 10.1152/ajpheart.2001.281.3.H1156. [DOI] [PubMed] [Google Scholar]
- Cohen R. Does EDCF contribute to diabetic endothelial cell dysfunction. Dialog Cardiovasc Med. 2002;7:225–231. [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Method. 2005;31:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Ellis EF, Jenkins LW, Povlishich JT, Rowe GT, et al. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ Res. 1985;57:142–151. doi: 10.1161/01.res.57.1.142. [DOI] [PubMed] [Google Scholar]
- Kukreja R, Kontos H, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NAD+ or NADPH. Circ Res. 1986;59:612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- Leung HS, Yao X, Leung FP, Ko WH, Chen ZY, Gollasch M, et al. Cilnidipine, a slow-acting Ca2+ channel blocker, induces relaxation in porcine coronary artery: role of endothelial nitric oxide and [Ca2+]i. Br J Pharmacol. 2006;147:55–63. doi: 10.1038/sj.bjp.0706450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Ratiometric confocal Ca2+-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993;14:359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Marie F, Bény JL. Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J Vascu Res. 2002;39:260–267. doi: 10.1159/000063691. [DOI] [PubMed] [Google Scholar]
- Mountian I, Baba-Aissa F, Jonas JC, De Smedt H, Wuytack F, Parys JB. Expression of Ca2+ transport genes in platelets and endothelial cells in hypertension. Hypertension. 2001;37:135–141. doi: 10.1161/01.hyp.37.1.135. [DOI] [PubMed] [Google Scholar]
- Nabha L, Garbern JC, Buller CL, Charpie JR. Vascular oxidative stress precedes high blood pressure in sponataneously hypertensive rats. Clin Exp Hypertens. 2005;27:71–82. doi: 10.1081/ceh-200044267. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Harada M, Osanai H, Hayashi K, Toki Y, Okumaura K, et al. Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovasc Res. 1999;41:682–688. doi: 10.1016/s0008-6363(98)00239-9. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiaoni L, Versari D, Salvetti A. Does EDCF play an important role in humans. Dialog Cardiovasc Med. 2002;7:237–243. [Google Scholar]
- Tang EHC, Feletou M, Huang Y, Man RYK, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005a;289:H2434–H2440. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Tang EHC, Ku DD, Tipoe GL, Feletou M, Man RYK, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild type and COX2−/− but not COX-1−/− knockout mice. J Cardiovasc Pharmacol. 2005b;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Sauve R, de Champlain J. Abnormal regulation of cytosolic free calcium in vascular endothelial cells from spontaneously hypertensive rats. J Hypertens. 1995;13:993–1001. [PubMed] [Google Scholar]
- Wu R, Lamontagne D, de Champlain J. Antioxidative properties of acetylsalicylic acid on vascular tissues from normotensive and spontaneously hypertensive rats. Circulation. 2002;22:387–392. doi: 10.1161/hc0302.102609. [DOI] [PubMed] [Google Scholar]
- Wu R, Millette E, Wu L, De Champlain J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2001;19:741–748. doi: 10.1097/00004872-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004a;18:321–326. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Nitric oxide and inactivation of the endothelium-dependent contracting factor released by acetylcholine in spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2004b;43:815–820. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]