Abstract
Background and purpose:
The aim of this report is to study mechanisms of G protein activation by agonists.
Experimental approach:
The association and dissociation of guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding at G proteins in membranes of CHO cells stably transfected with the human dopamine D2short receptor was studied in the presence of a range of agonists.
Key results:
Binding of [35S]GTPγS was dissociable in the absence of agonist and dissociation was accelerated both in rate and extent by dopamine, an effect which was blocked by the dopamine D2 receptor antagonist raclopride and by suramin, which inhibits receptor/G protein interaction. A range of agonists of varying efficacy increased the rate of dissociation of [35S]GTPγS binding, with the more efficacious agonists resulting in faster dissociation. Agonists were able to dissociate about 70% of the pre-bound [35S]GTPγS, leaving a component which may not be accessible to the agonist-bound receptor. The dissociable component of the [35S]GTPγS binding was reduced with longer association times and increased [35S]GTPγS concentrations.
Conclusions and implications:
These data are consistent with [35S]GTPγS binding being initially to receptor-linked G proteins and then to G proteins which have separated from the agonist bound receptor. Under the conditions used typically for [35S]GTPγS binding assays, therefore, much of the agonist-receptor complex remains in proximity to G proteins after they have been activated by agonist.
Keywords: D2 dopamine receptor, G proteins, [35S]GTPγS association, [35S]GTPγS dissociation, suramin, agonist mechanisms
Introduction
Approximately half of the currently prescribed drugs act on cell surface receptors, the majority of which are G protein-coupled receptors (GPCRs) (Fredriksson et al., 2003). Considerable effort is focussed on understanding the mechanisms of action of agonists at these receptors, and in particular, the differences between full and partial agonists.
G protein activation is the first step in the signal-transduction cascade of GPCRs. It is therefore of interest to look closely at this step in terms of how it contributes to agonist efficacy. It is currently thought that in the resting state, GDP is bound to the Gα subunit of the G protein heterotrimer (αβγ). When the receptor is activated, it promotes the dissociation of GDP and the association of GTP to the G protein. This induces the active state of the G protein, which leads to the heterotrimer dissociating into Gα-GTP and Gβγ. The GTPase activity of the Gα subunit hydrolyses the GTP to GDP causing the inactivation of the G protein. Gα-GDP and Gβγ then reassociate, returning to the resting state and the cycle is complete.
Several studies have suggested models of G protein activation, which differ from this. Biddlecome et al. (1996) proposed a model in which receptor, Gq and phospholipase C form a three protein complex in the presence of agonist which is responsible for phospholipase C signalling. Bunemann et al. (2003) suggested, using fluorescence resonance energy transfer (FRET) techniques, that the subunits of the Gi G protein do not actually separate upon agonist activation, but rather undergo a subunit rearrangement. Studies such as these are providing evidence that signal transduction from receptor to effector may involve multiprotein signalling complexes rather than separation of Gα-GTP and Gβγ subunits.
The ability of agonists to stimulate the dissociation of GDP and association of GTP from the G protein can be assessed by measuring the binding of guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS), a non-hydrolysable analogue of GTP. When the agonist binds to the receptor, GDP is released from the G protein and [35S]GTPγS binds. The G protein cycle is perturbed by this as the GTPase cannot hydrolyse the [35S]GTPγS, resulting in the accumulation of bound [35S]GTPγS. This assay has been used as a measure of agonist efficacy for many GPCRs, with the maximum effect providing a measure of the degree of agonism of the compound and the EC50 a measure of its potency (Harrison and Traynor, 2003).
Initial studies on [35S]GTPγS binding described the binding as irreversible and were carried out on purified Gα subunits (Bokoch et al., 1984; Sternweis and Robishaw, 1984), or on purified heterotrimeric G protein (Higashijima et al., 1987). The [35S]GTPγS binding was seen to be irreversible in the presence of millimolar concentrations of Mg2+, but became reversible when the Mg2+ concentration was lowered. Since then studies carried out in native membrane systems containing GPCRs have suggested that [35S]GTPγS binding is reversible and that the [35S]GTPγS binding reaches an equilibrium dependent on the association and dissociation of [35S]GTPγS. In support of this, agonists have been shown to stimulate the dissociation of [35S]GTPγS binding via muscarinic receptors in atrial porcine membranes (Hilf et al., 1989), the formyl peptide receptor in HL-60 cells (Kupprion et al., 1993), cannabinoid receptors in rat cerebellar membranes (Breivogel et al., 1998), β2-adrenergic receptors in Sf9 cells (Wenzel-Seifert and Seifert, 2000), muscarinic M1 receptors in CHO cells (Waelbroeck, 2001), 5 -HT1A receptors in CHO cells (Newman–Tancredi et al., 2002), and opioid receptors in SH-SY5Y cells (Alt et al., 2002).
In this study, we have taken the dopamine D2 receptor stably expressed in CHO cells as a system to further investigate the dissociation of [35S]GTPγS binding and the relation of this process to the G protein activation cycle. We have used a range of agonists of varying efficacy and examined the relationship between agonist efficacy and dissociation of [35S]GTPγS binding. The data show that [35S]GTPγS dissociation can be accelerated by agonists, and that a large proportion of bound [35S]GTPγS is to the agonist/receptor/G protein ternary complex which is relatively stable under the conditions used.
Materials and methods
Cell culture
CHO-K1 cells stably expressing the native human dopamine D2short receptor (1–2 pmol mg−1 protein) were made as described previously (Wilson et al., 2001). They were grown in Dulbecco's modified eagle's medium (DMEM) containing 2 mM L-glutamine, 1% non-essential amino acids, 5% foetal bovine serum and 200 μg ml−1 geneticin. Cells were grown at 37°C in an atmosphere of 5% CO2.
Membrane preparation
Membranes were prepared from CHO cells expressing the human D2short dopamine receptor as described previously (Castro and Strange, 1993). Confluent 175 cm2 flasks of cells were washed once with 5 ml 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (20 mM HEPES, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4). Cells were then removed from the surface of the flasks using a cell scraper into 10 ml HEPES buffer and were homogenized using an Ultra-Turrax homogeniser (4 × 5 s). This was followed by centrifugation at 250 g for 10 min at 4°C. Supernatants were collected and then centrifuged at 48 000 g for 60 min at 4°C. The resulting pellet was resuspended in HEPES buffer at a concentration of 2–4 mg protein ml−1 and stored in aliquots at −80°C until use. Protein concentration was determined by the method of Lowry et al. (1951).
Where Pertussis toxin was used cells were treated with 100 ng ml−1 of the toxin for 18 h before harvesting cells for membrane preparation.
[3H]NPA-binding assays
[3H]NPA binding was carried out as described previously (Roberts et al., 2004). Cell membranes (25 μg) were incubated with 0.4 nM [3H]NPA and competing drugs in HEPES buffer (20 mM HEPES, 1 mM EGTA, 1 mM EDTA, 10 mM MgCl2, 100 mM N-methyl-D-glucamine; pH 7.4 (using HCl) containing 0.1 mM dithiothreitol (DTT) in a final volume of 1 ml for 3 h at 25°C. The assay was terminated by rapid filtration (through Whatman GF/C filters) using a Brandel cell harvester followed by four washes with 4 ml ice-cold phosphate-buffered saline (0.14 M Sodium chloride (NaCl), 3 mM Potassium chloride (KCl), 1.5 mM potassium phosphate (KH2PO4), 5 mM disodium hydrogen phosphate (Na2HPO4); pH 7.4) to remove unbound radioactivity. Filters were soaked in 2 ml of scintillation fluid for at least 6 h and bound radioactivity was determined by liquid scintillation counting. High-affinity specific binding of [3H]NPA was defined as that inhibited by high concentrations of GTPγS or suramin, both of which gave similar definitions of [3H]NPA binding.
[35S]GTPγS dissociation
Assays were carried out in triplicate in 20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, 0.1 mM DTT, pH 7.4 in a final volume of 1 ml. Each tube contained 10 μM GDP, 0.2 nM [35S]GTPγS and 25 μg membrane protein from CHO cells expressing the D2 dopamine receptor. Assays were incubated at 30°C for 60 min before addition of 10 μM GTPγS and agonist to initiate dissociation. Assays were then terminated at the stated time and radioactivity determined as described above.
In some assays, both the association and dissociation of [35S]GTPγS were carried out in the presence of agonist. Association was performed using 0.1 nM [35S]GTPγS, 25 μg membranes, agonist (at the concentration stated) and 10 μM GDP, and dissociation was initiated by addition of 10μM GTPγS.
Data analysis
Data were analysed using GraphPad Prism (Graphpad San Diego, CA, USA). [35S]GTPγS dissociation data were analysed using one and two component exponential decay models and the fits compared using an F-test. All data were fitted best to a model of one site exponential decay with the equation ([35S]GTPγS bound)=Span e−kt+Plateau. Unless otherwise stated statistical analysis was carried out using unpaired, two-tailed t-tests with 95% confidence limits. All data presented are expressed as the mean±s.e.m. of at least three independent experiments.
Materials
(+)-Butaclamol, DMEM, dopamine, geneticin, GTPγS, L-glutamine, m-tyramine, non-essential amino acids, R-(+)-propylnorapomorphine (NPA), p-tyramine, raclopride and R(+)-3-(3-hydroxyphenyl)-N-propylpiperidine hydrochloride (R-(+)-3-PPP) were purchased from Sigma (Dorset, UK). GDP was from ICN Biomedicals (Hampshire, UK). Quinpirole was purchased from Tocris Cookson Ltd (Avonmouth, UK). NaCl, MgCl2 were from BDH (Loughborough, UK). Ultima Gold was from Perkin Elmer (Cambridge, UK). [35S]GTPγS (37 TBq mmol−1) was from Amersham (Bucks, UK) and [3H]NPA (1 TBq mmol1) was purchased from Perkin-Elmer Life Sciences.
Results
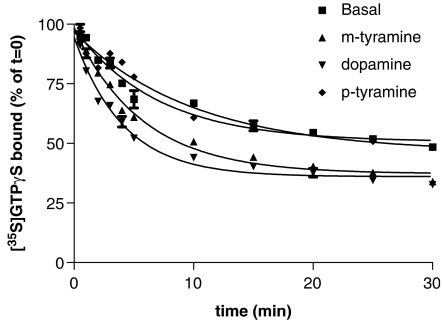
[35S]GTPγS dissociation from membranes of CHO cells expressing D2 dopamine receptors (CHO-D2 cells) was determined following a 60 min pre-labelling period with [35S]GTPγS. 10 μM non-radioactive GTPγS was added to stop nucleotide association and [35S]GTPγS dissociation was followed. About 40% of the bound [35S]GTPγS dissociated under these conditions and the dissociation data fitted well to a single exponential model with a t1/2 of 6.5 min. We then tested the effects of three agonists whose relative efficacies have been well defined in [35S]GTPγS-association experiments (dopamine (100%), m-tyramine (69%) and, p-tyramine (53%) (Payne et al., 2002)). The agonists were added at the same time as the non-radioactive GTPγS and were found to increase the rate and extent of [35S]GTPγS dissociation. The increase in the rate and extent was greater the more efficacious the agonist (Figure 1). The ability of dopamine to dissociate [35S]GTPγS binding under these assay conditions was inhibited by 10 μM raclopride (data not shown) showing that the effects of agonists on [35S]GTPγS dissociation are mediated via the D2 receptor. It is noteworthy, however, that the amount of additional [35S]GTPγS binding dissociated by the agonists is low and this presumably reflects the labelling of G proteins and other proteins inaccessible to the D2 receptor.
Figure 1.
Agonist-stimulated [35S]GTPγS dissociation from membranes of CHO cells expressing D2 dopamine receptors. Membranes were incubated with [35S]GTPγS (0.2 nM) for 60 min as described in the Materials and methods section. 1 mM dopamine, 1 mM m-tyramine, 1 mM p-tyramine or vehicle were then added to the assays, together with 10 μM GTPγS and the [35S]GTPγS dissociation determined. Data were fitted best by a model of a single exponential decay. t1/2 values obtained were 2.3±0.2 min for dopamine, 3.6±0.6 min for m-tyramine, 4.5±0.1 min for p-tyramine and 6.5±1.1 min for basal. Maximal [35S]GTPγS dissociated was 63.0±2.6% for dopamine, 62.2±1.8% for m-tyramine, 55.7±1.0% for p-tyramine and 51.1±1.7% for basal. This graph shows the data from a single representative experiment carried out three times with similar results.
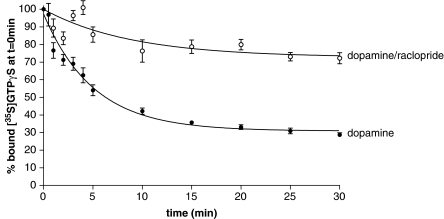
To increase the agonist-specific signal we, therefore, performed [35S]GTPγS association to CHO-D2 membranes in the presence of agonists before adding non-radioactive GTPγS to observe [35S]GTPγS dissociation. This resulted in higher levels of bound [35S]GTPγS at the start of the dissociation phase compared to experiments without agonist. Figure 2 shows the results of an experiment with dopamine. Basal levels of [35S]GTPγS dissociation determined in the absence of agonist were similar to those seen in Figure 1 and have been subtracted. In the experiment in Figure 2, basal [35S]GTPγS dissociation amounted to about a third of the agonist-stimulated dissociation. Under these conditions, about 70% of the bound [35S]GTPγS dissociated in a monexponential manner and the t1/2 for dissociation (3.0±0.3 min) was similar to that seen in experiments without dopamine in the association phase. To determine whether this effect of dopamine on [35S]GTPγS dissociation was a receptor-mediated event, we carried out agonist-stimulated dissociation of [35S]GTPγS in the presence of the D2 receptor antagonist raclopride. 100 μM raclopride was added to the assay after the 60 min association along with the 10 μM GTPγS. As shown in Figure 2 raclopride almost completely inhibited the effect of dopamine on the [35S]GTPγS dissociation. A high concentration of raclopride is used in this experiment in order to block the effects of dopamine rapidly and completely.
Figure 2.
Dopamine stimulated dissociation of [35S]GTPγS from CHO cell membranes expressing D2 dopamine receptors, effects of raclopride. [35S]GTPγS dissociation assays were performed as described in the Materials and methods section. Membranes were incubated with [35S]GTPγS (0.1 nM) in the presence of dopamine (10 μM) for 60 min and [35S]GTPγS dissociation was initiated by addition of 10 μM GTPγS. Parallel [35S]GTPγS dissociation assays were performed in the absence and presence of raclopride (100 μM), added at the same time as the GTPγS. Basal [35S]GTPγS dissociation has been subtracted from the data shown. Agonist-stimulated dissociation data were fitted best by a model of a single exponential decay. Data represented in this graph are from a single experiment replicated three times.
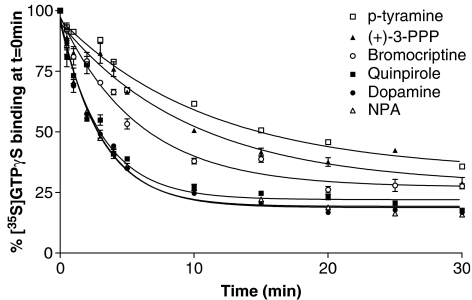
We then examined a range of agonists of varying efficacy for their ability to stimulate dissociation of the prebound [35S]GTPγS from the G proteins. Each agonist was tested at a maximally effective concentration as determined from the concentration/response dependence for the agonist in [35S]GTPγS association experiments (Gardner and Strange, 1998; Payne et al., 2002). The concentrations of agonists used here appear, therefore, rather high but they were chosen in order to provide a saturating response in the association part of the reaction, full occupancy of the receptors based on ligand-binding assays and a presumed maximal response in the dissociation phase. Using these concentrations, the present study provides relative efficacy data for association (see below) in good agreement with other studies. Also such concentrations of agonist do not give rise to unexpected effects in concentration/response curves. Indeed, we have shown for dopamine that similar, maximal effects are seen in association and dissociation assays for concentrations of 10 μM–1 mM (data not shown).
The efficacy of each agonist was determined in the present study as the amount of [35S]GTPγS bound in the association phase (60 min) of each experiment relative to that of 1 mM dopamine. Based on these data, the relative efficacy of the compounds ranged from 39 to 103%, and these data were similar to those found previously for this system (Gardner and Strange, 1998; Payne et al., 2002). Data from the dissociation experiments were best fitted to a model of a one site exponential decay (Figure 3). The t1/2 for the dissociation of [35S]GTPγS and the amount dissociated were calculated for each compound (Table 1). The t1/2 values for each of the compounds were converted to dissociation rate constants and were found to correlate well with the relative efficacy of the compounds in the association assay (r2=0.95). For dopamine, m-tyramine and p-tyramine, these t1/2 values are similar to those seen when the assay is performed without agonist present during the association phase (Figure 1, P>0.5, analysis of variance) suggesting that both assay formats provide similar measures of agonist efficacy. The extent of [35S]GTPγS dissociation also varied between the different agonists (Table 1). Although there was a trend for the most efficacious compounds to dissociate more [35S]GTPγS, the relative efficacy of the compounds did not show a significant correlation with the amount of [35S]GTPγS binding which the compounds were able to dissociate.
Figure 3.
Dissociation of [35S]GTPγS binding carried out in the presence of different agonists. Dissociation of [35S]GTPγS binding was carried out in the presence of a range of agonists as described in the Materials and methods section. Each agonist was present at a maximally effective concentration throughout the experiment that is, during [35S]GTPγS association and dissociation. Agonists tested were 1 mM dopamine, 1 mM quinpirole, 10 μM bromocriptine, 1 mM p-tyramine, 1 mM (+)-3-PPP and 10 μM NPA. Data are expressed as a percentage of [35S]GTPγS binding at time zero after the subtraction of basal levels of [35S]GTPγS dissociation. Dissociation data were fitted best by a model of a single exponential decay. Data shown are representative curves for experiments replicated at least three times.
Table 1.
Effect of a range of agonists of varying efficacy on the rate of [35S]GTPγS dissociation
| [35S]GTPγS dissociation assays | |||
|---|---|---|---|
| Compound | t1/2 (min) | Dissociable [35S]GTPγS (% of initial [35S]GTPγS bound) | Efficacy in [35S]GTPγS association assay (%, relative to dopamine) |
| NPA | 2.2±0.2 | 79.5±1.9 | 103.6±5.6 |
| Dopamine | 2.7±0.2 | 74.7±1.3 | 100 |
| Quinpirole | 2.9±0.8 | 70.9±3.5 | 80.5±1.7 |
| m-Tyramine | 4.3±1.0 | 60.3±7.7 | 62.5±2.6 |
| (+)-3-PPP | 6.3±0.8 | 73.3±1.9 | 55.6±0.4 |
| Bromocriptine | 5.3±0.7 | 70.8±2.6 | 50.5±2.4 |
| p-Tyramine | 7.1±0.9 | 61.4±3.5 | 39.9±8.6 |
Abbreviations: (+)-3-PPP, R(+)-3-(3-hydroxyphenyl)-N-propylpiperidine hydrochlorideNPA, R-(+)-propylnorapomorphine.
The effect of a range of agonists on the rate of dissociation of [35S]GTPγS was investigated as described in the Materials and methods section and in Figure 3. The relative efficacy of each agonist was calculated from the [35S]GTPγS association data relative to 1 mM dopamine, which was taken to be 100%. Agonists were added at maximally effective concentrations (1 mM dopamine, quinpirole, m-tyramine, (+)-3-PPP, and p-tyramine, 10 μM NPA and bromocriptine). t1/2 values were taken from the graphs of time versus amount of [35S]GTPγS bound, which fitted well to a model of a single exponential decay. The amount of dissociable [35S]GTPγS is shown as a percentage of the initial binding at t=0 min. Data are the mean±s.e.m. of at least three independent experiments.
In the [35S]GTPγS dissociation experiments, it was notable that about 30% of the bound [35S]GTPγS did not dissociate despite the presence of a 100 000 fold excess of non-radioactive GTPγS. The non-dissociable bound [35S]GTPγS was not non-specific binding as this was determined by including non-radioactive GTPγS (10 μM) throughout the experiment, and nonspecific binding amounted to 5% of the total [35S]GTPγS binding at the start of the dissociation. The majority of the bound [35S]GTPγS bound at the start of the dissociation was to Gi/o proteins as [35S]GTPγS binding to membranes from cells treated with Pertussis toxin was ∼5% of the value seen in membranes from untreated cells. This suggests that at the start of the dissociation there is more than one population of bound [35S]GTPγS, but most of this (∼95%) is to Gi/o proteins.
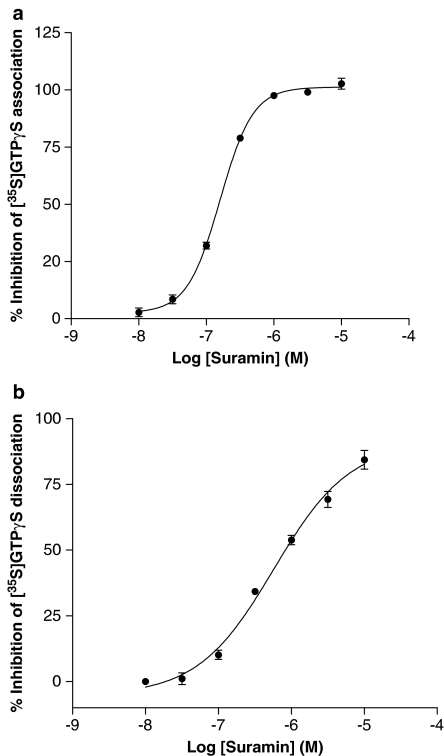
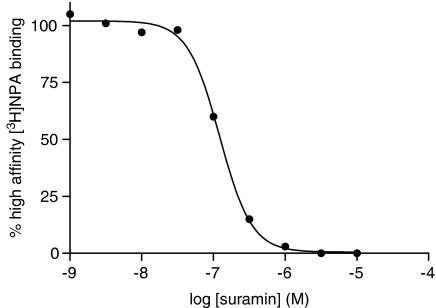
A large part of the bound [35S]GTPγS will dissociate in a manner sensitive to agonists and inhibited by antagonists suggesting that it is a receptor-mediated event. The fact that the receptor is required for this dissociation suggests that the receptor is still coupled to the G protein after agonist stimulation has occurred and that dissociation is occurring from the agonist/receptor/G protein(ARG)[35S]GTPγS species. To provide further evidence for an interaction between the receptor and G protein after agonist stimulation we examined the effect of suramin on both [35S]GTPγS association and dissociation as shown in Figure 4. Suramin inhibited both association and dissociation of [35S]GTPγS in a dose-dependent manner with pEC50 values of 6.79±0.01 and 6.13±0.43, respectively. In control experiments, it was shown that suramin did not inhibit [3H]spiperone binding to the receptor (C Meneveau and PG Strange, unpublished) indicating that suramin did not interact directly with the receptor-binding site. Taken together, these data suggest that the ARG complex persists after the agonist has stimulated binding of [35S]GTPγS to the G protein. This was examined further by determining the high-affinity binding of the agonist, [3H]NPA, which corresponds to labelling of the RG complex (Roberts et al., 2004) (Figure 5). Suramin inhibited high affinity [3H]NPA binding in a dose-dependent manner with a pEC50 of 6.90±0.08 which is similar to its effect on [35S]GTPγS association and dissociation.
Figure 4.
Effect of suramin on the association and dissociation of [35S]GTPγS binding to CHO cell membranes expressing the dopamine D2 receptor. Dopamine-stimulated [35S]GTPγS association and dissociation assays were carried out as described in the Materials and methods section. (a) Association assays were performed for 60 min in the presence of a range of concentrations of suramin. (b) Dissociation assays were performed as described in the Materials and methods section. Association was carried out for 60 min and suramin was added to the assay after this association phase with or without 10 μM non-radioactive GTPγS, and dissociation allowed to proceed for a further 30 min. Suramin added without the 10 μM non-radiolabelled GTPγS had no effect on dissociation, but did prevent any further association of the [35S]GTPγS. The graphs show data from representative experiments carried out three times with similar results.
Figure 5.
Inhibition of high-affinity [3H]NPA binding by suramin in CHO cell membranes expressing the dopamine D2 receptor. [3H]NPA binding was carried out as described in the Materials and methods section. The data are expressed as a percentage of the total high affinity [3H]NPA binding defined as the [3H]NPA binding inhibited by high concentrations of suramin. The data are from a single representative experiment repeated three times with similar results.
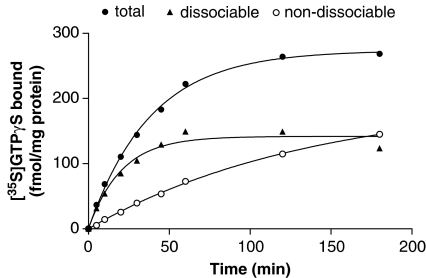
To investigate whether levels of dissociable [35S]GTPγS binding could be manipulated, we performed experiments with different association times. Total [35S]GTPγS binding increased with time reaching a plateau level at long incubation times (Figure 6). In separate assays at each incubation time, [35S]GTPγS association was terminated by addition of excess GTPγS and [35S]GTPγS dissociation assessed. The amounts of dissociable and non-dissociable [35S]GTPγS binding both increased with time. Whereas the dissociable portion increased quite quickly and reached a plateau level at long association times, the non-dissociable portion increased more slowly throughout the time course.
Figure 6.
[35S]GTPγS dissociation assays performed following different times of association. Membranes from CHO cells expressing the dopamine D2 receptor were incubated with 0.1 nM [35S]GTPγS in the presence and absence of 1 mM dopamine for different times and total bound [35S]GTPγS (total) was determined as described in the Materials and methods section. In parallel assays, [35S]GTPγS association was terminated at each time point by addition of 10 μM GTPγS and [35S]GTPγS dissociation determined after 30 min. Basal levels of [35S]GTPγS binding at each time point were subtracted from data. The figure shows the amount of [35S]GTPγS that was dissociable after 30 min at these different times (dissociable) and the amount that did not dissociate at these times (non-dissociable). Data shown are representative curves from an experiment performed independently three times with similar results.
This suggests that there are two species of [35S]GTPγS-labelled G proteins whose proportions change with association time. The dissociable component reached a plateau and the non-dissociable component increased as the association phase was allowed to proceed for longer times.
These data suggest that under typical [35S]GTPγS-binding assay conditions, breakdown of the ARG complex is low. This may be due to the low concentration of [35S]GTPγS (0.1 nM) used in these assays. To investigate this further, we performed dissociation assays using a higher concentration of [35S]GTPγS (1 nM) in the association and dissociation phases. At the higher [35S]GTPγS concentration, there is less dissociable binding (57.1±1.3%), that is, more breakdown of the ARG complex, as compared with that seen for the lower [35S]GTPγS concentration (69.8±2.4%, P<0.05).
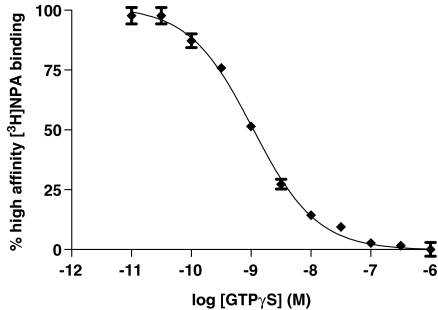
The effect of different concentrations of GTPγS on the stability of the ARG complex was examined using the high-affinity binding of the agonist [3H]NPA. GTPγS dose dependently inhibited high-affinity agonist binding, that is, the formation of the ARG complex (Figure 7).
Figure 7.
Inhibition of [3H]NPA binding by GTPγS in CHO cell membranes expressing the dopamine D2 receptor. [3H]NPA binding was carried out as described in the Materials and methods section. Data are expressed as a percentage of total high-affinity [3H]NPA binding defined as that inhibited by high concentrations of nucleotide. Data are from a single representative experiment, which was carried out three times with similar results.
Discussion
Studies carried out in the 1980s showed that the binding of [35S]GTPγS to purified Gαo and Gαi2 subunits of G proteins and to purified heterotrimeric Go protein was irreversible in the presence of millimolar concentrations of Mg2+ (Sternweis and Robishaw, 1984; Higashijima et al., 1987). More recent studies carried out in membrane systems containing GPCRs have shown, however, that [35S]GTPγS binding is reversible and that dissociation may be accelerated in the presence of an agonist (Kupprion et al., 1993; Breivogel et al., 1998; Waelbroeck, 2001; Alt et al., 2002). In this study, we have used the D2 dopamine receptor stably transfected in CHO cells to further investigate the dissociation of [35S]GTPγS from G proteins.
We observe that in CHO cell membranes transfected with the dopamine D2 receptor, [35S]GTPγS dissociation occurs under basal conditions (absence of agonist) and that the dissociation is markedly increased in rate and extent in the presence of dopamine. This agrees well with previous studies on the formyl peptide receptor (Kupprion et al., 1993), where a 50% decrease in bound [35S]GTPγS was observed over a 30 min period upon addition of 1 μM non-radiolabelled GTPγS. In that study, the dissociation was rapid and maximal in its extent in the first 10 min. Agonist independent [35S]GTPγS dissociation observed for cannabinoid receptors (Breivogel et al., 1998) showed a rapid dissociation phase with a t1/2 of 6.8 min. In addition, t1/2 values in the range 1–3 min have been reported for agonist stimulated dissociation of [35S]GTPγS in other systems for example, μ-opioid receptors (Traynor et al., 2002) and muscarinic acetylcholine receptors (Berstein et al., 1992), in agreement with this study.
We extended our study to investigate the effects of a range of agonists of varying efficacy on the dissociation of [35S]GTPγS. We examined the effect of agonists on [35S]GTPγS dissociation in two assay formats. In the first, [35S]GTPγS association occurred in the absence of agonist and [35S]GTPγS dissociation was started by the addition of excess non-radiolabelled GTPγS and agonist. In the second format, agonist was present during both the association and dissociation phase; otherwise the assay was the same. In both assay formats, the degree of acceleration of the [35S]GTPγS dissociation was dependent on and correlated well with the relative efficacy of the agonist, as measured in [35S]GTPγS-association experiments in this and other studies (Gardner and Strange, 1998; Payne et al., 2002). [35S]GTPγS dissociation reached a plateau where dissociation was incomplete and there was a tendency for the more efficacious agonists to dissociate more [35S]GTPγS. The two assay formats therefore, provide similar data showing that [35S]GTPγS dissociation may be accelerated by the agonist-bound dopamine D2 receptor.
It is surprising in the present study that not all of the bound [35S]GTPγS is dissociable, when a large excess of non-radiolabelled GTPγS is used to trigger dissociation. There appears to be about 30% of the bound [35S]GTPγS which is non-dissociable in experiments using 0.1 nM [35S]GTPγS and dopamine as agonist. There are various possible explanations for this. For example, the non-dissociable [35S]GTPγS could be bound to G proteins or other proteins not accessible to the D2 receptor. This seems unlikely as in the experiments where agonist is present in the association phase, there is a marked increase in [35S]GTPγS binding owing to the agonist and a proportion of this is also non-dissociable. Also, if we pretreat our cells with Pertussis toxin, we only observe a very small amount of agonist-induced [35S]GTPγS binding to membranes derived from the cells. This represents less than 5% of the signal we observe in membranes from control cells (data not shown), suggesting that the majority of [35S]GTPγS bound in these experiments is to Gi/o proteins. The bound [35S]GTPγS is also >95% specifically bound as determined in the presence of non-radioactive GTPγS. This shows clearly that the majority of bound [35S]GTPγS at the start of the dissociation is specifically bound to Gi/o proteins. The bound [35S]GTPγS seen at the end of the association phase could consist, therefore, of two distinct species of [35S]GTPγS bound to G protein. One of these could be [35S]GTPγS bound to G proteins which are still closely linked to the receptor (agonist/receptor/G[35S]GTPγS) and the other could be [35S]GTPγS bound to Gα subunits that have separated from the receptor (Gα [35S]GTPγS) (see e.g. (Birnbaumer et al., 1990)). [35S]GTPγS bound to the former species would be dissociable via the receptor. [35S]GTPγS bound to the G protein α subunits separated from the receptor is unlikely to be able to recombine easily with agonist bound receptors under the experimental conditions used here, as the large excess of non-radiolabelled GTPγS will lead to excess Gα GTPγS that will dominate any reverse reaction. Hence, [35S]GTPγS binding to free α subunits will appear irreversible.
It seems, therefore, that the following scheme may hold for these experiments.
We investigated this scheme by examining [35S]GTPγS dissociation following different association times. These experiments showed that the dissociable component of [35S]GTPγS binding reached an apparent plateau for the longer association times and the non-dissociable component increased. This would be consistent with [35S]GTPγS binding being first to G proteins linked to the receptor and subsequently to G protein α subunits separated from the receptor, formed slowly by breakdown of the ARG[35S]GTPγS. We also tested the effect of different concentrations of [35S]GTPγS in the assays. When the [35S]GTPγS concentration was increased to 1 nM, we observed less dissociable [35S]GTPγS binding suggesting that there is a greater breakdown of ARG in the presence of higher [35S]GTPγS concentrations. The effect of increasing the [35S]GTPγS concentration could also be consistent with labelling of different populations of RG complexes, so that one is labelled more at low concentrations of [35S]GTPγS and is dissociable and the other is labelled at higher concentrations of [35S]GTPγS and is non-dissociable. This scheme, however, seems unlikely as [35S]GTPγS saturation binding experiments indicate a single affinity (K Quirk and PG Strange unpublished).
To provide further support for the scheme where [35S]GTPγS labels both ARG and Gα, we investigated the effects of suramin, a compound that has been shown to inhibit RG coupling (Freissmuth et al., 1999). Suramin elicits a dose-dependent inhibition of [35S]GTPγS association and dissociation with pEC50 values which agree well with the ability of suramin to inhibit formation of the ARG species, as measured by its ability to inhibit high-affinity agonist ([3H]NPA) binding. These data support a role for the ARG complex in these assays and point to the fact that in the presence of low concentrations of [35S]GTPγS (0.1 nM) there is little breakdown of the ARG species. Further support for these ideas came from experiments where different concentrations of non-radioactive GTPγS were used to prevent high-affinity [3H]NPA binding, and this showed that ARG was fairly stable at low concentrations of GTPγS.
It seems likely, therefore, that the dissociation of [35S]GTPγS observed here in either assay format is occurring from agonist/receptor/G protein species. The rate we observe, (k=0.25 min−1), in the presence of a full agonist is similar to values reported for other GPCRs. The pharmacological profile of the dissociation reaction corresponds well to that of the observed association reaction. Although the observed association reaction will be dependent on both the association and dissociation rate constants, the correspondence in pharmacological profile between forward and back reactions is expected and reassuring.
We cannot relate the present observations directly to native systems. The principal G protein interacting with the D2 dopamine receptor in brain is Go, (Jiang et al., 2001) whereas in the CHO cells the principal inhibitory G proteins are Gi2 and Gi3 (Neubig et al., 1985; Raymond et al., 1993; Gettys et al., 1994). Whether these differences in G proteins make any difference to the data observed will require further experimentation. The R/G stoichiometry could also affect the data observed. R/G stoichiometry in tissues such as the brain is unknown, but dopamine/[3H]spiperone competition curves are similar for D2 in brain and expressed in CHO cells (Withy et al., 1981; Lin et al., 2006) suggesting that the R/G stoichiometry is not very different.
The data presented in this report, therefore, suggest that a large proportion of agonist/receptor/G protein (ARG) complexes remain intact after agonist has bound to the receptor and activated the G proteins. This is particularly clear when using the low concentrations of [35S]GTPγS (0.1 nM) typically used in [35S]GTPγS-binding assays. With higher concentrations of guanine nucleotides, more separation may occur. Models of G protein activation have assumed that the ARG complex dissociates upon binding of GTP or an analog. Studies in intact cells using FRET analyses have shown that for the G protein Gi, the subunits rearrange rather than separating (Bunemann et al., 2003). In addition, a study carried out on muscarinic m1 receptors suggested that the receptor, the G protein Gq and the effector phospholipase C form a three protein complex that is stable over multiple activation/deactivation cycles (Biddlecome et al., 1996). These data, taken together, provide support for the possibility that receptor and G protein may not immediately separate upon agonist activation, but this will be dependent on the conditions used for assays.
Agonist efficacy is dependent on the rate of the different reactions of the G protein cycle, with the slowest step in the cycle being the rate-determining step for that agonist. This assay shows us that the binding of [35S]GTPγS to the G protein α subunit is not an irreversible event and allows us to calculate a rate constant for this step in the cycle. The rate of dissociation of [35S]GTPγS is accelerated by the presence of an agonist and dependent upon the efficacy of the agonist. The data are consistent with bound [35S]GTPγS existing in two distinct species, in one of which the agonist/receptor/G[35S]GTPγS still remain closely linked. This assay provides us with a useful tool for further investigating the G protein cycle, which in turn aids in the understanding of the mechanisms of agonist efficacy.
Acknowledgments
We thank the BBSRC for funding this project.
Abbreviations
- ARG
agonist/receptor/G protein
- GPCR
G protein-coupled receptor
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thio)triphosphate
- NPA
R-(+)-propylnorapomorphine
- (+)-3-PPP
R(+)-3-(3-hydroxyphenyl)-N-propylpiperidine hydrochloride
Conflict of interest
These authors state no conflict of interest.
References
- Alt A, Clark MJ, Woods JH, Traynor JR. Mu and Delta opioid receptors activate the same G proteins in human neuroblastoma SH-SY5Y cells. Br J Pharmacol. 2002;135:217–225. doi: 10.1038/sj.bjp.0704430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein G, Blank JL, Smrcka AV, Higashijima T, Sternweis PC, Exton JH, et al. Reconstitution of agonist-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11, and phospholipase C-beta 1. J Biol Chem. 1992;267:8081–8088. [PubMed] [Google Scholar]
- Biddlecome GH, Berstein G, Ross EM. Regulation of phospholipase C-beta1 by Gq and m1 muscarinic cholinergic receptor. Steady-state balance of receptor-mediated activation and GTPase-activating protein-promoted deactivation. J Biol Chem. 1996;271:7999–8007. doi: 10.1074/jbc.271.14.7999. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Katada T, Northup JK, Ui M, Gilman AG. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984;259:3560–3567. [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro SW, Strange PG. Coupling of D2 and D3 dopamine receptors to G-proteins. FEBS Lett. 1993;315:223–226. doi: 10.1016/0014-5793(93)81168-y. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Waldhoer M, Bofill-Cardona E, Nanoff C. G protein antagonists. Trends Pharmacol Sci. 1999;20:237–245. doi: 10.1016/s0165-6147(99)01337-1. [DOI] [PubMed] [Google Scholar]
- Gardner B, Strange PG. Agonist action at D2(long) dopamine receptors: ligand binding and functional assays. Br J Pharmacol. 1998;124:978–984. doi: 10.1038/sj.bjp.0701926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettys TW, Sheriff-Carter K, Moomaw J, Taylor IL, Raymond JR. Characterization and use of crude alpha-subunit preparations for quantitative immunoblotting of G proteins. Anal Biochem. 1994;220:82–91. doi: 10.1006/abio.1994.1302. [DOI] [PubMed] [Google Scholar]
- Harrison C, Traynor JR. The [35S]GTPgammaS binding assay: approaches and applications in pharmacology. Life Sci. 2003;74:489–508. doi: 10.1016/j.lfs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Higashijima T, Ferguson KM, Sternweis PC, Smigel MD, Gilman AG. Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. J Biol Chem. 1987;262:762–766. [PubMed] [Google Scholar]
- Hilf G, Gierschik P, Jakobs KH. Muscarinic acetylcholine receptor-stimulated binding of guanosine 5′-O-(3-thiotriphosphate) to guanine-nucleotide-binding proteins in cardiac membranes. Eur J Biochem. 1989;186:725–731. doi: 10.1111/j.1432-1033.1989.tb15266.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupprion C, Wieland T, Jakobs KH. Receptor-stimulated dissociation of GTP[S] from Gi-proteins in membranes of HL-60 cells. Cell Signal. 1993;5:425–433. doi: 10.1016/0898-6568(93)90082-w. [DOI] [PubMed] [Google Scholar]
- Lin H, Saisch SG, Strange PG. Assays for enhanced activity of low efficacy partial agonists at the D(2) dopamine receptor. Br J Pharmacol. 2006;149:291–299. doi: 10.1038/sj.bjp.0706866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Neubig RR, Gantzos RD, Brasier RS. Agonist and antagonist binding to alpha 2-adrenergic receptors in purified membranes from human platelets. Implications of receptor-inhibitory nucleotide-binding protein stoichiometry. Mol Pharmacol. 1985;28:475–486. [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verriele L, Carpentier N, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes. J Pharmacol Exp Ther. 2002;303:815–822. doi: 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- Payne SL, Johansson AM, Strange PG. Mechanisms of ligand binding and efficacy at the human D2(short) dopamine receptor. J Neurochem. 2002;82:1106–1117. doi: 10.1046/j.1471-4159.2002.01046.x. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Olsen CL, Gettys TW. Cell-specific physical and functional coupling of human 5-HT1A receptors to inhibitory G protein alpha-subunits and lack of coupling to Gs alpha. Biochemistry. 1993;32:11064–11073. doi: 10.1021/bi00092a016. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Lin H, Strange PG. Investigation of the mechanism of agonist and inverse agonist action at D2 dopamine receptors. Biochem Pharmacol. 2004;67:1657–1665. doi: 10.1016/j.bcp.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- Traynor JR, Clark MJ, Remmers AE. Relationship between rate and extent of G protein activation: comparison between full and partial opioid agonists. J Pharmacol Exp Ther. 2002;300:157–161. doi: 10.1124/jpet.300.1.157. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M. Activation of guanosine 5′-[gamma-(35)S]thio-triphosphate binding through M(1) muscarinic receptors in transfected Chinese hamster ovary cell membranes; 1. Mathematical analysis of catalytic G protein activation. Mol Pharmacol. 2001;59:875–885. doi: 10.1124/mol.59.4.875. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Seifert R. Molecular analysis of beta(2)-adrenoceptor coupling to G(s)-, G(i)-, and G(q)-proteins. Mol Pharmacol. 2000;58:954–966. doi: 10.1124/mol.58.5.954. [DOI] [PubMed] [Google Scholar]
- Wilson J, Lin H, Fu D, Javitch JA, Strange PG. Mechanisms of inverse agonism of antipsychotic drugs at the D(2) dopamine receptor: use of a mutant D(2) dopamine receptor that adopts the activated conformation. J Neurochem. 2001;77:493–504. doi: 10.1046/j.1471-4159.2001.00233.x. [DOI] [PubMed] [Google Scholar]
- Withy RM, Mayer RJ, Strange PG. Use of [3H]spiperone for labelling dopaminergic and serotonergic receptors in bovine caudate nucleus. J Neurochem. 1981;37:1144–1154. doi: 10.1111/j.1471-4159.1981.tb04664.x. [DOI] [PubMed] [Google Scholar]