Abstract
Background and purpose:
Agonist apparent affinities (pKI′) in histamine H3-receptor binding assays were higher than expected from apparent affinity values (pKapp) estimated in bioassay. Here, we investigate whether the degree of pKI′ overestimation is related to agonist intrinsic efficacy, by studying the effect of buffer composition on the pKI′ of ligands with varying intrinsic activity.
Experimental approach:
In the guinea-pig ileum bioassay, intrinsic activity (α) was determined from the maximal inhibition of the contraction produced by increasing agonist concentration. pKapp values were estimated using the method of Furchgott. The pKL of [3H]clobenpropit in guinea-pig cerebral cortex was estimated by saturation analysis in 20 mM HEPES-NaOH buffer (buffer B(0,0,0)), or buffer B(0,0,0) containing 70 mM CaCl2, 100 mM NaCl and 100 mM KCl (buffer B(0.07,0.1,0.1)). PKI values were determined in competition studies in both buffers.
Key results:
[3H]clobenpropit saturation isotherms had n H values of unity in both buffers. In buffer B(0.07,0.1,0.1), agonist pKI′ values were closer to pKapp values than in buffer B(0,0,0) but were associated with n H values <1. A two-site analysis of agonist data in buffer B(0.07, 0.1, 0.1) provided a better fit than a one-site fit and low affinity values (pKIL) were comparable to pKapp. Differences between the pKI′ in buffer B(0,0,0) and pKIL values in buffer B(0.07,0.1,0.1) (ΔpK) were correlated with α.
Conclusions and implications:
H3-receptor binding assays conducted in buffer B(0,0,0) and buffer B(0.07,0.1,0.1) can provide a measure of ligand affinity (pKapp) and intrinsic efficacy. The assay predicts that some ligands previously classified as H3-receptor antagonists may possess residual intrinsic efficacy.
Keywords: [3H]clobenpropit, histamine H3-receptors
Introduction
Tissue- or assay-dependent expression of intrinsic efficacy is a well-recognized phenomenon and as such, ligands that act as antagonists or partial agonists in one tissue of a species may behave as full agonists in another tissue from the same species. For instance, Black (1988) found that dichloroisoprenaline was an agonist at β-adrenoceptors in the spontaneously beating guinea-pig heart preparation, but an antagonist in the guinea-pig cardiac papillary muscle. Similarly, Kenakin and Beek (1980) showed that prenalterol was almost a full agonist at β2-adrenoceptors in guinea-pig trachea, a partial agonist in guinea-pig left atria and an antagonist in guinea-pig extensor digitorum longus.
Some years ago, before the histamine H3-receptor was cloned (Lovenberg et al., 1999) and as part of a programme aimed at the development of high-affinity and selective H3-receptor antagonists, we developed a radioligand binding assay using the H3-receptor agonist, [3H]R-α-methylhistamine ([3H]R-α-MH; Harper et al., 1997a, 1997b; 1999a). In the course of evaluating ligands in this assay, we found that the affinity estimates of ligands, previously characterized as competitive antagonists, were on the whole, comparable to those reported in histamine H3-receptor isolated tissue bioassays. However, we also noticed that there were a number of ligands that expressed a considerably higher affinity in the radioligand binding assays than they expressed in the isolated tissue bioassays. Moreover, for some of the ligands, the degree of affinity overestimation appeared to correlate with the ligand's intrinsic activity (α) measured in the functional assay, as though the overestimation provided a measure of agonist intrinsic efficacy. This observation raised the possibility that the H3-receptor radioligand binding assay might be a sensitive method of detecting residual intrinsic efficacy of H3-receptor ligands and, moreover, that it could allow identification of H3-receptor partial agonism that had remained undetected in the isolated tissue bioassay.
To facilitate estimation of both the apparent affinity (pKapp) and intrinsic efficacy of H3-receptor ligands, in the radioligand-binding assay, we considered that it would be necessary to manipulate the conditions of the assay to provide ‘high' and ‘low' affinity estimates for each ligand, the low affinity estimate being equivalent to the ligand's pKapp value estimated in isolated tissue bioassays and the difference between the ‘low' and ‘high' affinity estimates being a measure of the ligand's intrinsic efficacy.
We chose to try to manipulate the radioligand-binding assay, to provide the ‘low' affinity (pKapp) estimates for each ligand, by adding salts to the assay buffer rather than by adding guanine nucleotide analogues or using the guinea-pig ileum Krebs–Henseleit (K–H) buffer. This was because it was noted that the affinity of agonists in H3-receptor radioligand-binding assays, in the presence of high concentrations of guanine nucleotides or in Krebs buffer (e.g. Arrang et al., 1990; [3H]R-α-MH pKL, 100 μM Gpp(NH)=9.05, Krebs=9.41) were still considerably higher than pEC50 values obtained in functional assays of the same tissue (pEC50=8.40) and therefore could not be equivalent to the agonist pKapp. In addition, in the process of developing the guinea-pig cortex H3-receptor assay, we had found that increasing the buffer concentrations of a variety of salts (e.g. NaCl, CaCl2, MgSO4, NaH2PO4 and KCl) produced a concentration–dependent decrease in the specific binding of the agonist radioligand, [3H]R-α-MH (data not shown). Saturation studies performed in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES–NaOH) buffer (buffer B) containing 70 mM CaCl2, 100 mM NaCl and 100 mM KCl, alone and in combination (buffer B(0.07,0.1,0.1)), indicated that the salts reduced the specific binding of [3H]R-α-MH by decreasing the pKL without any change in Bmax and the greatest decrease in pKL was obtained in the presence of 100 mM NaCl, 100 mM KCl and 70 mM CaCl2 (data not shown). Although competition studies performed in buffer B and in buffer B(0.07,0.1,0.1) indicated that antagonist affinity was unchanged by buffer composition (see Table 1) and that ‘low' affinity estimates could be obtained for H3-receptor agonists (see Table 1), which were comparable to those obtained in the guinea-pig ileum bioassay, we were concerned that these studies required high concentrations of radioligand (20 nM) and that it was necessary to increase the tissue concentration to obtain a suitable ‘window' of specific binding. Consequently, we chose to try and use the H3-receptor antagonist, [3H]clobenpropit, to perform the studies on the assumption that an antagonist's affinity would not change appreciably, when the assay conditions were manipulated (see Table 1, Harper et al., 1997c, 1999b).
Table 1.
Parameter estimates for histamine H3-receptor ligands, obtained in buffer B(0,0,0) and buffer B (0.07,0.1,0.1), in guinea-pig cerebral cortex using [3H]R-α-MH as radioligand
| Ligand | Buffer B(0,0,0) | Buffer B(0.07,0.1,0.1) | n | |||
|---|---|---|---|---|---|---|
| pKI′ | nH | pKI′ | nH | |||
| Antagonists | thioperamide | 8.59±0.27 | 0.84±0.09 | 8.76±0.14 | 0.84±0.06 | 3 |
| clobenpropit | 10.50±0.08 | 1.22±0.06 | 9.82±0.18 | 1.04±0.16 | 3 | |
| Agonist | iodoproxyfan | 9.58±0.05 | 0.96±0.10 | 8.47±0.14 | 1.04±0.09 | 4 |
| proxyfan | 8.89±0.34 | 0.95±0.02 | 7.40±0.05 | 1.11±0.06 | 3 | |
Data are the mean±s.e.m. from the number of assays shown (n). Competition experiments were conducted as described by Harper et al. (1999a). A final assay tissue concentration of 6 mg and 0.1 nM concentration of [3H]R-α-MH was used in buffer B(0,0,0). In buffer B(0.07,0.1,0.1), the concentration of [3H]R-α-MH was 20 nM and a tissue concentration of 12 mg was used to achieve sufficient specific binding.
In this study, we first report the effect that including NaCl, KCl and CaCl2 (buffer B(0.07,0.1,0.1)) in the assay buffer has on the affinity of [3H]clobenpropit at H3-receptors in guinea-pig cortex. We subsequently report the effect that this buffer has on the ‘behaviour' of a series of H3-receptor antagonists and agonists (see Figure 1), which had been described at the time, with varying α as defined by assays performed in the guinea-pig ileum bioassay. Some of these data were previously presented to the British Pharmacological Society (Harper et al, 1997d; Watt et al., 1997).
Figure 1.
Chemical structures of histamine H3-receptor ligands.
Methods
Preparation of guinea-pig cerebral cortex membranes
Adult male Dunkin–Hartley guinea pigs (200–300 g) were killed by cervical dislocation and the whole brain was removed and immediately placed in ice-cold 20 mM HEPES–NaOH buffer (pH7. 4, 4°C). The cortex was dissected, weighed and homogenized in ice-cold 20 mM HEPES–NaOH buffer (pH7.4, 4°C) (1 g 15 ml−1) using a polytron homogenizer (Kinematica AG, GmbH, Lucerne, Switzerland; PT-DA 3020/2TS; ∼3 s × 3). The homogenate was centrifuged at 100 g for 5 min and the supernatants pooled and stored at 4°C. The pellets were rehomogenized in fresh ice-cold buffer (80 ml) and recentrifuged at 100 g for 5 min at 4°C. The supernatants were centrifuged at 39800 g for 12 min at 4°C and the final pellet was resuspended in 20 mM HEPES–NaOH buffer containing 3 mM metyrapone (21°C) (Harper et al., 1997c), to the required tissue concentration, using a Teflon-in-glass homogenizer (setting 5, 3 ×).
[3H]clobenpropit – saturation studies
Guinea-pig cortical membranes (1.6 mg) were incubated for 165 min at 21±3°C, in a final volume of 0.5 ml with HEPES–NaOH buffer (buffer B(0,0,0)) and 0.004 to 3 nM [3H]clobenpropit. Total and nonspecific binding of [3H]clobenpropit were defined using HEPES–NaOH buffer and 1μM thioperamide (pKI at histamine H3-receptors in guinea-pig cortex ∼9.0, Harper et al., 1999a), respectively. The assay was terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.3% polyethyleneimine, which were washed (3 × 3 ml) with ice-cold 50 mM Tris–HCl (pH7.4, 4°C) using a Brandell Cell Harvester (Brandell, Gaithersburg, MD, USA). Filters were transferred into scintillation vials, 4 ml Meridian Gold–Star liquid scintillation cocktail added and after 3 h the bound radioactivity was determined by counting (3 min) in a Beckman liquid scintillation counter.
To determine the effect of modifying the assay buffer on the binding of [3H]clobenpropit, we performed saturation analysis in buffer B(0,0,0), and in this buffer containing final assay concentrations of 70 mM CaCl2, 100 mM NaCl and 100 mM KCl (buffer B(0.07,0.1,0.1); with final ionic concentrations (M) Ca2+, 0.07; Na+, 0.1; K+, 0.1; Cl−, 0.27=buffer B(0.07, 0.10, 0.10, 0.27)). In a further series of experiments, [3H]clobenpropit saturation analysis was performed in the presence of increasing (0, 30, 70, 100, 200, 300 mM) final assay concentrations of CaCl2 (buffer B(0,0,0), B(0.03,0,0), B(0.07,0,0), B(0.1,0,0), B(0.2,0,0) and B(0.3,0,0), respectively).
[3H]clobenpropit competition studies
Competition studies were conducted using a membrane concentration, which was previously found to result in zone A conditions for [3H]clobenpropit binding at a 0.2 nM concentration (see Harper et al., 1999b). In addition, the competition assay incubation time was previously shown to be sufficient for equilibrium of both radioligand and competitor (see Harper et al., 1999b). Guinea-pig cerebral cortex membranes were resuspended in 20 mM HEPES–NaOH buffer containing 0.3 mM metyrapone. Membranes (1.6 mg) were incubated for 165 min at 21±3°C in a final volume of 0.5 ml with 20 mM HEPES–NaOH buffer containing [3H]clobenpropit (0.2 nM), histamine H3-receptor ligands and either 20 mM HEPES–NaOH, buffer B(0.07,0.1,0.1), buffer B(0.03,0,0), buffer B(0.07,0,0), buffer B(0.1,0,0), buffer B(0.2,0,0) or buffer B(0.3,0,0). Total and nonspecific binding of [3H]clobenpropit were defined using HEPES–NaOH buffer and 1μM thioperamide, respectively.
Guinea-pig ileum assay
Measurement of intrinsic activity
Adult male Dunkin–Hartley guinea pigs (300–500 g) were killed by cervical dislocation. The ileum was removed at a point 20 cm from the caecum and flushed with and placed in modified K–H buffer of the following mM composition: 118 NaCl, 5.9 KCl, 1.2 CaCl2, 1.2 MgSO4, 1, Na2HPO4, 25 NaHCO3 and 10 D-glucose. Ileum segments (2.5–3 cm) were suspended in 20 ml organ baths containing K–H buffer maintained at 37±1°C and gassed with 95% O2/5% CO2. The initial resting tension was adjusted to 1 g and the tissues field stimulated (0.1 Hz and 0.5 ms) at supramaximal voltage. A single cumulative concentration–effect curve (E/(A)) curve was obtained in each tissue. Decreases in tissue twitch tension (% inhibition) were recorded using isometric transducers (Grass FTO3). Mepyramine (3 μM) and famotidine (10 μM) were added to the K–H buffer to block postsynaptic H1 and presynaptic H2 receptors, respectively.
Measurement of antagonist affinity
Thioperamide, JB16132, JB96134, iodophenpropit, JB97034, GT-2227, JB95130 and GR175737 were preincubated with tissues for 1 h before the change in tension in response to increasing concentrations of the H3-receptor agonist, R-α-MH, was determined. Clobenpropit was preincubated with tissues for 3 h.
Estimation of apparent agonist affinity (pKapp)
Measurement of the pKapp of agonists at H3-receptors in guinea-pig ileum was achieved using the method of Furchgott with the irreversible H3-receptor antagonist, N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ, see Taylor and Kilpatrick, 1992). Agonist E/(A) curves were constructed in untreated tissues and in tissues which had been incubated (15 min) with EEDQ (0.3 μM) and washed (six times at 10 min intervals) before use. In each experiment the effect of EEDQ treatment on agonist E/(A) curves was ascertained for R-α-MH and at least three other agonists.
Data analysis
All data are presented as the mean±s.e.m. unless otherwise stated.
Functional data–agonist concentration effect curves
To obtain estimates of pEC50, and maximal asymptote (α), the Hill equation was fitted to agonist dose–response data, expressed as percentage inhibition of the electrically induced contraction of the ileum. To permit comparison of the agonist α values in different experiments, the α-values for each agonist, in each experiment were expressed as a percentage of the mean α-value obtained for R-α-MH in that experiment. R-α-MH was assigned a α-value of 1.0
Functional data – Schild analysis
When the minimum criteria for competitive antagonism were satisfied, that is, the antagonist produced a parallel, rightward shift in the R-α-MH concentration effect curve with no change in maximum asymptote, data were analysed according to the methods described by Black et al. (1985). pA2 values were estimated by fitting the individual pEC50 values, obtained in the presence (pEC50′) and absence (pEC50) of antagonist to the following derivative of the Schild equation
If the Schild slope parameter (b) was not significantly different from unity, it was constrained to unity and the data refitted to provide a pKB estimate.
Functional data – estimation of apparent agonist affinity (pKapp)
Mean data sets obtained for at least three agonists and R-α-MH, in control tissue and in EEDQ-pretreated tissues, were fitted to the operational model of agonism (see Black and Leff, 1983) with shared values of maximal effect (Emax) and transducer slope parameter (n). pKapp values were obtained by fitting all the data to the model using a derivative-free nonlinear, regression programme (BMDP Statistical Software, Module AR: Dixon, 1992).
Radioligand binding – saturation analysis
The Hill equation was fitted to saturation data (Eq. (2)) using Graph-Pad prism where the Hill slope (nH) was permitted to vary and where this parameter was constrained to unity.
In this equation, L is the radioligand concentration, Bmax the receptor density and KL the equilibrium dissociation constant of the radioligand.
Radioligand binding – competition curve data
To obtain pIC50 and nH parameter estimates, competition data were fitted to the Hill equation and to the Hill equation with nH constrained to unity, using Graph-Pad Prism software. When nH parameter estimates were less than unity and the unconstrained Hill equation provided a significantly better fit of the data than the constrained equation, as determined using an F-test, a two-site model was also fitted to the data.
Notwithstanding the finding of nH values that were significantly less than unity, dissociation constants were subsequently determined from pIC50 values using the Cheng and Prusoff (1973) to correct for the different receptor occupancy of [3H]clobenpropit in the different buffers. The parameter pKI′ has been assigned to dissociation constants which were derived from pIC50 values, where competition curve nH parameter estimates were significantly less than unity. The pKL values that were used to correct pIC50 values obtained in buffer B(0,0,0) and Buffer B(0.07,0.1,0.1) were 10.36 and 9.82, respectively. pKL values used to correct for the occupancy of [3H]clobenpropit in the presence of increasing CaCl2 concentrations are presented in Table 3.
Table 3.
Effect of increasing CaCl2 concentration on the affinity (pKL), nH and Bmax of [3H]clobenpropit at histamine H3-receptors in guinea-pig cerebral cortex
| Buffer | pKL | Bmax (fmol mg−1) | nH |
|---|---|---|---|
| B(0,0,0) | 10.34±0.10 | 3.19±0.25 | 1.23±0.11 |
| B(0.03, 0,0) | 10.16±0.22 | 3.54±0.05 | 1.11±0.05 |
| B(0.07,0,0) | 10.05±0.06 | 4.17±0.38 | 1.08±0.09 |
| B(0.1,0,0) | 9.84±0.15 | 4.14±0.23 | 1.04±0.14 |
| B(0.2,0,0) | 9.70±0.05 | 3.53±0.32 | 1.06±0.12 |
| B(0.3,0,0) | 9.60±0.02 | 4.10±0.23 | 1.06±0.02 |
Data are the mean±s.e.m. of three separate experiments. The concentrations of CaCl2 used in the buffers were (M): 0, 0.03, 0.07, 0.1, 0.2, 0.3. The s.e.m. of the nH parameter from individual data sets was between 0.36 and 0.09.
Statistical analysis
The effect of antagonist treatment on pEC50 and α-values was assessed by analysis of variance (ANOVA) and the Bonferroni-modified t-test for multiple comparisons. Differences in [3H]clobenpropit pKL values were determined by ANOVA. P-values of less than 0.05 were considered significant.
Statistical comparison of ligand pKI′ values obtained in buffer B(0,0,0) and buffer B(0.07,0.1,0.1) were performed using Minitab version 13 (Mintab Inc.) by fitting a general linear model with corresponding ANOVA, according to Rosendaal and Stone (2003). This approach was used to avoid the situation that often occurs when dealing with comparisons of large complex data sets by the use of multiple t-tests, that is, by chance significant differences will be found.
Materials
[3H]clobenpropit (VUF9153) was prepared to a specific activity of 1.67TBq mmol−1. by Amersham International plc. (Little Chalfont, Buckinghamshire, UK).
Iodophenpropit, proxyfan, 4-iodoproxyfan and the chloro and bromo derivatives, JB96132, JB96134, JB97034, GR175737, JB95130 and GT-2227 were synthesized by James Black Foundation chemists. Histamine, 2-methyl-1,2-di-3-pyridyl-1-propanone (metyrapone), HEPES, EEDQ and Trizma base were obtained from Sigma Chemical Co., Poole, Dorset, UK. R-α-MH, S-α-methylhistamine (S-α-MH), thioperamide and imetit were obtained from Research Biochemicals Inc. (Poole, Dorset, UK). N-α-methylhistamine (N-α-MH) was obtained from Tocris Cookson Ltd. (Bristol, UK). All other materials were obtained from Fisher Scientific (Loughborough, Leicestershire, UK).
Results
Guinea-pig ileum bioassay
Histamine, S-α-MH, R-α-MH, N-α-MH, imetit, proxyfan, chloroproxyfan, iodoproxyfan and bromoproxyfan produced dose–dependent inhibition of the electrically induced twitch of the guinea-pig ileum (e.g. Figure 2). Agonist potency values (pEC50) and maximal inhibitory effects (α) are shown in Table 2.
Figure 2.
Effect of histamine H3-receptor ligands on the electrically induced contraction of the guinea-pig ileum. Each point represents the mean±s.e.m. of determinations in at least three separate preparations (see Table 2).
Table 2.
pEC50, α, pKapp and pKB values for histamine H3-receptor ligands in the guinea-pig ileum bioassay
| H3-receptor agonist | pEC50 (n) | α (n) | pKapp | H3-receptor antagonist | pA2 (n) |
|---|---|---|---|---|---|
| imetit | 7.95±0.04 (12) | 0.90±0.09 (12) | 7.61±0.16 | thioperamide | 8.53±0.08 (20) |
| proxyfan | 7.29±0.19 (4) | 0.35±0.07 (4) | 7.66±0.49 | iodophenpropit | 8.82±0.34 (4)c |
| 7.34±0.10 (4)a | |||||
| chloroproyfan | 7.85±0.14 (4) | 0.45±0.12 (4) | 8.04±0.25 | JB96132 | 8.58±0.10 (54) |
| bromoproxyfan | 8.27±0.10 (9) | 0.69±0.07 (9) | 7.76±0.33 | JB96134 | 6.96±0.10 (6)c |
| iodoproxyfan | 8.33±0.11 (10) | 0.90±0.05 (10) | 8.11±0.21 | JB97034 | 7.22±0.16 (5)c |
| R-α-MH | 7.64±0.06 (25) | 1.00±0.05 (25) | 7.19±0.20 (3)b | JB95130 | 5.44±0.26 (7)c |
| N-α-MH | 7.41±0.07 (5) | 0.93±0.10 (5) | 7.16e | GR175737 | 8.29±0.28 (4)c |
| S-α-MH | 6.52±0.03 (10) | 0.99±0.08 (10) | 5.59±0.14 | GT-2227 | 6.82±0.11 (70) |
| histamine | 7.40±0.04 (6) | 1.16±0.11 (6) | 6.37±0.16 | clobenpropit | 9.93±0.12 (3)d |
Abbreviations: N-α-MH, N-α-methylhistamine; R-α-MH, R-α-methylhistamine; S-α-MH, S-α-methylhistamine.
Data shown are the mean±s.e.m.; n=number of tissues used unless stated otherwise. The error on the pKapp values is the fitting error.
The pA2 of proxyfan determined in four separate experiments±s.e.m.
Mean pKapp from three separate experiments±s.e.m.
pA2 determined from a single antagonist concentration.
pA2 determined in three separate experiments.
Thioperamide, clobenpropit, GR175737, iodophenpropit, JB95130, GT-2227, JB96132, JB96134 and JB97034 had no effect on the electrically induced twitch of the guinea-pig ileum but produced parallel rightward shifts in R-α-MH E/(A) curves without change in maximal response or slope (nH) (Table 2). Antagonist pKB or pA2 values are shown in Table 2.
EEDQ treatment of tissues resulted in an increase in agonist pEC50 and decrease in the maximal inhibitory response to each agonist (Figure 3). The effect of EEDQ on agonist E/(A) curves was prevented following receptor protection with H3-receptor antagonists (thioperamide 1 μM, pA2=8.53; clobenpropit 30 nM, pA2=10.1; data not shown). Agonist pKapp values, shown in Table 2, were obtained by global fitting of the data using the model of agonism (Black and Leff, 1983) and a derivative-free nonlinear, regression programme (BMDP Statistical Software, Module AR: Dixon, 1992).
Figure 3.
Effect of EEDQ (0.3 μM) on (a) R-α-MH, (b) imetit, (c) S-α-MH and (d) histamine-induced inhibition of the electrically induced contraction of the guinea-pig ileum. Each point is the mean±s.e.m. of determinations in at least six separate preparations.
Effect of buffer composition on [3H]clobenpropit saturation analyses
The effect of 70 mM CaCl2, 100 mM KCl and 100 mM NaCl (buffer B(0.07,0.1,0.1)) or the effect that increasing concentrations of CaCl2 had on the binding of [3H]clobenpropit to guinea-pig cerebral cortex membranes, was investigated. This was carried out so that it was possible to correct the H3-receptor ligand pIC50 values, obtained in competition studies, to account for changes in the radioligand receptor occupancy that would result from a change in the radioligand's affinity (pKL).
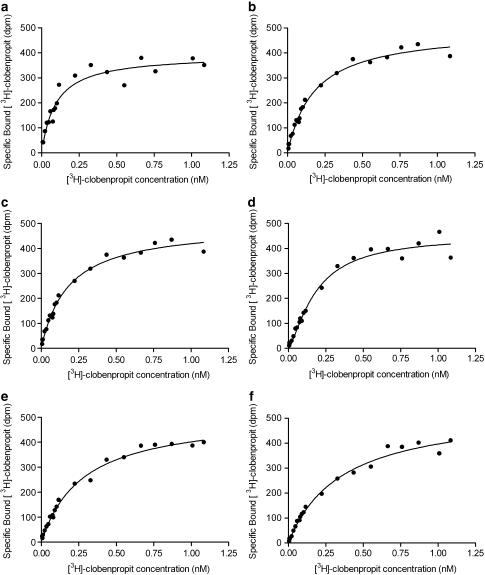
The binding of [3H]clobenpropit to guinea-pig cerebral cortex membranes was saturable in buffer B(0,0,0) and buffer B(0.07,0.1,0.1) (Figure 4). Saturation isotherms were monophasic and nH parameter estimates were not significantly different from unity in both buffers (buffer B(0,0,0) nH=1.14±0.09, buffer B(0.07, 0.1, 0.1) nH=0.95±0.06; n=4, t-test P>0.05). In addition, there was no significant difference between the goodness of fit to the Hill equation and that to the Hill equation with nH constrained to unity (F-test, P>0.05). The affinity (pKL) of [3H]clobenpropit was significantly lower in buffer B(0.07, 0.1, 0.1) (pKL=9.82±0.07; n=4) than in buffer B(0,0,0) (pKL=10.36±0.07; n=4; ANOVA P<0.002) but there was no significant difference in the H3-receptor density estimates in buffer B(0,0,0) (Bmax=4.46±0.53 fmol mg−1 original wet weight) and buffer B(0.07,0.1,0.1) (Bmax=5.59±0.66 fmol mg−1 original wet weight; ANOVA P>0.05).
Figure 4.
Representative saturation isotherms of [3H]clobenpropit to sites in guinea-pig cerebral cortex membranes in buffer B(0,0,0) (a and c) and buffer B(0.07,0.1,0.1) (b and d). The lines shown superimposed on the data points are the saturation isotherm obtained by fitting the Hill equation with nH constrained to unity to the data. Guinea-pig cortical membranes (1.6 mg) were incubated for 165 min at 21±3°C in a final volume of 0.5 ml with HEPES–NaOH buffer and [3H]clobenpropit. Total and nonspecific binding of [3H]clobenpropit were defined using buffer B(0,0,0) or buffer B(0.07,0.1,0.1) and 1 μM thioperamide, respectively. All determinations were made in triplicate.
Binding of [3H]clobenpropit to guinea-pig cerebral cortex membranes was also saturable in buffer containing concentrations of CaCl2 up to and including 300 mM (buffers B(0.03,0,0), B(0.07,0,0), B(0.1,0,0), B(0.2,0,0) and B(0.3,0,0); Figure 5). Saturation isotherms were monophasic and nH parameter estimates were not significantly different from unity at all CaCl2 concentrations (n=3; P>0.05, t-test). There was no significant difference between the goodness of fit to the Hill equation and that to the Hill equation with nH constrained to unity in all buffers (F-test, P>0.05). CaCl2 concentration had no significant effect on H3-receptor density (Table 3, Figures 5 and 6; ANOVA P>0.05). The pKL of [3H]clobenpropit was significantly decreased in the presence of increasing CaCl2 concentration (Table 3, Figures 5 and 6; ANOVA P<0.01). The decrease in pKL appeared to be saturable (Figure 6) such that the greatest change in this parameter was obtained in the presence of 100 mM CaCl2 (0.51±0.10, n=3) and increasing this concentration by a further 200 mM produced only a further 0.24±0.19 decrease (n=3).
Figure 5.
Representative saturation isotherms of [3H]clobenpropit to sites in guinea-pig cerebral cortex membranes in (a) buffer B(0,0,0), (b) buffer B(0.03,0,0), (c) buffer B(0.07,0,0), (d) buffer B(0.1,0,0), (e) buffer B(0.2,0,0) and (f) buffer B(0.3,0,0). The lines shown superimposed on the data points are the saturation isotherm obtained by fitting the Hill equation with nH constrained to unity to the data. Guinea-pig cortical membranes (1.6 mg) were incubated for 165 min at 21±3°C in a final volume of 0.5 ml with HEPES–NaOH buffer and [3H]clobenpropit. Total and nonspecific binding of [3H]clobenpropit were defined using appropriate buffer and 1 μM thioperamide, respectively. All determinations were made in triplicate.
Figure 6.
Effect of increasing concentration of CaCl2 in buffer B on (a) pKL and (b) Bmax of [3H]clobenpropit at sites in guinea-pig cerebral cortex membranes. The line shown superimposed on the data was obtained by fitting a hyperbolic function.
Effect of buffer composition on specific binding of 0.2 nM [3H]clobenpropit
In buffer B(0,0,0), the percentage specific binding of [3H]clobenpropit (35±1%, n=65) was significantly less than that obtained in the presence of buffer B(0.07,0.1,0,1) (73±1%, n=65; ANOVA P<0.001; see Figure 7). In contrast, the percentage coefficient of variation, calculated from all the triplicate data points forming each competition curve, was significantly greater in buffer B(0.07,0.1,0.1) (6.68±0.24, n=65) than in buffer B(0,0,0) (4.68±0.14, n=65; t-test, P<0.001).
Figure 7.
Competition curves for H3-receptor agonists and antagonists at sites labelled with [3H]clobenpropit in guinea-pig cerebral cortex. (a) Effect of increasing concentrations of ligands on [3H]clobenpropit binding (dpm). Data were obtained in a single experiment in buffer B(0,0,0) and buffer B(0.07,0.1,0.1) and errors are the mean±s.e.m. of triplicates. (b) Mean competition curve data for ligands, expressed as percentage specific binding, in buffer B(0,0,0) or (c) buffer B(0.07,0.1,0.1). Guinea-pig cortical membranes (1.6 mg) were incubated for 165 min at 21±3°C in a final volume of 0.5 ml with HEPES–NaOH buffer, [3H]clobenpropit (0.2 nM) and increasing concentrations of ligands. Total and nonspecific binding of [3H]clobenpropit were defined using appropriate buffer and 1 μM thioperamide, respectively. Data are the mean±s.e.m. of between four and six experiments (see Table 4). The lines shown superimposed on the data for imetit, chloroproxyfan and S-α-methylhistamine were obtained using a two-site fit. The line shown superimposed on the thioperamide data was obtained using a one-site fit.
Changes in ligand pKapp obtained in buffer B(0,0,0) and buffer B(0.07,0.1,0.1)
In both buffer B(0,0,0) and buffer B(0.07,0.1,0.1) each histamine H3-receptor ligand produced a concentration–dependent inhibition of the specific binding of [3H]clobenpropit to H3-receptors in guinea-pig cerebral cortex membranes (e.g. Figure 7). In buffer B(0,0,0), the estimated mid-point slope parameter estimates (nH) for the agonist ligands; imetit, N-α-MH, S-α-MH, iodoproxyfan and chloroproxyfan were all significantly less than unity whereas in B(0.07,0.1,0.1) buffer, the nH values were significantly less than unity for all the agonists (Table 4, t-test P<0.05). The nH values, estimated from competition curves, for all the antagonist ligands obtained in both buffers were not significantly different from unity (Table 4, t-test, P<0.05).
Table 4.
Parameter estimates for histamine H3-receptor ligands obtained from analysis of competition experiments performed in buffer containing 3 mM metyrapone (buffer B(0,0,0)) and in buffer B(0.07,0.1,0.1)
| Ligand | n | Buffer B (0,0,0) | Buffer B (0.07,0.1,0.1) | ΔpKI′ | ΔpK | ||||
|---|---|---|---|---|---|---|---|---|---|
| pKI or pKI′ | nH | pKIH and pKIL | pKI or pKI′ | nH | pKIH and pKIL | ||||
| Histamine H3-receptor agonists | |||||||||
| imetit | 4 | 9.22±0.30 | 0.63±0.03* | 10.20±0.31 | 8.06±0.07 | 0.66±0.07* | 10.05±0.51 | 1.16±0.23 | 1.52±0.29 |
| 8.04±0.34 | 7.71±0.03 | ||||||||
| proxyfan | 4 | 8.34±0.10 | 0.83±0.23 | 7.68±0.11 | 0.70±0.09* | 9.53±0.11 | 0.66±0.06 | 0.98±0.21 | |
| 7.36±0.11 | |||||||||
| chloroproxyfan | 4 | 8.98±0.24 | 0.77±0.05* | 9.47±0.23 | 8.41±0.02 | 0.72±0.06* | 9.94±0.26 | 0.58±0.24 | 0.92±0.20 |
| 8.22±0.36 | 8.06±0.07 | ||||||||
| bromoproxyfan | 3 | 8.93±0.25 | 0.82±0.22 | 8.37±0.09 | 0.63±0.03* | 10.34±0.19 | 0.55±0.26 | 0.99±0.25 | |
| 7.94±0.09 | |||||||||
| iodoproxyfan | 5 | 9.54±0.22 | 0.71±0.04* | 10.04±0.37 | 8.73±0.12 | 0.70±0.03* | 10.21±0.22 | 1.21±0.23 | 1.16±0.13 |
| 8.27±0.36 | 8.37±0.10 | ||||||||
| R-α-MH | 4 | 9.74±0.15 | 1.02±0.31 | 7.80±0.07 | 0.61±0.07* | 9.07±0.37 | 1.94±0.11 | 2.51±0.29 | |
| 7.24±0.15 | |||||||||
| N-α-MH | 6 | 9.58±0.19 | 0.70±0.04* | 10.08±0.10 | 8.09±0.29 | 0.51±0.04* | 9.82±0.26 | 1.45±0.23 | 2.15±0.13 |
| 8.19±0.30 | 7.43±0.23 | ||||||||
| S-α-MH | 6 | 7.67±0.14 | 0.64±0.03* | 8.81±0.18 | 6.29±0.14 | 0.49±0.02* | 8.08±0.22 | 1.37±0.15 | 1.98±0.19 |
| 6.90±0.19 | 5.68±0.12 | ||||||||
| histamine | 4 | 8.87±0.32 | 0.83±0.14 | 7.04±0.15 | 0.54±0.02* | 8.44±0.13 | 1.50±0.43 | 2.24±0.32 | |
| 6.30±0.09 | |||||||||
| Histamine H3-receptor antagonists | |||||||||
| thioperamide | 4 | 8.41±0.06 | 0.99±0.08 | 8.93±0.06 | 0.92±0.04 | −0.52±0.07 | −0.52±0.07 | ||
| iodophenpropit | 4 | 9.72±0.05 | 1.08±0.09 | 9.62±0.14 | 1.03±0.08 | 0.10±0.14 | 0.10±0.14 | ||
| JB96132 | 4 | 8.54±0.09 | 1.23±0.22 | 8.94±0.07 | 0.95±0.03 | −0.40±0.13 | −0.40±0.13 | ||
| JB96134 | 4 | 7.79±0.12 | 1.15±0.30 | 7.44±0.03 | 0.91±0.15 | 0.35±0.16 | 0.35±0.16 | ||
| JB97034 | 3 | 7.53±0.24 | 1.01±0.10 | 7.58±0.19 | 0.91±0.10 | −0.06±0.05 | −0.06±0.05 | ||
| JB95130 | 3 | 6.16±0.31 | 1.02±0.25 | 5.97±0.11 | 1.08±0.03 | −0.18±0.20 | −0.18±0.20 | ||
| GR175737 | 3 | 8.43±0.03 | 0.82±0.06 | 7.95±0.07 | 0.93±0.07 | 0.48±0.07 | 0.48±0.07 | ||
| GT-2227 | 3 | 7.33±0.21 | 1.26±0.28 | 6.88±0.21 | 1.13±0.08 | 0.45±0.14 | 0.45±0.14 | ||
Abbreviations: N-α-MH, N-α-methylhistamine; R-α-MH, R-α-methylhistamine; S-α-MH, S-α-methylhistamine.
Data are the mean±s.e.m. from the number of assays shown (n). When the mean nH parameter estimate for a ligand was not different from unity, an affinity (pKI) value is provided. When nH is significantly less than unity, pIC50 values were corrected using the Cheng–Prusoff equation but affinity values were assigned the parameter, pKI′. pKIH and pKIL values were obtained by fitting a two-site model to the data. ΔpKI′ is the difference between pKI or pKI′ values in buffer B(0,0,0) to pKIL in buffer B(0.07,0.1,0.1). ΔpK is the difference between the pKI or pKI′ in buffer B(0,0,0) to pKIL in buffer B(0.07,0.1,0.1).
nH significantly different from unity P<0.05, t-test.
Notwithstanding the non-unit nH values, obtained with several agonist ligands in both buffer B(0,0,0) and buffer B(0.07,0.1,0.1), in the first instance, we investigated the relationship between the apparent affinity values (pKI or pKI′), obtained in the two buffers. The pKI or pKI′ values for 14 of the 17 ligands, in buffer B(0,0,0), were higher than those obtained in buffer B(0.07,0.1,0.1) (Figure 8). All the agonist ligands expressed a lower pKI or pKI′ in buffer B(0.07,0.1,0.1) than in buffer B(0,0,0). In addition, the difference between the estimated pKI or pKI′ (ΔpKI′) of ligands in buffer B(0,0,0) and in buffer B(0.07,0.1,0.1), was significantly less (P<0.0001 t-test) for the antagonist ligands (see Figure 8 and Table 4, mean ΔpKI′=0.03±0.13, n=8; for instance, JB97034 had ΔpKI′=−0.06±0.05) than for the ligands classified as agonists in the guinea-pig ileum bioassay (mean ΔpKI′=1.16±0.16, n=9; S-α-MH and R-α-MH, ΔpKI′=−1.37±0.15 and ΔpKI′=1.94±0.11, respectively).
Figure 8.
Comparison of the apparent affinities (pKI or pKI′) of histamine H3-receptor agonists and antagonists obtained in buffer B(0,0,0) and in buffer B containing 70 mM CaCl2, 100 mM KCl and 100 mM NaCl (buffer B(0.07,0.1,0.1)). The broken line represents the line of identity.
There was a significant effect of tissue preparation on agonist affinity (pKI or pKI′) (P<0.001) in buffer B(0,0,0) but not in buffer B(0.7,0.1,0.1) (P>0.1, ANOVA). ANOVA indicated that there was a significant effect of tissue preparation and agonist on the magnitude of the ΔpKI′ (tissue preparation P<0.025, agonist P<0.001). There was no significant effect of tissue preparation on antagonist affinity (pKI) when determined in buffer B(0,0,0) or buffer B(0.07,0.1,0.1) (ANOVA, P>0.1). There was a significant effect of antagonist but no significant effect of tissue preparation on the magnitude of the ΔpKI′ (antagonist P<0.001, tissue preparation P>0.1 ANOVA).
Comparison of ligand pKapp values (pKI′), obtained in buffer B(0,0,0) and buffer B(0.07,0.1,0.1), with pKapp, pA2 or pKB values obtained in the guinea-pig ileum bioassay
When the pKI or pKI′ value, obtained for each ligand in buffer B(0,0,0), was compared with the pKapp or pA2 value estimated in the guinea-pig ileum bioassay, the data points for the ligands appeared randomly scattered and 14 of the 17 data points lay below the expected line of identity (y=x), that is, the ligands expressed an affinity that was higher than expected from the pKapp or pA2 value obtained in the functional bioassay (Figure 9a). When the same comparison was made using pKI or pKI′ values, obtained in buffer B(0.07,0.1,0.1), the data points were more linearly distributed and closer to the line of identity (Figure 9b). The mean deviation from the line of identity (∑(pKi – pKapp, pA2 or pKB)/n) was greatest when the pKI or pKI′ values were estimated in buffer B(0,0,0) rather than buffer B(0.07,0.1,0.1) (B(0,0,0), mean deviation from pKapp, pA2 or pKB=1.09±0.21; B(0.07,0.1,0.1) mean difference from pKapp, pA2 or pKB=0.44±0.08; Figure 9a and b).
Figure 9.
Comparison of the affinities of histamine H3-receptor agonists (pKapp) and antagonists (pA2 or pKB) at H3-receptors in the guinea-pig ileum bioassay (see Table 2) with those estimated in radioligand binding assays (see Table 4). pKI or pKI′ values for (a) agonists and antagonists in standard buffer (B(0,0,0)), (b) agonists and antagonists in B(0.07,0.1,0.1), (c) agonists in B(0,0,0), (d) agonists in B(0.07,0.1,0.1), (e) antagonists in B(0.07,0.1,0.1) and (f) antagonists in B(0.07,0.1,0.1). The broken line represents the line of identity (y=x).
When the antagonist pKI values, were compared with pKB or pA2 values estimated in the guinea-pig ileum assay, there was no significant change in the deviation from y=x when pKI values were estimated in buffer B(0,0,0) or buffer B(0.07,0.1,0.1) (0.41±0.14 and 0.30±0.12, respectively; t-test, P>0.05; Figure 9e and f). In contrast, when only the agonist pKI or pKI′ values, obtained in buffer B(0,0,0) were compared with pKapp values estimated in the guinea-pig ileum assay, the deviation from y=x was 1.69±0.24. When the same comparison was performed using pKI′ values obtained in buffer B(0.07,0.1,0.1), the data points were significantly closer to y=x (0.55±0.08; t-test, P<0.0005; Figure 9c and d) but 8 of the 9 data points still lay below the line of identity. In buffer B(0,0,0), the mean deviation from y=x, was significantly greater for agonists than antagonists (t-test, P<0.005) but there was no significant difference in the degree of deviation from y=x in buffer B(0.07,0.1,0.1) (t-test, P>0.05).
In light of this finding and the observation that all the mean agonist nH parameter estimates were significantly less than unity in buffer B(0.07,0.1,0.1), the agonist competition data were analysed further. The goodness of fit of the Hill equation and of the Hill equation with nH constrained to unity, were compared. The Hill equation with unconstrained slope provided a significantly improved fit to the data for all agonists (F-test, P<0.05) and therefore a two-site model was fitted to the data. The two affinity values (pKIL and pKIH) that were obtained are presented in Table 4. When the pKIL values, obtained from the two-site fit of the agonist competition curves in buffer B(0.07,0.1,0.1), were compared with pKapp values estimated in the guinea-pig ileum assay, the data points were evenly distributed about the line of identity and the deviation from y=x (0.08±0.06) was significantly less than when pKI′ values were compared (0.55±0.08, Figure 10).
Figure 10.
Comparison of (a) pKI′ and (b) pKIL values for agonists in buffer B(0.07,0.1,0.1) with ileum pKapp estimates.
Comparison of α-values obtained in the guinea-pig ileum with ΔpK values from radioligand binding
There appeared to be a relationship between agonist ΔpK values and α-values estimated in the guinea-pig ileum bioassay such that agonists with higher α expressed higher ΔpK values (Figure 11).
Figure 11.
Comparison of ligand ΔpK values and α measured in the guinea-pig ileum bioassay.
Discussion
In this study, we have used a guinea-pig ileum bioassay to determine the affinity (pA2) of histamine H3-receptor antagonists and also the α and pKapp of a series of histamine H3-receptor agonists at guinea pig H3-receptors. In addition, we have investigated the effect of altering buffer composition on the apparent affinity (pKI′) that ligands, with known α, express in competition studies performed in the absence (buffer B(0,0,0)) and presence of buffer salts (buffer B(0.07,0.1,0.1)); with the aim of establishing whether assays of this type can be used to measure the pKapp and to detect residual intrinsic efficacy of ligands. We refer to ileum H3-receptor affinity values estimated by the method of Furchgott, as pKapp values, in order to take account of the problem that defining this parameter as pKA assumes that activation of the receptor has no effect on binding and this is not the case for currently proposed models of agonist action (see Colquhoun, 1998). The term pKapp is used to indicate that the measurement is a macroscopic equilibrium constant describing the overall constant for the binding of agonist to receptor and subsequent isomerization of this receptor to form AR* (see Neubig et al., 2003).
The pA2 values of the previously described H3-receptor antagonist ligands, thioperamide, clobenpropit and GR175737, in the guinea-pig ileum, were comparable to those reported previously (Arrang et al., 1987, 1990; Van der Goot et al., 1992; Barnes et al., 1993; Clitherow et al., 1996; Valentine et al., 1999). The affinity of iodophenpropit and GT-2227 were underestimated compared with those reported previously (iodophenpropit pA2=9.6, Jansen et al., 1992; GT-2227, pA2=7.9, Tedford et al., 1998); this underestimation may have been a consequence of the 1 h antagonist preincubation being insufficient for equilibration of these antagonists.
In the radioligand-binding studies, the competition curves for all the agonist ligands, with the exception of R-α-MH and histamine, in buffer B(0,0,0) and for all the agonists in buffer B(0.07,0.1,0.1), were associated with nH parameter estimates that were significantly less than unity. This behaviour has been well described for the binding of agonists at many types of receptor and a number of models have been proposed to explain the phenomenon (e.g. Lefkowitz et al., 1993; Samama et al., 1993; Weiss et al., 1996). In light of nH values less than unity, it could be argued that only the pIC50 values for R-α-MH and histamine should be corrected using the Cheng–Prusoff equation because the derivation of this correction relies on simple competition between two ligands at a homogenous receptor population and therefore should be applied only when nH is not different from unity. However, in this study we have corrected all pIC50 values using the Cheng–Prusoff equation to correct for the differential occupancy of ∼0.2 nM [3H]clobenpropit in the two buffers (buffer B(0,0,0), pKL=10.36 and buffer B(0.07,0.1,0.1), pKL=9.82). Ideally, in order for this not to be a confounding problem, we would have performed competition studies in both buffers at a concentration of [3H]clobenpropit, which was equivalent to its pKL. However, this was not possible because the low specific activity of the radioligand resulted in too small a specific-binding window in buffer B(0,0,0) at a ∼0.04 nM concentration. We also corrected all pIC50 values irrespective of nH parameter because although R-α-MH and histamine had nH values that were not different from unity, it appeared from functional pEC50 values that their pIC50 values were likely to have been overestimated by as much if not more than other agonists, where the nH parameter was significantly less than unity (e.g. chloroproxyfan). Thus, the pIC50 value of R-α-MH in buffer B(0,0,0) was 8.96±0.16 and the pEC50 value in the functional assay was 7.64, whereas the pIC50 value of chloroproxyfan was 8.17±0.24 and the pEC50 value was 7.85. Clearly, if the same mechanism underlies the binding of all agonists it is equally inappropriate to correct an agonist pIC50 value when the nH value is not different from unity as when the nH is less than unity. To make a distinction between pIC50 values that have been corrected for [3H]clobenpropit occupancy where nH was equal to unity and those where nH was less than unity and where strictly speaking the Cheng–Prusoff equation should not have been applied, we have assigned the latter the parameter pKI′.
In the radioligand-binding studies, affinity values (pKI or pKI′) for the agonist ligands, in the absence of buffer salts (buffer (B(0,0,0))), were higher than their estimated pKapp or pA2 values obtained in bioassay studies (Figure 9a), which confirmed our previous observations where we used [3H]R-α-MH as the radioligand (Harper et al., 1999a). This indicated that, despite using an antagonist radioligand, it was still possible to obtain overestimated ‘high' affinity binding values for agonists. The finding that the apparent affinity values (pKI′) of ligands in buffer B containing salts (buffer B(0.07,0.1,0.1)) were reduced relative to those obtained in buffer B(0,0,0) was also consistent with our preliminary studies in which [3H]R-α-MH was used as the radioligand and indicated that it was also possible to obtain ‘low' affinity estimates for agonist ligands when using the antagonist radioligand, [3H]clobenpropit.
The observation that the change in pKI′ between that obtained in buffer B(0,0,0) and that obtained in buffer B(0.07,0.1,0.1) (ΔpKI′) was greater for agonists than antagonists (agonists=1.16; antagonists=0.03) and that there was a significant effect of agonist but not antagonist on the ΔpKI′ (agonist P<0.001, antagonist P>0.1) suggested that the change in pKI′ value, brought about by modification of the buffer composition, was related to a property of the ligands, which was only expressed by agonists. It is possible to explain the high- and low-affinity binding of agonists in H3-receptor radioligand binding assays by considering the extended ternary complex model (TCM) developed by Lefkowitz and colleagues (Samama et al., 1993; Lefkowitz et al., 1993; see Figure 12a) and also by the cubic TCM that was described some years later (Weiss et al., 1996; see Figure 12b). In the extended TCM, it is proposed that the receptor can exist in a low-affinity agonist state (R) and a high-affinity state (R*), which can also interact with a G-protein (R*G) in the absence of agonist. Thus, the high-affinity H3-receptor agonist binding may be a consequence of the agonist binding to R* or R*G. In the cubic TCM, it is postulated that the receptor can exist in a low-affinity state (Ri equivalent to R in the TCM) and a high-affinity state (Ra, equivalent to R* in the TCM) and that both R and R* can exist as high-affinity states as a consequence of interaction with a G-protein (RG and R*G). Therefore, according to this model, high-affinity H3-receptor agonist binding could result from either binding to preformed high-affinity receptor states (R* and R*G) or from the induction of these high-affinity states through binding to low-affinity receptors (R). Low-affinity agonist binding in buffer B(0.07,0.1,0.1) can be explained by considering that, under these conditions, the agonist binds only to the low-affinity receptor state (R) and cannot bind to or induce the formation of R* or R*G. Although, at the time these studies were performed it was not definitely known that the H3-receptor was G-protein coupled because it had not yet been cloned, the possibility that agonists could induce H3-receptor ternary complex formation was supported by studies suggesting that these receptors were linked to effector systems through G-proteins (Arrang et al., 1990; West et al., 1990; Zweig et al., 1992; Clark et al., 1993; Litosch et al., 1993; Clark and Hill, 1995; Clark and Hill, 1996).
Figure 12.
(a) The extended TCM as described by Samama et al. (1993). (b) The cubic TCM of Weiss et al. (1996). In both models, H is hormone, G is G protein and R is the inactive receptor state and R* the ‘active' state. To allow simple comparison of the models, the terms used for ‘inactive', Ri and ‘active' receptor states (Ra) in the model of Weiss et al. (1996) have been modified to R and R*, respectively.
It seems unlikely that the apparent changes in ligand affinity obtained using buffer B(0,0,0) and buffer B(0.07,0.1,0.1) could have arisen simply as a consequence of highly variable data because although the percentage specific binding of [3H]clobenpropit was higher in buffer B(0.07,0.1,0.1) (73±1%) compared with that obtained in buffer B(0,0,0) (35±1%), the % coefficient of variation of the data points in buffer B(0,0,0) was lower than that in buffer B(0.07,0.1,0.1). Furthermore, we previously found that although the percentage specific binding of [3H]clobenpropit in buffer B(0,0,0) is low (∼40%), there was a close correlation between histamine H3-receptor pKI values estimated using this radioligand and a radioligand exhibiting considerably higher percentage specific binding ([3H]R-α-MH with 89%; Harper et al., 1999a, 1999b).
The pretext for performing competition studies in assay buffer containing salts, that is, that it could allow estimation of agonist affinity (pKapp), was supported by comparisons made between the radioligand binding pKI′ values and the pKapp or pA2 values obtained in the guinea-pig ileum bioassay (Figure 9). Thus, the deviation from the expected y=x for all the ligands, was significantly lower in modified buffer (buffer B(0,0,0)=1.09±0.21; B(0.07,0.1,0.1)=0.44±0.08; Figure 9a and b). In addition, when the same analysis was performed on subsets of ligands, characterized as agonists (Figure 9c and d) or antagonists (Figure 9e and f), in the guinea-pig ileum, the deviation from the expected y=x was increased for the agonists (buffer B(0,0,0)=1.69±0.24, Figure 9c) and unchanged for the antagonists (buffer B(0,0,0)=0.41±0.14, Figure 9e) relative to the complete data set. In addition, the deviation from y=x was significantly decreased when agonist but not antagonist pKI values were determined in the buffer containing salts (buffer B(0.07,0.1,0.1) agonists=0.55±0.08, Figure 9d; antagonists=0.30±0.12, Figure 9e) and, furthermore, the mean deviation from y=x was only significantly greater for agonists than that for antagonists when the pKI′ or pKI values were estimated in buffer B(0,0,0).
Notwithstanding the finding that the deviation from the expected y=x for the antagonists was unchanged by buffer, we noticed that for one antagonist, iodophenpropit, there was a large difference between the pA2 and pKI obtained in both buffers (∼1 log unit; pA2=8.82±0.34; pKI buffer B(0,0,0)=9.72±0.05, buffer B(0.07,0.1,0.1)=9.62±0.14). This discrepancy could be explained by considering that the pA2 value of iodophenpropit was underestimated because it had not been preincubated with tissue for long enough to reach equilibrium. In support of this possibility, Jansen et al. (1992) reported a pA2 of 9.6 for iodophenpropit and we found that the pA2 value of another H3-receptor antagonist with similar structure, clobenpropit, was increased when preincubated with tissue for 3 h.
Despite the similarity between pKapp values in the ileum bioassay and the pKI or pKI′ values of ligands in buffer B(0.07,0.1,0.1) (see Figure 9b), it was still apparent that eight of the nine agonist data points still lay below the expected line of identity (y=x, Figure 9d). It seems unlikely that we found that the ileum pKapp values were lower than the pKI′ values in buffer B(0.07,0.1,0.1) simply because of experimental error and because they were estimated, albeit with the exception of R-α-MH, in a single experiment. This is because, for one agonist, proxyfan, the pKapp (7.66±0.49) from model fitting was comparable to the pA2 determined by investigating the effect of this ligand on R-α-MH concentration effect curves (7.34±0.10, n=4; Table 4 and Schlicker et al., 1996, pA2=7.12). In addition, the pKapp values for R-α-MH and histamine were comparable to those previously reported by Taylor and Kilpatrick (1992) (R-α-MH=7.01; histamine=6.13).
The possibility that, even in buffer B(0.07,0.1,0.1), the agonist pKI′ values were not equivalent to the pKapp estimated in the ileum bioassay is supported by the observation that all the agonist competition curves in this buffer had nH parameter estimates, which were significantly less than unity (Table 4). The cubic TCM (Weiss et al., 1996; see Figure 12b) predicts this behaviour if it is considered that the change in composition of the assay buffer (buffer B(0.07,0.1,0.1)) is not sufficient to prevent the agonist inducing some high-affinity receptor states (ARG and/ or AR*G) or from binding to pre-existing high-affinity receptors (R* or RG). Therefore, according to this model, the flat competition curves result from competition for labelled R (low-affinity binding) and some induction or binding to R*G or RG (high-affinity binding component). The data cannot be explained by competition for labelled R and R* because in this situation competition curves would have unit slope. Interestingly, in support of this explanation, when the buffer B(0.07,0.1,0.1) agonist competition data were analysed using a two-site model, and the low-affinity estimates (pKIL) were compared with the ileum pKapp values, the data points were closer to y=x (Figure 10).
A possible explanation for the decrease in agonist affinity in buffer B(0.07,0.1,0.1) is that, in this buffer, the metal ions interfere in some way with the ability of agonists to induce high-affinity ternary complex receptor states. Indeed, many studies have demonstrated that agonist, but not neutral antagonist, binding is sensitive to metal ions (Limbird et al., 1982; Puttfarcken et al., 1986; Gowraganahalli et al., 1990) and have suggested that the effect that metal ions have on agonist binding is owing to them, in some way, preventing the formation of ternary complex between agonist, receptor and G-protein (Childers and La Riviere, 1984; Lambert and Childers, 1984; Kim and Neubig, 1985; Lynch et al., 1985; Demoliou-Mason and Barnard, 1986; Puttfarcken et al., 1986; Carraway et al., 1992). However, there are two other possible explanations to explain why the modified buffer reduces the high-affinity agonist binding and why some high-affinity binding remains in the modified buffer (B(0.07,0.1,0.1)). This is because adding NaCl, CaCl2 and KCl, also alters the osmotic and ionic strength of buffer B(0,0,0 (buffer B(0.07,0.1,0.1) osmotic strength=290 mM; ionic strength=430 mM; buffer B(0,0,0) osmotic strength=20 mM; ionic strength=20 mM). In fact, buffer ionic strength has been previously shown to reduce agonist affinity (Arias, 1996). However, our previous observation that 70 mM CaCl2 produced a greater reduction in [3H]R-α-MH pKL than 100 mM NaCl (data not shown) suggests that the osmotic strength of the buffer does not result in low-affinity agonist binding. Nonetheless, in retrospect, it would have been interesting to perform further competition studies to establish whether osmotic strength contributed to the reduction in agonist affinity, by adding glucose to buffer B(0,0,0).
To establish whether agonist competition curves with unit slope could be obtained, which at the same time had affinity estimates equal to ileum pKapp estimates and therefore to test the hypothesis that flat agonist competition curves, obtained in buffer B(0.07,0.1,0.1), resulted from some formation of ARG and/or AR*G, we performed further competition experiments in which the concentration of just one of the salts, CaCl2, was increased. The results we obtained were consistent with this hypothesis. The pKI′ of R-α-MH decreased with increasing CaCl2 concentration, whereas the pKI of thioperamide remained unchanged (Table 5), indicating that CaCl2 alone had the same effect as the combination of NaCl, KCl and CaCl2 in buffer B(0.07,0.1,0.1). In addition, the decrease in R-α-MH pKI′ was associated with an increase in nH such that at the highest CaCl2 concentration (300 mM) the nH parameter estimate was not different from unity and, moreover, at this CaCl2 concentration, the pKI of R-α-MH (7.24±0.06, n=3) was not different to the pKIL estimated from a two-site analysis of data obtained in buffer B(0.07,0.1,0.1) (7.24±0.15, n=4). An alternative approach to elucidate whether the nonunit agonist competition curves in buffer B(0.07,0.1,0.1) had resulted from the agonists binding to, or inducing the formation of ternary complex (ARG), would have been to add guanine nucleotides to the assay buffer. In addition, when these studies were performed, it had been suggested that the H3-receptor was coupled to either Gi (Clark et al., 1993; Litosch et al., 1993; Clark and Hill, 1996) or Gs-proteins (Cherifi et al., 1992). Therefore, it would also have been interesting to establish if treatment of tissues with pertussis or cholera toxin resulted in unit nH parameter estimates for agonists in buffer B(0.07,0.1,0.1).
Table 5.
Apparent affinity (pKI′) and nH values expressed by R-α-MH and thioperamide at H3-receptors in guinea-pig cortex in buffer containing increasing concentrations of CaCl2
| Buffer | R-α-MH | Thioperamide | ||
|---|---|---|---|---|
| pKI′ | nH | pKI | nH | |
| B(0,0,0) | 9.03±0.08 | 0.54±0.04* | 8.28±0.08 | 1.04±0.28 |
| B(0.03,0,0) | 8.59±0.21 | 0.52±0.04* | 8.55±0.09 | 0.93±0.14 |
| B(0.07,0,0) | 8.45±0.07 | 0.54±0.02* | 8.46±0.08 | 0.90±0.04 |
| B(0.1,0,0) | 8.09±0.13 | 0.60±0.06* | 8.38±0.16 | 0.87±0.05 |
| B(0.2,0,0) | 7.68±0.07 | 0.68±0.03* | 8.44±0.08 | 1.14±0.13 |
| B(0.3,0,0) | 7.24±0.06 | 0.88±0.08 | 8.39±0.06 | 0.95±0.02 |
Data are the mean±s.e.m. of three experiments. The concentrations of CaCl2 used in the buffers were the same as those in the experiments described in Table 3.
nH significantly different from unity, P<0.05 t-test.
The observation that CaCl2 decreased the pKL of [3H]clobenpropit (Figure 6) was consistent with the effect that buffer containing 70 mM CaCl2, 100 mM KCl and 100 mM NaCl (buffer B(0.07,0.1,0.1)) had on this parameter. That this effect appeared saturable was consistent with the possibility that the increased buffer CaCl2 concentration or increased buffer ionic strength prevented clobenpropit from binding to or inducing high-affinity receptor states. These states cannot be equivalent to R*G or RG because the nH parameter estimates for clobenpropit at all CaCl2 concentrations were not significantly different from unity. However, it is possible that the higher affinity of [3H]clobenpropit, in buffer B(0,0,0), results from it inducing or binding to R*.
The finding of a relationship between α measured in the ileum bioassay and the ΔpK (Tables 2 and 4 and Figure 11) suggests that H3-receptor radioligand binding assays can be used to detect residual agonist efficacy. Thus, ligands with ΔpK values of less than 1.00 were partial agonists (α=0.35–0.90) in the guinea-pig ileum assay and those ligands with ΔpK values of >1.1 were full agonists (α∼1.00). The finding that GR175737, an antagonist ligand as defined by the guinea-pig ileum bioassay, had a ΔpK value of 0.48 can be accounted for by considering that the H3-receptor radioligand binding assay detects intrinsic efficacy that has remained undetected in the bioassay. Thus, if a full agonist (α∼1.0, R-α-MH) has a ΔpK of 2.51 and expresses a pKI′ value approximately 2.5 log units higher than its pKapp, a partial agonist (α∼0.45, chloroproxyfan) has a ΔpK of 0.92 and expresses a pKI′ value approximately 1 log unit higher than its pKapp, then it is possible that a weaker partial agonist, which acts as a competitive antagonist in the functional assay, could express a pKI′ value that is still significantly higher than its pKB. Interestingly, since these studies were conducted, GR175737 has been shown to be a partial agonist (α∼0.4) (Wulff et al., 2002) in a cAMP assay which also found that proxyfan, a partial agonist in the guinea-pig ileum assay (α=0.35, pEC50=7.29), was a full agonist with over 1 log unit higher potency (pEC50∼8.4).
Conclusion
We have manipulated the conditions of the H3-receptor radioligand-binding assay to provide a method of obtaining a measure of both the pKapp and intrinsic efficacy of novel H3-receptor ligands. The assay predicts that some ligands previously classified as H3-receptor antagonists may possess residual agonist efficacy, so that under certain conditions radioligand-binding assays may be a more sensitive detector of agonist intrinsic efficacy than functional in vitro assays. The prospect that radioligand-binding assays can be used to detect intrinsic efficacy may be useful for the study of human receptors in native tissue, where it may not be possible to develop a functional assay. Studies of this type may be useful for excluding the possibility that, in human tissues, the receptor dimerizes with other receptor types or interacts with tissue-dependent factors (e.g. RAMP and scaffolding proteins) that modify the receptor pharmacology, such that ligands defined in recombinant systems as antagonists are found to express intrinsic efficacy.
Acknowledgments
This work was funded by Johnson and Johnson. We are grateful to Professor Mervyn Stone for his statistical advice and to Gillian Watt and Eric Griffin for experimental contributions.
Abbreviations
- EEDQ
N-ethoxycarbonyl-2-ethoxy-1, 2-dihydroquinoline
- N-α-MH
N-α-methylhistamine
- PEI
polyethyleneimine
- R-α-MH
R-α-methylhistamine
- S-α-MH
S-α-methylhistamine
Conflict of interest
The authors state no conflict of interest.
References
- Arias HR. Temperature and ionic strength dependence of quinacrine binding and quinacrine displacement elicited by high concentrations of agonists on the nicotinic acetylcholine receptor. Arch Biochem Biophys. 1996;333:1–11. doi: 10.1006/abbi.1996.0357. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Lancelot J-C, Lecomte J-M, Pollard H, Robba M, et al. Highly potent and selective ligand for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- Arrang J-M, Roy J, Morgat J-L, Schunack W, Schwartz J-C. Histamine H3 receptor binding sites in rat brain membranes: modulations by guanine nucleotides and divalent cations. Eur J Pharmacol. 1990;188:219–227. doi: 10.1016/0922-4106(90)90005-i. [DOI] [PubMed] [Google Scholar]
- Barnes JC, Brown JD, Clarke NP, Clapham J, Evans DJ, O'Shaughnessy CTO. Pharmacological activity of VUF9153, an isothiourea histamine H3 receptor antagonist. Eur J Pharmacol. 1993;250:147–152. doi: 10.1016/0014-2999(93)90632-r. [DOI] [PubMed] [Google Scholar]
- Black J. The Nobel Prizes 1988. Almqvist & Wiksell International: Stockholm Sweden; 1988. Drugs from emasculated hormones: the principles of syntopic antagonism. [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc Roy Soc (Lond B) 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P, Shankley NP. Further analysis of anomalous pKB values for histamine H2-receptor antagonists in the isolated mouse stomach. Br J Pharmacol. 1985;86:581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway RE, Mitra SP, Honeyman TW. Effect of GTP analogs and metal ions on the binding of neurotensin to porcine brain membranes. Peptide. 1992;14:37–45. doi: 10.1016/0196-9781(93)90008-5. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Prusoff WH. Relationship between the inhibition constant Ki and the concentration of inhibitor which causes 50% inhibition IC50 of an enzymic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cherifi Y, Pigeon C, Le Romancer M, Bado A, Reyl-Desmars F, Lewin MJ. Purification of a histamine H3 receptor negatively coupled to phosphoinositide turnover in the human gastric cell line HGT1. J Biol Chem. 1992;267:25315–25320. [PubMed] [Google Scholar]
- Childers SR, La Riviere G. Modification of guanine nucleotide-regulatory components in brain membranes. II Relationship of guanosine 5′-triphosphate effects on opiate receptor binding and coupling of receptors with adenylate cyclase. J Neurosci. 1984;4:2764–2771. doi: 10.1523/JNEUROSCI.04-11-02764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Hill SJ. Differential effect of sodium ions and guanine nucleotides on the binding of thioperamide and clobenpropit to histamine H3-receptors in rat cerebral cortex membranes. Br J Pharmacol. 1995;114:357–362. doi: 10.1111/j.1476-5381.1995.tb13234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Hill SJ. Sensitivity of histamine H3 receptor agonist-stimulated [35S]GTPã[S] binding to pertussis toxin. Eur J Pharmacol. 1996;296:2230225. doi: 10.1016/0014-2999(95)00800-4. [DOI] [PubMed] [Google Scholar]
- Clark MA, Korte A, Egan RW. Guanine nucleotides and pertussis toxin reduce the affinity of histamine H3 receptors on AtT-20 cells. Agents Actions. 1993;40:129–134. doi: 10.1007/BF01984051. [DOI] [PubMed] [Google Scholar]
- Clitherow JW, Beswick P, Irving RW, Scopes DIC, Barnes JC, Clapham J, et al. Novel 1,2,4-oxadiazoles as potent and selective H3 receptor antagonists. Bioorgan Med Chem Lett. 1996;6:833–838. [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: The interpretataion of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoliou-Mason CD, Barnard EA. Characterisation of opioid receptor subtypes in solution. J Neurochem. 1986;46:1129–1137. doi: 10.1111/j.1471-4159.1986.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. BMDP Statistical Software. University of California Press, Los Angeles; 1992. [Google Scholar]
- Gowraganahalli J, Cragoe EJ, Deth RC. Modulation of bovine aortic alpha-2 receptors by Na+. 5′-guanylylimidodiphosphate, amiloride and ethylisopropylamiloride: evidence for receptor g-protein precoupling. J Pharmacol Expt Ther. 1990;252:1184–1196. [PubMed] [Google Scholar]
- Harper EA, Gardner B, Griffin EP, Shankley NP, Black JW. Characterisation of histamine H3-receptor ligands in guinea-pig cortex and ileal longitudinal muscle myenteric plexus. Br J Pharmacol. 1997b;122:431P. [Google Scholar]
- Harper EA, Shankley NP, Black JW. Development of histamine H3-receptor radioligand binding assays in guinea-pig cerebral cortex and ileal longitudinal muscle myenteric plexus. Br J Pharmacol. 1997a;122:430P. doi: 10.1038/sj.bjp.0702861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EA, Shankley NP, Black JW. Characterisation of the binding of the histamine H3-receptor antagonist, [3H]clobenpropit, to sites in guinea-pig cerebral cortex membranes. Br J Pharmacol. 1997c;122:432P. doi: 10.1038/sj.bjp.0702860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EA, Shankley NP, Black JW. Development of H3-receptor radioligand binding assays in guinea-pig cerebral cortex membranes for the detection of agonist efficacy. Br J Pharmacol. 1997d;122:429P. [Google Scholar]
- Harper EA, Shankley NP, Black JW. Evidence that histamine homologues discriminate between H3-receptors in guinea-pig cerebral cortex and ileum longitudinal muscle myenteric plexus. Br J Pharmacol. 1999a;128:751–759. doi: 10.1038/sj.bjp.0702861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EA, Shankley NP, Black JW. Characterisation of the binding of [3H]clobenpropit to histamine H3-receptors in guinea-pig cerebral cortex membranes. Br J Pharmacol. 1999b;128:881–890. doi: 10.1038/sj.bjp.0702860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen FP, Rademaker B, Bast A, Timmerman H. The first radiolabeled histamine H3 receptor antagonist. [125I]iodophenpropit: saturable and reversible binding to rat cortex membranes. Eur J Pharmacol. 1992;217:203–205. doi: 10.1016/0014-2999(92)90851-t. [DOI] [PubMed] [Google Scholar]
- Kenakin TP, Beek D. Is prenalterol (H133/80) really a selective beta 1 adrenoceptor agonist? Tissue selectivity resulting from differences in stimulus-response relationships. J Pharmacol Expt Ther. 1980;213:406–413. [PubMed] [Google Scholar]
- Kim MH, Neubig RR. Parallel inactivation of α2-adrenergic agonist binding and Ni by alkaline treatment. FEBS Lett. 1985;192:321–325. doi: 10.1016/0014-5793(85)80134-4. [DOI] [PubMed] [Google Scholar]
- Lambert SM, Childers SR. Modification of guanine nucleotide-regulatory components in brain membranes, I. Changes in guanosine 5′-triphosphate regulation of opiate receptor binding sites. J Neurosci. 1984;4:2755–2763. doi: 10.1523/JNEUROSCI.04-11-02755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- Limbird LE, Speck JL, Smith SK. Sodium ion modulates agonist and antagonist interactions with the human platelet alpha 2-adrenergic receptor in membrane and solubilised preparations. Mol Pharmacol. 1982;21:609–617. [PubMed] [Google Scholar]
- Litosch I, Sulkholutskaya I, Weng C. G-protein-mediated inhibition of phospholipase C activity in a solubilized membrane preparation. J Biol Chem. 1993;268:8692–8697. [PubMed] [Google Scholar]
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, et al. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- Lynch CJ, Charest R, Blackmore PF, Exton JH. Studies on the hepatic α1-adrenergic receptor. J Biol Chem. 1985;160:1593–1600. [PubMed] [Google Scholar]
- Neubig RR, Spedding M, Kenakin T, Christopoulus A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- Puttfarcken P, Werling LL, Brown SR, Cote TE, Cox BM. Sodium regulation of agonist binding at opioid receptors. I—Effects of sodium replacement on binding at μ- and δ-type receptors in 7315c and NG108-15 cells and cell membranes. Mol Pharmacol. 1986;30:81–89. [PubMed] [Google Scholar]
- Rosendaal M, Stone M. Enhancement of repopulation haemopoiesis by heterozygous connexion 43 stem cells seeded on wild-type connexion 43 stroma. Clin Sci. 2003;105:561–568. doi: 10.1042/CS20030151. [DOI] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor. EXTENDING THE TERNARY COMPLEX MODEL. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- Schlicker E, Kathman M, Bitshnau H, Marr L, Reidemeister S, Stark H, et al. Potencies of antagonists chemically related to iodoproxyfan at histamine H3 receptors in mouse brain cortex and guinea-pig ileum: evidence for H3 receptor heterogeneity. Naunyn–Schmeidebeg's Arch. Pharmacol. 1996;353:482–488. doi: 10.1007/BF00169166. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Kilpatrick GJ. Characterisation of histamine H3 receptors controlling non-adrenergic non-cholinergic contractions of the guinea-pig isolated ileum. Br J Pharmacol. 1992;105:667–674. doi: 10.1111/j.1476-5381.1992.tb09036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford CE, Hoffman M, Seyedi N, Maruyama R, Levi R, Yates SL, et al. High antagonist potency of GT-2227 and GT-2331, new histamine H3 receptor antagonists, in two functional models. Eur J Pharmacol. 1998;351:307–311. doi: 10.1016/s0014-2999(98)00396-3. [DOI] [PubMed] [Google Scholar]
- Valentine AF, Rizzo CA, Rivelli MA, Hey JA. Pharmacological characterisation of histamine H3 receptors in human and guinea-pig ileum. Eur J Pharmacol. 1999;366:73–78. doi: 10.1016/s0014-2999(98)00904-2. [DOI] [PubMed] [Google Scholar]
- Van Der Goot H, Schepers MJP, Sterk GJ, Timmerman H. Isothiourea analogues of histamine as potent agonists or antagonists of the histamine H3 receptor. Eur J Med Chem. 1992;27:511–517. [Google Scholar]
- Watt GF, Sykes DA, Roberts SP, Shankley NP, Black JW. Estimation of agonist affinity and efficacy parameters of histamine H3-receptor ligands in guinea-pig ileum. Br J Pharmacol. 1997;122:435P. [Google Scholar]
- Weiss JM, Morgan PH, Lutz MW, Kenakin TP. The Cubic Ternary Complex Receptor – Occupancy Model I. Model Description. J Theor Biol. 1996;178:151–167. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- West RE, Zweig A, Shih NY, Siegel MI, Egan RW, Clark MA. Identification of two H3-receptor subtypes. Mol Pharmacol. 1990;38:610–613. [PubMed] [Google Scholar]
- Wulff BS, Hastrup S, Rimvall K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur J Pharmacol. 2002;453:33–41. doi: 10.1016/s0014-2999(02)02382-8. [DOI] [PubMed] [Google Scholar]
- Zweig A, Siegel MI, Egan RW, Clark MA, Shorr RGLn, West RE., Jr Characterisation of a digitonin-solubilised bovine brain H3-histamine receptor coupled to a guanine nucleotide binding protein. J Neurochem. 1992;59:1661–1666. doi: 10.1111/j.1471-4159.1992.tb10996.x. [DOI] [PubMed] [Google Scholar]