Abstract
Background and purpose:
The pro-inflammatory cytokine, interleukin-1β (IL-1β), has been implicated in the pathogenesis of atherosclerosis, potentially via its release from vascular endothelium. Endothelial cells (EC) synthesize IL-1β in response to inflammatory stimuli, but the demonstration and mechanism of release of IL-1 from ECs remains unclear. In activated monocytes, efficient release of bioactive IL-1β occurred via activation of ATP-gated P2X7 receptors (P2X7Rs). Activation of P2X7R in ECs from human umbilical vein (HUVECs) released IL-1 receptor antagonist (IL-1Ra). The purpose of this study was to provide a quantitative investigation of P2XR expression and function, in parallel with IL-1β and IL-1Ra synthesis, processing and release, in HUVECs under pro-inflammatory conditions.
Experimental approach:
Quantitative RT-PCR, immunoblotting, ELISA, flow cytometry, and whole-cell patch clamp recordings were used to determine protein expression and receptor function. IL-8-luciferase-reporter was used as an IL-1 sensitive bioassay.
Key results:
HUVECs expressed P2X4R and P2X7R subtypes and both were significantly up-regulated under inflammatory conditions. P2X7R currents were increased 3-fold by inflammatory stimuli, whereas no P2X4R-mediated currents were detected. Caspase-1, but not IL-1β, was present intracellularly under basal conditions; inflammatory stimuli activated the synthesis of intracellular pro-IL-1β and increased caspase-1 levels. Activation of P2X7Rs resulted in low-level release of bioactive IL-1β and simultaneous release of IL-1Ra. The net biological effect of release was anti-inflammatory.
Conclusions and implications:
Endothelial P2X7Rs induced secretion of both pro- and anti-inflammatory IL-1 receptor ligands, the balance of which may provide a means for altering the inflammatory state of the arterial vessel wall.
Keywords: P2X receptors, IL-1, IL-1Ra, endothelial cells, cytokines, inflammation
Introduction
The interleukin-1 (IL-1) family of cytokines are important regulators of the acute inflammatory response (Dinarello, 1996) with two identified agonists IL-1α and IL-1β as well as a naturally occurring inhibitor of IL-1 signalling IL-1 receptor antagonist (IL-1Ra). IL-1β is a leaderless protein, thus lacking the signal peptide generally found in prototypically secreted proteins responsible for shuttling secreted proteins through the organized endoplasmic reticulum (ER)-Golgi secretory pathway (Zocchi and Rubartelli, 2001). In inflammatory cells such as the monocyte/macrophage, pro-inflammatory stimuli, notably bacterial lipopolysaccharide (LPS), induce high levels of pro-inflammatory IL-1β (pro-IL1β) synthesis but, in the absence of a secondary stimulus, little IL-1β is released into the medium (Hogquist et al., 1991; Perregaux and Gabel, 1994; Ferrari et al., 1997a; Di Virgilio et al., 1998; Sanz and Di Virgilio, 2000). One of the best demonstrated physiological mechanisms for release of bioactive IL-1β from activated macrophages is the purinergic P2X7 receptor (Ferrari et al., 2006). The P2X7 receptor is a cation-selective ion channel activated by extracellular adenosine 5′-triphosphate (ATP) but, in striking contrast to other ion channels, its activation also leads to the opening of larger pores able to pass molecules up to 900 Da (Virginio et al., 1999). It is thought that activation of this so-called large pore triggers changes in intracellular ionic homeostasis sufficient to activate the caspase-1 cascade leading to proteolytic cleavage of pro-IL-1β and immediate release into the external environment. Although more than one mechanism of release of processed IL-1β in response to P2X7 receptor activation has been documented, there is now substantial evidence that it is the P2X7, and not other P2X receptors, which is responsible for IL-1β release from activated monocytes and macrophages (Perregaux and Gabel, 1994; Griffiths et al., 1995; Ferrari et al., 1997b; Di Virgilio et al., 1998; Perregaux et al., 2000; Sanz and Di Virgilio, 2000; Mehta et al., 2001; Le Feuvre et al., 2002). The most unequivocal evidence for this receptor's role in ATP-mediated IL-1β release comes from studies with transgenic mice lacking the P2X7 receptor gene. In LPS-stimulated macrophages from these mice, no IL-1β is released in response to application of ATP, although LPS-induced synthesis of both caspase-1 and pro-IL-1β are unaltered (Solle et al., 2001). These mice also exhibit attenuated inflammatory responses (Labasi et al., 2002).
A unique feature of IL-1 receptors is the counterbalance of the actions of the pro-inflammatory IL-1α (pro-IL-1α) and pro-IL-1β by two inhibitory mechanisms: a soluble non-signalling/decoy receptor (IL-1RII) plus a naturally occurring inhibitory form of IL-1, IL-1Ra, which competitively inhibits binding of IL-1α and IL-1β to IL-1RI. IL-1Ra does not activate the receptor, as its binding prevents recruitment of the accessory protein to the receptor complex (Arend, 2002). There is evidence that the balance between IL-1 and IL-1Ra determines the overall inflammatory response (Arend, 2002).
A key feature of any organism's inflammatory response is endothelial cell (EC) activation resulting in recruitment of inflammatory cells (Bevilacqua et al., 1984). There are many reports that ECs synthesize IL-1β (Warner et al., 1987; Galea et al., 1996) but little demonstration of IL-1 release from these cells. Production of inflammatory cytokines by resident non-leukocytes has been hypothesized as a mechanism of chronic inflammation. IL-1β synthesis by EC occurs at sites of chronic inflammation; we have previously shown that EC upregulated IL-1β in human coronary atherosclerosis (Galea et al., 1996; Chamberlain et al., 1999, 2006). Neointima development in animal models is retarded by IL-1 inhibitory strategies (Morton et al., 2005; Chamberlain et al., 2006). We have shown previously that ECs synthesize only the intracellular splice variant of IL-1Ra (Dewberry et al., 2000) and also demonstrated that this particular splice variant (also lacking a signal peptide sequence) is released from EC following P2X7 receptor stimulation in the absence of induced cell death (Wilson et al., 2004).
In this study, we characterize P2X receptor expression and responses in ECs, the conditions under which IL-1β is synthesized by ECs and examine how release occurs to determine the relative importance of this in the context of concomitant intracellular IL-1Ra release.
Materials and methods
Cells and solutions
Human umbilical vein ECs (HUVECs) were isolated by 0.1% Type IV collagenase (Sigma) treatment from umbilical cords collected following individual informed consent from the Sheffield Teaching Hospitals' maternity unit. Freshly isolated cells were cultured at 37°C on gelatin-coated flasks in medium 199 (Sigma, Dorset, UK). From passage 1, cells were grown in RPMI medium (Invitrogen, Paisley, UK), to avoid culture in media supplemented with ATP (i.e. M199, a common EC growth medium), which might downregulate ATP-mediated events. Media were supplemented with 20 μg ml−1 EC growth supplement (Totam Biologicals, Peterborough, UK), 90 μg ml−1 heparin (Sigma), 1% Fungizone (Life Technologies) 10% fetal calf serum (FCS) and 10% newborn calf serum (Life Technologies) (Jaffe et al., 1973). HeLa cells were purchased from ATCC (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM glutamine, 10% heat-inactivated FCS (BioWhitaker, Surrey, UK), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Some HUVECs were purchased from PromoCell (Heidelberg, Germany) and cultured in the supplied media, according to the manufacturer's instructions. HeLa cells were transfected using PolyFect transfection reagent (Qiagen, W Sussex, UK) according to manufacturer's instructions. Human embryonic kidney (HEK293) cells (ATCC) were transfected with Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. Human tamm-horsefall protein-1 (THP-1) monocytes (ATCC) were differentiated with 0.5 μM phorbol-12-myristate 13-acetate (Sigma) and plated into six-well plates as described previously (MacKenzie et al., 2001).
Control experiments used rP2X7, hP2X7, hP2X7±C-terminal EE (EYMPME) or green fluorescent protein (GFP) tags, stably or transiently expressed in HEK293 cells as described previously (Virginio et al., 1999). Adenoviral constructs were generated for hP2X7-GFP or hP2X7-EE using the BD-Clontech Adeno-X™ Expression System 1 (Clontech, BD, Oxford, UK). Virus produced from these constructs and from empty-vector controls was used to infect HUVECs in complete growth medium, by overnight incubation.
Stimulation conditions used were LPS (recombinant from Escherichia coli, serotype 0111:B4 (055:B5; Sigma) at 1 μg ml−1, interferon γ (IFNγ; Calbiochem, Nottingham, UK) at 100 ng ml−1, tumour necrosis factor-α (TNFα; Calbiochem) at 10 ng−1 and IL-1β; R&D systems, Abingdon, UK) at 10 ng ml−1 (unless otherwise indicated). Incubations were at 37°C in normal growth media. Cells were then washed with phosphate-buffered saline (PBS) and 3′-O-(4-benzoyl)benzoyl-ATP (Bz-ATP) at 300 μM or ATP (Sigma) at 3 mM, was added in extracellular solution, consisting of 147 mM N-methyl-D-glucamine, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES and 12 mM glucose, for 60 min unless otherwise stated, at 37°C. Supernatants were collected, cells were washed with PBS, and then extracted by scraping in PBS plus 1% Triton or 1% dodecyl maltoside, plus protease inhibitors (Cocktail III, Calbiochem).
Reverse transcription to detect P2X receptor message
Reverse transcription (RT) from total RNA was made using the Superscript III System (Invitrogen). Primer pairs used were as shown in Table 1. Thermal cycling was performed using a Hybaid PCR machine, for 94°C, 1 min; 35 cycles of 94°C, 30 s; 55°C, 30 s; 72°C, 1 min, then a final step to 72°C for 5 min. Products were run on 1.5% agarose gel containing ethidium bromide.
Table 1.
Primer pairs used in RT-PCR and q-PCR
Quantitative RT-polymerase chain reaction
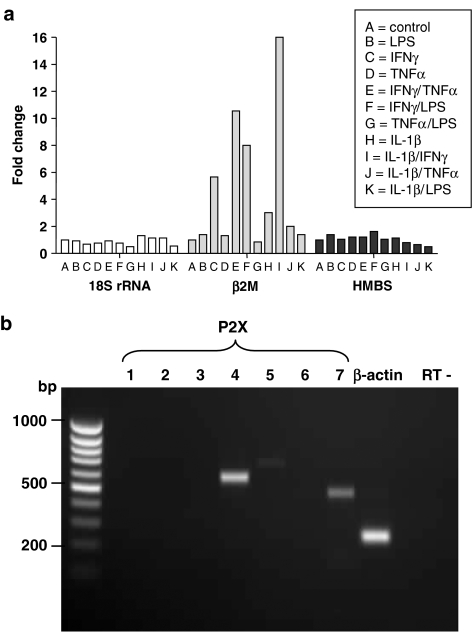
SYBR-green probes (Molecular Probes) were used with an iCycler (Bio-Rad, Herts, UK) thermal cycler, for primers pairs as shown in Table 1. 18S-rRNA, β2M and human hydroxymethylbilane synthase (HMBS) were used as housekeeping genes, which have been suggested to be more appropriate controls that are not susceptible to tissue variation or complex transcriptional regulation (Vandesompele et al., 2002); their expression was compared using a cocktail of different inflammatory stimuli (Figure 1a), to assess whether these were prone to variable expression under such conditions. Both 18S-rRNA and HMBS exhibited unaltered levels of expression in the presence of these stimuli, whereas the expression of β2M was highly variable, being expressed at much higher levels in the presence of IFNγ, and combinations containing this agent. The latter was therefore deemed an unsuitable housekeeping control. In all further quantitative RT-polymerase chain reaction (qRT-PCR) experiments, both 18S-rRNA and HMBS were used as housekeeping controls, where relative expression levels of these two messages were normalized and averaged. We considered this the most reliable means to avoid introducing bias-related variations due to inflammatory stimulating conditions. Each primer pair-stimulatory condition was assayed in triplicate and melt curves obtained routinely to check primer specificity.
Figure 1.
RT-PCR assay of P2X receptor subtypes in HUVEC. (a) Quantitative RT-PCR was optimized for ‘housekeeping genes' to detect human 18S rRNA, β2-microglobulin (β2M) and human hydroxymethylbilane synthase (HMBS), under various stimulatory conditions. HUVECs were cultured in the presence of pro-inflammatory mediators for 24 h before total RNA was extracted and cDNA synthesized. h18S rRNA, hβ2M and hHMBS qRT-PCR primers were then tested against these cDNA samples to observe changes in expression levels. (b) RT-PCR on total RNA from unstimulated HUVECs using standard P2X receptor and β-actin primers, separated by agarose gel electrophoresis. RT-control is for thermal cycling in the absence of template.
Probes were tested by melt curve analysis to ensure single products were detected and that no signal from the template was detected due to primer–dimer formation. The efficiency and specificity of all primer sets were tested on plasmids encoding P2X4, P2X7, 18S-rRNA, β2-M and HMBS, subcloned into pcDNA3.1- (Invitrogen) or pGEM-T (Promega, Southampton, UK). Primer pairs were used for quantification where efficiencies of ⩾93% were obtained, and given by Efficiency (%)=(10−1/slope−1) × 100 (Rasmussen, 2001). Thermal cycling parameters were: 94°C, 1 min; 25 cycles of 94°C, 30 s; 55°C, 30 s; 68°C, 90 s; then 68°C for 5 min.
Flow cytometry
Detection of P2X7 receptors on the cell surface of HUVEC was performed by flow cytometry using a mouse monoclonal anti-human P2X7 receptor antibody (gift from I Chessell, Neurology and GI Centre of Excellence for Drug Discovery, GlaxoSmithkline Pharmaceuticals, New Frontiers Science Park, Essex, UK) at 6.8 μg ml−1, or mouse monoclonal IgG2a (Dako, Cambridgeshire, UK) as isotype control at 1 μg ml−1, and F(ab′)2 rabbit anti-mouse IgG-fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Serotec, Oxford, UK) at 10 μg ml−1.
Electrophysiology
HUVECs were plated onto coverslips and cultured in complete media for at least 24 h before whole-cell patch-clamp recordings were made. Recordings were made at room temperature using an EPC9 amplifier (HEKA Electronics, Lambrecht, Germany) and Pulse acquisition software. Internal solution was (in mM) 145 NaCl, 10 HEPES, 10 EGTA (pH 7.3); standard external solution was (in mM) 145 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 13 glucose (pH 7.3) and osmolarity of 300–310 mosmol l−1. Low divalent cation external solution (LDV) contained 0 MgCl2 and 0.2 mM CaCl2. Agonists were applied using a fast flow system (Rapid Solution Changer, IntraCel, Royston, UK) and the cells were held at a holding potential of −60 mV. Agonists used were at 30 μM and Bz-ATP at 300 μM. Ivermectin (Sigma) at 3 μM was used as a tool to potentiate P2X4 responses (Khakh et al., 1999) and the LDV was used to potentiate P2X7 responses, their effects being confirmed in HEK293 cells stably expressing P2X4 or P2X7 receptors, respectively. The specific P2X7 receptor antagonist AZ11645373, (Stokes et al., 2006) used at 100 nM and KN-62 used at 1 μM were applied to the cell for 3 min before agonist.
For adenoviral-mediated gene delivery, HUVECs were plated onto coverslips and cultured in complete media for at least 24 h. The P2X7 receptor adenovirus construct was added for 24–48 h and cells were patched 48 h post-infection.
Immunoblotting
Supernatant media from cell incubations were analysed directly or concentrated by centrifugation using Nano-sep 10 kDa cutoff filters (VWR). Supernatants from stimulated cells and whole-cell extracts were analysed by immunoblotting after electrophoresis on 12% sodium dodecyl sulphate (SDS)–polyacrylamide gel (PAGE) and transfer onto polyvinylidene fluoride (PVDF) membrane. Samples were loaded at equal concentrations, as determined by protein assay. Antibodies were diluted in PBS with 0.1% Tween20 (PBS-T) or Tris-buffered saline (TBS) with 0.1% Tween-20 (TBS-T), 2% nonfat milk. Primary antibodies (all polyclonal) used were (r=rat, h=human): rabbit anti-rP2X4 (Alomone Labs, Jerusalem, Israel) used at 0.3 μg ml−1 (crossreacts with human); rabbit anti-rP2X7 (Alomone Labs) used at 0.3 μg ml−1; goat anti-hP2X7 (Santa Cruz, Santa Cruz, CA, USA) used at 0.1 μg ml−1; rabbit anti-hIL-1β (Santa Cruz) used at 0.5 μg ml−1; goat anti-hIL-1α (R&D systems) used at 0.2 μg ml−1; rabbit anti-hCaspase-1 (Santa Cruz) used at 0.5 μg ml−1; goat anti-hIL-1ra (R&D Systems) at 0.1 μg ml−1. Positive controls used were: hP2X4, lysates of HEK293 cells stably expressing hP2X4 receptor; hP2X7, lysates of HEK293 cells stably expressing hP2X7 receptor; hIL-1β, recombinant human IL-1β (R&D systems); hIL-1α, recombinant human IL-1α (R&D systems); hIL-1Ra, recombinant IL-1Ra (R&D Systems); hCaspase-1, lysates of HL60 cells. These primary antibodies were incubated for 1 h at room temperature or overnight at 4°C then washed four times in PBS-T or TBS-T. The corresponding species of secondary antibody as HRP conjugate (Dako) at 1:2000 dilution, incubated for 1 h at room temperature, followed by four washes for 15 min in TBS-T. Protein band detection used the Western Lightning chemiluminescent detection system (Perkin-Elmer, Beaconsfield, Buchs, UK). If necessary membranes were stripped in Restore Western-blot stripping buffer (Perbio, Northumberland, UK), according to manufacturer's instructions. Band densities from blots were compared for semiquantitative analysis using National Institutes of Health (NIH) image.
Surface protein biotinylation
Cells were plated into 60 mm Petri dishes (three dishes per treatment) and cultured for 48 h in the presence or absence of IFNγ+TNFα. Cells were washed once with PBS and 2 ml (0.5 mg ml−1) EZ-link Sulfo-NHS-LC-biotin (Perbio) was added to each dish of cells for 30 min at room temperature. Glycine was added to quench the biotinylation reaction. Cells were pelleted and lysed in 2% Triton X-100-containing lysis buffer plus complete protease inhibitors (Calbiochem). Equal amounts of protein (determined as below) were used in the pull-down of biotinylated cell surface proteins using Immuno-pure immobilized streptavidin beads (PerBio). Samples were incubated with streptavidin beads overnight at 4°C by rotating. Beads were washed three times with lysis buffer then protein eluted by addition of SDS–PAGE sample buffer heated to 100°C for 5 min, followed by separation on 8% polyacrylamide gels.
Protein assay
Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Pierce) or the Bio-Rad protein assay.
LDH assay
Lactate dehydrogenase (LDH) activity in supernatants or 1% Triton-X lysed cells was determined by measuring the change in absorbance over time at 340 nm using an LDH kit (Sigma), according to manufacturer's instructions.
IL-8 luciferase used as an IL-1-sensitive bioassay
For IL-8 luciferase reporter assays, HeLa cells were transfected with the pIL-8-luc construct (Kiss-Toth et al., 2000) and pTK-luc (Promega) in 96-well plates at approximately 1 × 104 cells per well (around 80% confluence). Transfection efficiency was estimated using pEGFP-N1 (Clontech, Basingstoke, UK) transfection by counting the percentage of green cells in three separate fields by fluorescence microscopy ( × 20 objective), and by measuring Renilla luciferase. Cells were used only where transfection efficiencies were ⩾80%. Incubations were with or without human recombinant IL-1β (at 10 pg ml−1, R&D Systems) for 6 h at 37°C. Cells were then lysed with passive lysis buffer (Promega), transferred into microtitre plates (DYNEX Technologies, Worthing, UK) and assayed for luminescence intensity using Standard Luciferase Assay and Stop and Glo reagents (Promega) on a Packard Bioscience Fusion plate reader.
Luciferase activity was calculated by normalizing to the transfection efficiency based on the Renilla luminescence measured in each well. Ratios were taken for each individual experiment. For comparison between experiments, the activity of the IL-1β-stimulated control was taken as the maximal (100%) value, and activities expressed as a percentage of this.
Measurement of IL-1β and IL-1Ra levels by ELISA
Concentrations of human IL-1β, IL-1Ra and soluble Type II IL-1 receptor (sIL-1RII) in cell extracts and supernatants were quantified by enzyme-linked immunosorbent assay (ELISA). For IL-1β, a standard kit (Endogen, Perbio) or a high sensitivity assay was used (R&D Systems, QuantiGlo). IL-1Ra and sIL-1RII levels were quantified using ELISA Quantikine kits (R&D).
Statistical analysis
Unless otherwise stated, results are mean±s.e.m. of 3–6 experiments. Paired t-tests were used to compare P2X4 and P2X7 mRNA expression levels within the same samples. One-way analysis of variance (ANOVA) with appropriate post-tests was used to compare samples as stated.
Results
P2X receptor expression in HUVECs
mRNA levels
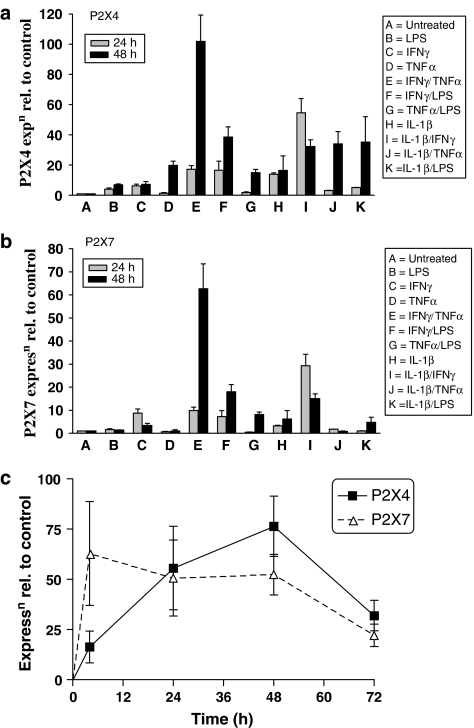
RT-PCR was initially carried out on unstimulated HUVECs using the standard P2X receptor and β-actin PCR primers. mRNA encoding P2X4 and P2X7 receptors, but not P2X1, P2X2, P2X3, or P2X6 receptors, were clearly detected (Figure 1b). A very low level of P2X5 receptor transcript was detected in some RT-PCRs. LPS, IFNγ, TNFα and IL-1β itself, alone or in combination significantly increased mRNA levels of both P2X4 and P2X7 receptors, with combined treatment using IFNγ and TNFα inducing the greatest increase in both of these receptors (Figures 2a and b). IFNγ+TNFα treatment resulted in a 100-fold and a 65-fold upregulation of P2X4 receptor and P2X7 receptor message, respectively, at 48 h (Figures 2a and b). Levels of mRNA for P2X7 receptors reached their maximum within 6 h of treatment with IFNγ+TNFα and remained elevated at approximately the same level for 48 h, whereas P2X4 receptor mRNA gradually increased to reach its maximum levels at 48 h (Figure 2c).
Figure 2.
Effect of pro-inflammatory cytokines on expression of P2X4 and P2X7 receptors in HUVEC. (a) qRT-PCR for P2X4 receptor message relative to untreated control under various stimulatory conditions for 24 and 48 h. (b) q-RT-PCR for P2X7 receptor message relative to untreated control under various stimulatory conditions for 24 and 48 h. (c) Relative expression of P2X4 and P2X7 receptor message compared to untreated control, for IFNγ (100 ng ml−1)+TNFα (10 ng ml−1) treatment over 72 h. Values shown are (in a and b) means±s.d., n=2; (in c) means±s.e.m, n=6.
Protein expression
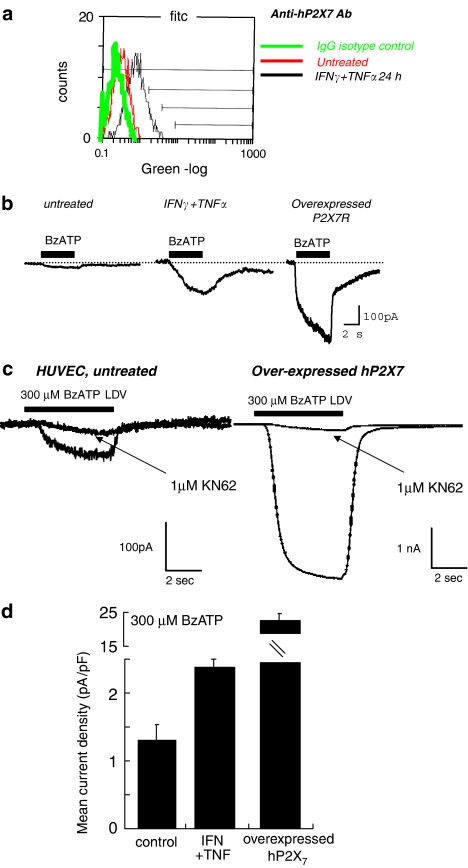
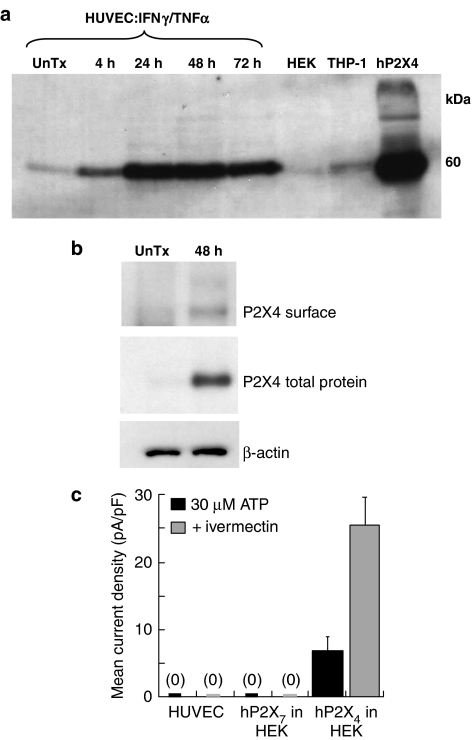
Fluorescence-activated cell scanning (FACS) analysis using a monoclonal anti-human P2X7 receptor ectodomain antibody (Buell et al., 1998) revealed an increase in surface expression of P2X7 receptors at 24 h treatment with IFNγ+TNFα (Figure 3a). We were unable to examine total P2X7 human protein by Western blotting because of limitations in the antibodies currently available. We used antibodies to examine both total and surface membrane expression of P2X4 receptors using Western blotting and biotinylation protocols (Ennion and Evans, 2001). Total protein was significantly increased within 4 h of IFNγ+TNFα treatment, with maximum protein expression at 48 h (Figure 4a). In agreement with the increased surface expression of P2X7 receptors following treatment with IFNγ+TNFα, there was an increase in surface P2X4 receptor, as measured with surface biotinylation (Figure 4b).
Figure 3.
Expression of P2X7 receptor protein and functional responses in HUVEC. (a) Flow cytometry using an antibody to the extracellular domain of the human P2X7 receptor to detect protein expression at 24 h for IFNγ (100 ng ml−1) and TNFα (10 ng ml−1) stimulation. (b) Functional whole cell electrophysiology responses in HUVECs. Typical membrane currents for 5 s application of Bz-ATP (300 μM) in low divalent cation solution (LDV), recorded from untreated HUVEC (left), HUVEC treated for 48 h with IFNγ (100 ng ml−1) and TNFα (10 ng ml−1) (centre) and HUVEC over-expressing hP2X7 receptor by adenoviral transfer (right). (c) Whole cell electrophysiology responses to Bz-ATP (300 μM) in LDV, in the absence and presence of KN-62 (1 μM). (d) Mean (±s.d.) current density values from untreated HUVEC (nine cells), HUVEC treated for 48 h with IFNγ (100 ng ml−1) + TNFα (10 ng ml−1) (seven cells) and HUVEC overexpressing hP2X7 receptor by adenoviral transfer (eight cells).
Figure 4.
Expression of P2X4 receptor protein and functional responses in HUVEC. (a) Immunoblotting to detect P2X4 receptor protein, for IFNγ (100 ng ml−1)+TNFα (10 ng ml−1) treatment over time. 20 μg protein was loaded in each lane. (b) Cell surface-labelled P2X4 receptor protein blotting using biotinylation. (c) Histogram showing mean current densities from HUVEC, or HEK293 cells overexpressing hP2X7 or hP2X4 receptors for stimulation with 30 μM ATP in the absence (black) or presence (grey) of ivermectin (3 μM), which acts to enhance hP2X4 responses. Values shown are means±s.d. from n=4, HEK/P2X7; n=6, HEK/P2X4; HUVEC, n=12.
P2X-activated membrane currents
The actions of ATP and the stable analogue Bz-ATP on membrane currents were examined in untreated HUVECs and after 24–48 h treatment with IFNγ+TNFα using recording conditions to block voltage-dependent and potential P2Y receptor-mediated potassium currents (Bowler et al., 2003) to isolate ATP-gated P2X currents. P2X4 receptor-mediated currents can be distinguished from P2X7 receptor-activated currents in three ways: concentrations of ATP <100 μM do not activate human P2X7 receptors but will maximally activate P2X4 receptors; ivermectin strongly potentiates P2X4 receptors but has no effect at P2X7 receptors; and Bz-ATP is a weak partial agonist at P2X4 receptors but produces a larger maximum response than does ATP at P2X7 receptors (Khakh et al., 1999; North and Surprenant, 2000; North, 2002). As we could not demonstrate expression of P2X receptors other than P2X4 and P2X7 in HUVECs (Figure 1b), these criteria should determine presence of functional P2X4 and P2X7 responses in HUVECs.
No change in membrane current occurred in response to application of 30 μM ATP in untreated or IFNγ+TNFα-treated HUVECs, nor did ivermectin potentiate currents produced by ATP (Figure 4c). In addition, ivermectin did not alter the Bz-ATP evoked current in untreated or IFNγ+TNFα-treated HUVEC. Parallel experiments performed on HEK293 cells stably expressing hP2X4 receptors resulted in half-maximal concentration (EC50 value) of ATP of 30 μM with a 3–7-fold increase in ATP-evoked currents in the presence of ivermectin (Figure 4c). These results, therefore, provided no evidence for the presence of functional ATP-gated P2X4 receptor channels, or P2X4/7 heteromers, in HUVEC.
In contrast, Bz-ATP (100–300 μM) evoked small but reproducible currents in untreated HUVECs, which were significantly increased after IFNγ+TNFα treatment (Figures 3b and d). These values were approximately 14-fold lower than P2X7 receptor-mediated currents recorded from human alveolar macrophages (33.4±5.03 pA pF−1, n=5). Ectopic expression of hP2X7 receptors (using adenoviral constructs) in untreated or LPS-treated HUVECs resulted in Bz-ATP-mediated currents that were 2–4-fold larger (6±1 pA pF−1, n=8) than Bz-ATP-activated currents recorded from IFNγ+TNFα-treated, untransfected cells (Figures 3b and d). Maximum current amplitudes in response to Bz-ATP (300 μM) were significantly greater than maximum amplitudes evoked in response to ATP (3–5 mM). KN62 (1 μM), which blocks the human P2X7 receptor (North and Surprenant, 2000), inhibited Bz-ATP-evoked currents by >65% (Figure 3c). Thus, these results show functional P2X7 receptors in HUVECs, whose current density is exceedingly low in untreated HUVECs but is significantly increased by inflammatory stimuli, although lower than found in macrophages.
IL-1β synthesis by HUVECs
HUVECs were cultured in the presence of pro-inflammatory mediators for 24 h. Whole-cell lysates were collected and assayed for intracellular IL-1β levels by ELISA (Table 2). IL-1β was not produced constitutively in unstimulated HUVEC, but was synthesized in significant quantities in response to pro-inflammatory stimulation. Stimulated HUVEC lysates were also tested for the presence of IL-1α by immunoblotting, but this was never detected (data not shown). IL-1β itself induced its own synthesis both alone and in combination with other pro-inflammatory mediators. Lysates from THP-1 monocytes, used as a positive control, produced approximately five times more IL-1β than the maximal production seen with stimulated HUVECs (Table 2).
Table 2.
IL-1β produced in HUVECs and THP-1 monocytes measured by ELISA
| Cell type | Treatment | IL-1β (pg/ml) |
|---|---|---|
| Mean±s.e.m. (n=3) | ||
| HUVECs | Untreated | 0 |
| LPS | 124±65 | |
| IFNγ | 0 (NS) | |
| TNFα | 86±29 | |
| IFNγ/TNFα | 158±92 | |
| IFNγ/LPS | 410±113 | |
| TNFα/LPS | 708±330 (NS) | |
| IL-1β | 611±92 | |
| IL-1β/IFNγ | 821±73 | |
| IL-1β/TNFα | 1880±477 | |
| IL-1β/LPS | 1570±565 | |
| THP-1 monocytes | Untreated | 0 |
| LPS | 8750±751 |
Levels of IL-1β produced in HUVECs and THP-1 monocytes following treatment with various combinations of stimuli. IL-1β levels from whole cell lysates were determined by the R&D QuantiGlo ELISA kit. Values are the mean±s.e.m. for n=3 separate determinations; P<0.05; unpaired Student's t-test, except where shown as NS.
Abbreviations: ELISA, enyme-linked immunosorbent assay; HUVEC, human umbilical vein endothelial cell; IFNγ, interferon γ; IL-1, interleukin-1; LPS, lipopolysaccharide; NS, nonsignificant; TNFα, tumour necrosis factor-α.
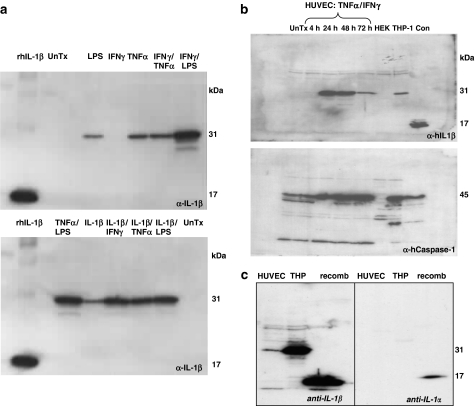
The identity of the IL-1β protein synthesized in stimulated HUVEC was confirmed by immunoblotting (Figure 5a). No detectable IL-1β was observed in untreated cells but full-length pro-IL-1β (31 kDa) was detected after inflammatory stimuli (Figure 5a). The time course for induction of both IL-1β and caspase-1 expression in HUVEC lysates was determined by immunoblotting, following stimulation with IFNγ and TNFα (Figure 5b). These demonstrate a gradual time-dependent increase in IL-1β that was maximal between 24 and 48 h. Pro-caspase-1 (45 kDa) was constitutively expressed by HUVEC and increased in a time-dependent manner following pro-inflammatory stimulation (lower blot, Figure 5b).
Figure 5.
IL-1β and IL-1α production in HUVEC. (a) The expression of IL-1β in HUVECs under various inflammatory stimuli was detected by immunoblotting cell extracts. The 31 kDa band represents the unprocessed biologically inactive pro-IL-1β, and the 17 kDa band corresponds to processed biologically active IL-1β. rhIL-1β is recombinant human IL-1β run as control, UnTx are untreated HUVEC controls. Twenty micrograms of protein was loaded in each lane. (b) Stimulation of IL-1β and caspase-1 in HUVECs and THP-1 monocytes. The time-course of induction of IL-1β and caspase-1 was determined by immuno-blotting, for HUVECs treated with TNFα (10 ng ml−1) and IFNγ (100 ng ml−1). Differentiated THP-1 monocytes and HEK293 cells treated for 24 h with the respective inflammatory stimuli, were used as controls. The positive control (con) is recombinant human IL-1β (20 μg), or HL-60 cell lysates for caspase-1. For each set of conditions, initially samples were blotted using anti-human IL-1β, and were then stripped and re-probed with anti-caspase-1 antibodies. Twenty micrograms of protein was loaded into each lane. (c) HUVEC supernatants were compared for IL-1β (left) and IL-1α production (right) following IFNγ/TNFα treatment. Positive controls are recombinant human IL-1β and IL-1α (R&D) and THP-1 cells treated for 24 h with LPS. Twenty micrograms of protein was loaded in each lane.
Secretion of IL-1β
Although LPS alone induced the synthesis of pro-IL-1β (Table 2), no IL-1β release into the medium was detected from LPS-primed HUVEC in response to 30, 60 or 120 min application of ATP (3 mM) or Bz-ATP (300 μM) (n=3, data not shown). Similar experiments were then carried out using LPS-primed HUVECs after 48 h prior treatment with IFNγ and TNFα. Supernatants were collected and concentrated by immunoprecipitation and then assayed for IL-1β by ELISA as were cell lysates from the same experiments (Figures 6a and b). There was significant variability in responses from individual donors which is in agreement with previous work on loss-of-function and/or gain-of-function polymorphisms of P2X7 receptors in the human population (Lammas et al., 1997; Li et al., 2002; Saunders et al., 2003; Wiley et al., 2003; Gu et al., 2004; Sluyter et al., 2004a, 2004b; Cabrini et al., 2005; Skarratt et al., 2005; Shemon et al., 2006) but, in general, Bz-ATP-evoked release of IL-1β into the supernatant was only just above threshold for detection using the sensitive ELISA assay (Figure 6b). Where IL-1β release was detected, Bz-ATP evoked higher levels of IL-1β in the medium than did ATP, in keeping with P2X7 receptor function.
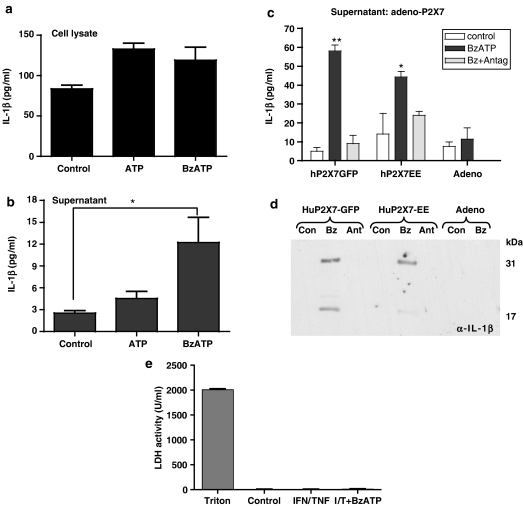
Figure 6.
Released IL-1β from stimulated HUVEC. Cell lysates (a) and supernatants (b), collected from HUVECs treated with TNFα (10 ng ml−1) and IFNγ (100 ng ml−1) for 48 h were tested by ELISA for the presence of IL-1β. (c) Release of IL-1β from HUVEC over-expressing P2X7 receptors. HUVECs were transformed with adenoviral human P2X7-GFP, adenoviral human P2X7-EE or adenovirus alone (Adeno) and treated for 48 h with TNFα (10 ng ml−1) and IFNγ (100 ng ml−1), then for 60 min with buffer (control) or 300 μM Bz-ATP (Bz), or 300 μM Bz-ATP in the presence of 100 nM AZ11645373 P2X7 receptor antagonist (Ant). (a) Supernatants were collected and tested by ELISA (c) or (d), by immunoblotting for the presence of IL-1β. The LDH activity of the HUVEC supernatants was tested as a measure of cell death (e), compared to control Triton X (1%, v/v)-treated cells. Data are mean±s.e.m. for six separate wells. **P<0.01 and *P<0.05 vs control, where data were analysed by one-way ANOVA with Tukey's post-test (b), or by two-way ANOVA with Bonferroni's post-test (c).
In view of the relatively weak release of IL-1β from HUVECs in response to Bz-ATP in spite of the high levels of intracellular IL-1β induced by inflammatory stimuli, we examined whether this was owing to a dysfunction in the inflammasome per se or to low levels of functional P2X7 receptors, by overexpressing P2X7 receptors in HUVECs using adenoviral delivery. Release experiments were carried out from the same preparations that were studied by whole-cell patch-clamp recordings (i.e. Figure 3b). In HUVEC with ectopic expression of P2X7 receptors, Bz-ATP resulted in significant and higher levels of IL-1β being released into the supernatant media (Figure 6c). Furthermore processing to the active 17 kDa form of IL-1β was demonstrated by Western blot (Figure 6d). Concomitant with release of IL-1β into the medium by Bz-ATP stimulation, total intracellular IL-1β content was decreased in response to Bz-ATP stimulation (325±52 vs 123±20 pg ml−1 mean±s.e.m, n=3; P<0.05, Student's t-test). Bz-ATP did not evoke IL-1β release in control experiments using an empty-vector adenoviral construct although LPS-induced synthesis of intracellular IL-1β was normal. We also measured LDH activity in the supernatants from all experiments as an assay for cell death, and compared values to those obtained by Triton-induced cell lysis, but in no case was LDH release into the medium detected (Figure 6e).
Bioactivity of secreted IL-1β
To determine whether the low level of released IL-1β from HUVECs was biologically active, an IL-8 luciferase reporter assay was used. We have previously reported that the IL-8 promoters linked to luciferase and expressed in HeLa cells can be used as a bioassay for IL-1 activation (Kiss-Toth et al., 2000), and is sensitive to picomolar concentrations of IL-1β. The assay provides a linear response between 10 and 100 pg ml−1 (Evans et al., 2006). Upon addition of HUVEC supernatants following control, ATP or Bz-ATP treatment to the IL-1 bioassay, activation of IL-8 luciferase was not observed (clear bars, Figure 7a), compared to addition of 1 pg ml−1 IL-1β that caused strong reporter activation (shaded bar, for buffer addition, Figure 7a). Of considerable interest was the observation that these same HUVEC supernatant samples inhibited the agonist response to exogenously applied IL-1β to the reporter assay (shaded bars, Figure 7a). This indicated that an IL-1 inhibitory factor was present in the HUVEC-released fraction/supernatant samples. The presence of induced release of IL-1Ra was confirmed in the HUVEC supernatants by ELISA (Figure 7b) and by Western blotting (Figure 7c). Application of 100 ng ml−1 recombinant IL-1Ra protein to the IL-1 bioassay was equally effective at causing inhibition of IL-1β mediated IL-8 luciferase activity compared to addition of the HUVEC supernatants (Figure 7a). Increased levels of IL-1Ra were detected in supernatants from stimulated HUVEC transformed with P2X7 adenovirus, corresponding to those samples tested for IL-1β release in Figure 6c. These samples from native and P2X7-adeno transformed HUVECs were tested for the presence of another IL-1 inhibitory factor, sIL-1RII by ELISA. However, no sIL-1RII was detected (not shown).
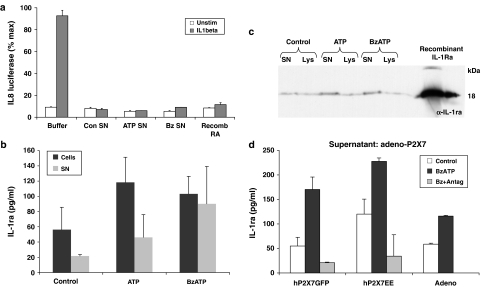
Figure 7.
Biological activity of supernatants from stimulated HUVEC at the IL-1 receptor. (a) Effect of supernatants from stimulated HUVECs on IL-1 bioassay. Buffer only (Buffer) or supernatants from HUVEC treated for 48 h with TNFα (10 ng ml−1)+IFNγ (100 ng ml−1), then for 60 min with extracellular solution (Con SN), ATP (3 mM, ATP SN) or Bz-ATP (300 μM, Bz SN) were added to the IL-1-sensitive IL-8 luciferase reporter assay in HeLa cells in the absence (clear bars) or presence (filled bars) of 1 pg ml-1 IL-1β. The effect of the HUVEC samples on the IL-8 luciferase activity was compared to the effect of exogenously applied recombinant IL-1Ra (Recomb RA) at 100 ng ml-1. HUVEC cell lysates (Cells) and supernatants (SN) from these same treatments in (a) were tested for the presence of IL-1Ra by ELISA (b) and Western blotting (c) Values in (a) and (b) are means±s.e.m., n=4. (d) HUVEC were transformed with adenoviral human P2X7-GFP, adenoviral human P2X7-EE or adenovirus alone (Adeno) and treated for 48 h with TNFα (10 ng ml−1) and IFNγ (100 ng ml−1), then for 60 min with buffer (control) or 300 μM Bz-ATP (Bz), or 300 μM Bz-ATP in the presence of 100 nM AZ11645373 P2X7 receptor antagonist (Ant). Supernatants were collected and tested by ELISA for IL-1Ra levels. These samples correspond to those tested for IL-1β levels in Figure 6c. Values shown are means±s.e.m., n=3.
Discussion
We have tested the hypothesis that IL-1β is released from ECs via a P2X-dependent process. The data presented here demonstrate the presence of P2X4 and P2X7 receptors at low levels in unstimulated HUVECs and that these are induced by similar inflammatory stimulants to those that induce the synthesis of IL-1β.
Levels of P2X4 receptors appeared to be in excess of P2X7 receptors under both basal and stimulated conditions. Interestingly, the P2X4R and P2X7 receptor genes are located on chromosome 12 within 130 kb of each other and it is certainly possible that they share common promoter elements explaining how their expression levels appear to be so closely linked. P2X4 receptor protein was easily detected in HUVECs by Western blotting. Whole-cell electrophysiology responses attributable to P2X4 receptor activation were barely detectable, when assessed with low micromolar concentrations of ATP, and by the addition of the P2X4 receptor-potentiating compound, ivermectin. It is unclear why P2X4 receptors on HUVEC do not appear to have electrophysiological function.
P2X7 receptor protein was detected in HUVECs by immunoprecipitation with cell surface expression of P2X7 receptor confirmed by FACS analysis. Functional P2X7 receptors on HUVEC were confirmed by whole-cell electrophysiology recording, with inward currents in response to both ATP and Bz-ATP application, which were potentiated in low divalent cation solutions. Responses were significantly increased in HUVECs that had been treated with IFNγ/TNFα consistent with the observed upregulation in expression of P2X7 receptors. The endogenous P2X7 receptor ion channel responses were approximately 14-fold lower compared to endogenous currents from human lung macrophages and 2–4-fold lower than in experiments where P2X7 was overexpressed by viral transfection.
IL-1β synthesis was detected by ELISA immunoassay and Western blotting on HUVEC whole-cell extracts, the latter confirming intracellular production of 31 kDa Pro-IL-1β. IL-1β synthesis was negligible in unstimulated HUVECs but was induced by pro-inflammatory stimulation in a similar fashion to P2X receptor expression and over a similar time course. Interestingly, the greatest induction of IL-1β synthesis was produced by combinations of pro-inflammatory mediators containing IL-1β itself. Western blotting for the 45 kDa precursor form of caspase-1 showed that it was constitutively present in HUVECs but was similarly induced by pro-inflammatory stimulation. The lower band present in the caspase-1 blot of HUVEC samples may be attributable to active caspase-1. Processing of IL-1β in the same samples of cell lysates is not observed. This is likely to be owing to the fact that generation of active IL-1β is intimately coupled to its release from the cell, hence any production is only detected in the supernatant fraction (Hazuda et al. 1988; Perregaux et al. 2002).
A key objective was to test whether HUVECs process and release IL-1β in a P2X receptor-dependent manner. Here we confirm that IL-1β synthesis and release plays a key regulatory role in vascular ECs. Consistent with this we, and others, found that IL-1β synthesis was negligible in unstimulated HUVEC (Marceau et al., 1992; Schumann et al., 1998). We have shown that under inflammatory conditions, using LPS, IFNγ, TNFα and IL-1β, either singly or in double combinations mature IL-1β, but not IL-1α, is synthesized in reasonably large amounts (about one-fifth of that observed from monocytes). We used a combination of IFNγ and TNFα to stimulate IL-1β release, owing to its effect in upregulating both P2X4/7 receptor expression and IL-1β synthesis. We used a variety of techniques to try and detect IL-1β release from HUVECs including immunoprecipitation and Western blotting. However, we were only able to detect IL-1β release by testing supernatants in a highly sensitive IL-1β ELISA, with approximately 12 pg ml−1 detected. This occurred in the absence of cell death. We were consistently unable to detect any IL-1β release by immunoprecipitation or Western blotting most likely due to the levels being below the threshold for detection (lower limit of detection by immunoprecipitation 30 pg ml−1 rhIL-1β and 3 ng ml−1 rhIL-1β by immunoblotting). The only other report demonstrating mature IL-1β release from human ECs was in response to CD40 ligation by a thymocyte proliferation assay (Schonbeck et al., 1997). We tested whether CD40 ligand, low-density lipoprotein (LDL) and oxidized LDL (oxLDL) were effective in altering P2X receptor expression, but no significant difference from control was observed (data not shown). Much of the initial work on P2X7 receptor-dependent IL-1β release was performed in THP-1 monocytes. HUVECs synthesized approximately 10–50-fold less IL-1β than THP-1 cells and expressed fewer P2X7 receptors, even after pro-inflammatory stimulation. As the amount of IL-1β released from HUVEC was only just detectable, to confirm a role for the P2X7 receptor in mediating this secretion, we artificially increased the expression of P2X7 receptors in HUVECs, by transfecting them with adenoviral P2X7 receptor. High levels of expression were confirmed by measuring functional P2X7 receptor responses. Transformed cells still required pro-inflammatory stimulation to induce IL-1β synthesis and release. Levels of released IL-1β were approximately three or four times higher in P2X7 receptor transformed compared to native cells. In addition, incubation with the specific P2X7 receptor antagonist (AZ11645373) attenuated release of IL-1β from P2X7 receptor-transformed HUVEC, providing further support for the role of this receptor in secretion of leaderless IL-1β from ECs.
These experiments indicateed that an intact inflammasome is active within HUVEC and that to some extent IL-1β secretion is limited by low-level P2X7 receptor expression in EC. Although we cannot exclude other mechanisms in EC that might be able to induce the release of IL-1β at a higher level than is achieved by P2X7 receptor stimulation, this is one of the best-characterized processes known to influence the release of this cytokine. Therefore, we conclude that the low level of IL-1β release from HUVEC is in part because of the low level of P2X7 receptor expression and in part the lower synthetic capacity of HUVEC for IL-1β compared to monocyte/macrophages. We cannot exclude the possibility that IL-1β might be released at higher levels from HUVEC by other unidentified stimuli. The measured release of 12 pg ml−1 IL-1β from untransfected HUVEC, corresponding to approximately 1 pM concentration, is an adequate concentration to easily affect a range of targets and is also effective in the IL-8-luciferase reporter assay, when applied exogenously (Dower et al., 1986; Kiss-Toth et al., 2000).
We utilized an IL-8 reporter assay to determine the bioactivity of the released products from stimulated ECs. This assay is sensitive to low picomolar concentrations of IL-1β. However, the overall effect of application of HUVEC supernatants resulted in an inhibition of IL-1-induced activity. The presence of IL-1Ra in the supernatants was confirmed, by immunoblotting and ELISA, whereas addition of recombinant IL-1Ra to the IL-1 reporter bioassay mimicked the effect of the HUVEC supernatants. The concentration of exogenous recombinant IL-1Ra required to cause the same extent of inhibition as the HUVEC supernatants was almost 100-fold higher than that detected by ELISA in the HUVEC supernatants. These data are consistent with our previous observations of P2X7 receptor-mediated release of IL-1Ra from HUVEC (Evans et al., 2006). It is unclear why IL-1Ra is released in greater amounts that IL-1β in response to ATP. It may just be the ratio of synthetic capacity for these two IL-1 products in HUVEC, but we cannot exclude the possibility of subtle differences in the P2X7 receptor – dependent mechanisms of IL-1β and IL-1Ra release and that these may be cell-type-specific. We have noticed that EC do not bleb in response to ATP, a feature that has been seen in monocyte/macrophages and has been linked to IL-1β processing. We have attempted to neutralize IL-1Ra in the samples using neutralizing antibodies, to reveal IL-1β activity but were unable to achieve effective neutralization with the antibodies currently available for this purpose.
Under the conditions we have studied, the net effect of P2X7 receptor activation is anti-inflammatory due to the dominant-released factor being intracellular IL-1Ra rather than IL-1β. This is consistent with the idea that the predominant role of ECs in vivo is anti-inflammatory, with mechanisms in place to protect ECs in the face of blood borne inflammatory insults, for example, the induction of decay accelerating factor by cytokines or the membrane attack complex protecting endothelial cells against complement deposition (Mason et al., 1999). The role of ectonucleotidases should also be considered in protecting against P2X receptor-mediated cytokine release in EC, which may be altered under different inflammatory conditions (Imai et al., 2000). These workers also showed that cell-associated IL-1α release occurs from LPS-stimulated HUVEC (Imai et al., 2000). In this study, we were unable to detect any free IL-1α in the stimulated HUVEC supernatants by Western blotting.
This work has demonstrated that the net effect of P2X7 receptor stimulation in EC is anti-inflammatory. This may be important for the therapeutic use of agents that inhibit P2X7 receptors. We have reported previously that P2X7 receptor gene deletion in the mouse does not reproduce an IL-1-deficient phenotype in a vascular injury model (Chamberlain et al., 2006), and the data reported here may add to a growing impression that, within the vascular wall, IL-1Ra expression is an important control mechanism.
In conclusion, HUVEC express P2X4 and P2X7 receptors under inflammatory conditions, but are mostly responsive to P2X7 receptor stimulation. EC do synthesize IL-1β but only when stimulated by inflammatory mediators. P2X7 receptor-dependent release of IL-1β can be elicited from HUVEC but this is at low levels and under the conditions tested is biologically balanced by concomitant IL-1Ra release.
Acknowledgments
This work was supported by the Wellcome Trust and British Heart Foundation. We are grateful to Gary Shaw for isolation of HUVECs, to Dr Jon Ward for discussion, and to Vicky Porteous and Daniele Estoppey for assistance with cell culture.
Abbreviations
- Bz-ATP
3′-O-(4-benzoyl)benzoyl-ATP
- IFNγ
interferon γ
- IL-1β
interleukin-1β
- IL-1α
interleukin-1α
- IL-1Ra
interleukin 1 receptor antagonist
- LPS
lipopolysaccharide
- TNFα
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler JW, Bailey RJ, North RA, Surprenant A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol. 2003;140:567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. [PubMed] [Google Scholar]
- Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, et al. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, et al. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–1403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J, Gunn J, Francis S, Holt C, Crossman D. Temporal and spatial distribution of interleukin-1 beta in balloon injured porcine coronary arteries. Cardiovasc Res. 1999;44:156–165. doi: 10.1016/s0008-6363(99)00175-3. [DOI] [PubMed] [Google Scholar]
- Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–2400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, et al. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dower SK, Call SM, Gillis S, Urdal DL. Similarity between the interleukin 1 receptors on a murine T-lymphoma cell line and on a murine fibroblast cell line. Proc Natl Acad Sci USA. 1986;83:1060–1064. doi: 10.1073/pnas.83.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. Agonist-stimulated internalisation of the ligand-gated ion channel P2X(1) in rat vas deferens. FEBS Lett. 2001;489:154–158. doi: 10.1016/s0014-5793(01)02102-0. [DOI] [PubMed] [Google Scholar]
- Evans I, Dower SK, Francis SE, Crossman DC, Wilson HL. Action of intracellular IL-1Ra (Type 1) is independent of the IL-1 intracellular signalling pathway. Cytokine. 2006;33:274–280. doi: 10.1016/j.cyto.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997a;159:1451–1458. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 Receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- Griffiths RJ, Stam EJ, Downs JT, Otterness IG. ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol. 1995;154:2821–2828. [PubMed] [Google Scholar]
- Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, et al. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287–31295. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- Hazuda DJ, Lee JC, Young PR. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. J Biol Chem. 1988;263:8473–8479. [PubMed] [Google Scholar]
- Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Goepfert C, Kaczmarek E, Robson SC. CD39 modulates IL-1 release from activated endothelial cells. Biochem Biophys Res Commun. 2000;270:272–278. doi: 10.1006/bbrc.2000.2410. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss-Toth E, Guesdon FM, Wyllie DH, Qwarnstrom EE, Dower SK. A novel mammalian expression screen exploiting green fluorescent protein-based transcription detection in single cells. J Immunol Methods. 2000;239:125–135. doi: 10.1016/s0022-1759(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, Mccurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- Le Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol. 2002;447:261–269. doi: 10.1016/s0014-2999(02)01848-4. [DOI] [PubMed] [Google Scholar]
- Li CM, Campbell SJ, Kumararatne DS, Bellamy R, Ruwende C, Mcadam KP, et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis. 2002;186:1458–1462. doi: 10.1086/344351. [DOI] [PubMed] [Google Scholar]
- Mackenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Marceau F, Grassi J, Frobert Y, Bergeron C, Poubelle PE. Effects of experimental conditions on the production of interleukin-1 alpha and -1 beta by human endothelial cells cultured in vitro. Int J Immunopharmacol. 1992;14:525–534. doi: 10.1016/0192-0561(92)90113-y. [DOI] [PubMed] [Google Scholar]
- Mason JC, Yarwood H, Sugars K, Morgan BP, Davies KA, Haskard DO. Induction of decay-accelerating factor by cytokines or the membrane-attack complex protects vascular endothelial cells against complement deposition. Blood. 1999;94:1673–1682. [PubMed] [Google Scholar]
- Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- Morton AC, Arnold ND, Gunn J, Varcoe R, Francis SE, Dower SK, et al. Interleukin-1 receptor antagonist alters the response to vessel wall injury in a porcine coronary artery model. Cardiovasc Res. 2005;68:493–501. doi: 10.1016/j.cardiores.2005.06.026. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Perregaux DG, Bhavsar K, Contillo L, Shi J, Gabel CA. Antimicrobial peptides initiate IL-1 beta posttranslational processing: a novel role beyond innate immunity. J Immunol. 2002;168:3024–3032. doi: 10.4049/jimmunol.168.6.3024. [DOI] [PubMed] [Google Scholar]
- Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. Rapid Cycle Real-Time PCR, Methods and Applications. Springer Press: Heidelberg; 2001. Quantification on the LightCycler; pp. 21–34. [Google Scholar]
- Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss-of-function polymorphism in the human P2X7 rece ptor abolishes ATP-mediated killing of mycobacteria. J Immunol. 2003;171:5442–5446. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Bonnefoy JY, Loppnow H, Flad HD, Libby P. Ligation of CD40 activates interleukin 1beta-converting enzyme (caspase-1) activity in vascular smooth muscle and endothelial cells and promotes elaboration of active interleukin 1beta. J Biol Chem. 1997;272:19569–19574. doi: 10.1074/jbc.272.31.19569. [DOI] [PubMed] [Google Scholar]
- Schumann RR, Belka C, Reuter D, Lamping N, Kirschning CJ, Weber JR, et al. Lipopolysaccharide activates caspase-1 (interleukin-1-converting enzyme) in cultured monocytic and endothelial cells. Blood. 1998;91:577–584. [PubMed] [Google Scholar]
- Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, et al. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- Skarratt KK, Fuller SJ, Sluyter R, Dao-Ung LP, Gu BJ, Wiley JS. A 5′ intronic splice site polymorphism leads to a null allele of the P2X7 gene in 1–2% of the Caucasian population. FEBS Lett. 2005;579:2675–2678. doi: 10.1016/j.febslet.2005.03.091. [DOI] [PubMed] [Google Scholar]
- Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004a;5:588–591. doi: 10.1038/sj.gene.6364127. [DOI] [PubMed] [Google Scholar]
- Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol. 2004b;172:3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, et al. Characterization of a selective and potent antagonist of human P2X(7) receptors, AZ11645373. Br J Pharmacol. 2006;149:880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes Genome Biol 20023RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, Mackenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519 (Part 2):335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner SJ, Auger KR, Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987;139:1911–1917. [PubMed] [Google Scholar]
- Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, et al. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173:1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- Zocchi MR, Rubartelli A.Nonclassical mechanisms of secretion in the physiopathology of the immune system Frontiers of Life 2001Academic Press, MA, USA; 477–488.In: Baltimore D, Dulbecco R, Jacob F, Levi-Montalcini R (eds) [Google Scholar]