Abstract
Background and purpose:
Parabens are commonly added in pharmaceutical, cosmetic and food products because of their wide antibacterial properties, low toxicity, inertness and chemical stability, although the molecular mechanism of their antibacterial effect is not fully understood. Some agonists of the transient receptor potential (TRP) A1 channels are known to have strong antibacterial activities. Therefore, a series of experiments was conducted to find out the effects of parabens on TRP channels expressed in sensory neurons, particularly the TRPA1 channels.
Experimental approach:
Effects of parabens, especially of methyl p-hydroxybenzoate (methyl paraben) on TRP channel activities were examined using Ca2+-imaging and patch-clamp methods. In addition, an involvement of methyl paraben in the development of pain-related behavior in mice was investigated.
Key results:
Methyl paraben specifically activated TRPA1 in both HEK293 cells expressing TRPA1 and in mouse sensory neurons with an EC50 value of 4.4 mM, an attainable concentration in methyl paraben-containing products. Methyl paraben caused pain-related behavior in mice similar to that caused by allyl isothiocyanate, which was blocked by the TRP channel blocker, ruthenium red.
Conclusions and implications:
Our data indicate that methyl paraben is able to activate TRPA1 channels and can cause pain sensation. As such, methyl paraben provides a useful tool for investigating TRPA1 function and development of antinociceptive agents acting on TRPA1 channels.
Keywords: TRPA1, methyl paraben, nociception
Introduction
Transient receptor potential (TRP) channels were first described in Drosophila, where photoreceptors carrying trp gene mutations exhibited an abnormal transient responsiveness to continuous light (Montell and Rubin, 1989). In mammals, TRP channels comprise six related protein families (TRPC, TRPV, TRPM, TRPA, TRPML, TRPP) (Minke and Cook, 2002; Clapham, 2003; Montell, 2005). TRP channels are well recognized for their contributions to sensory transduction, responding to a wide variety of stimuli including temperature, nociceptive stimuli, touch, osmolarity and pheromones. The involvement of TRP channels in nociception has been extensively studied since the cloning of the capsaicin receptor, TRPV1 (Caterina et al., 1997; Tominaga and Caterina, 2004). TRPA1 (formerly ANKTM1), another member of the TRP channel super family, has also attracted attention for its potential role in nociception (Story et al., 2003; Bautista et al., 2006; Kwan et al., 2006). Heterologously expressed TRPA1 channels can be activated by isothiocyanate or thiosulphinate compounds, which constitute the pungent ingredients of mustard oil and garlic, respectively (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2005; Macpherson et al., 2005). Topical application of these compounds is known to excite sensory neurons, thereby producing acute pain and neurogenic inflammation through release of several substances including substance P and CGRP from the activated nerve endings.
Parabens are alkyl esters of p-hydroxybenzoate (Figure 1) and commonly added in pharmaceutical, cosmetic and food products because of their wide antibacterial properties, low toxicity, inertness and chemical stability (Soni et al., 2002). Parabens have been reported to cause contact dermatitis reactions and have been implicated in numerous cases of contact sensitivity on cutaneous exposure, when parabens have been applied to damaged or broken skin. Allergic reactions to ingested parabens have also been reported. Although the molecular mechanisms underlying the antibacterial action of this family of compounds have not been well clarified, parabens are hypothesized to act by inhibiting synthesis of DNA and RNA (Nes and Eklund, 1983) or of some key enzymes such as ATPases and phosphotransferases in some bacterial species (Ma and Marquis, 1996) or by disrupting membrane transport processes (Freese et al., 1973). It has recently been reported that propyl p-hydroxybenzoate (propyl paraben) induces K+ efflux in Escherichia coli (Bredin et al., 2005) and the mechanosensitive channel of large conductance (MscL: large prokaryotic mechanosensitive channel) underlies this action of propyl paraben (Nguyen et al., 2005). Methyl p-hydroxybenzoate (methyl paraben) has also been reported to be an activator of Ca2+ release mediated by ryanodine receptors in skeletal muscle terminal cisternae, a finding which might be related to the development of malignant hyperthermia (Cavagna et al., 2000). However, to date, it is not clear whether these actions are the mechanisms underlying the antibacterial effects of parabens (Nelson, 1919). Allyl isothiocyanate, cinnamaldehyde and allicin, the main pungent ingredients of mustard oil, cinnamon and garlic, respectively, are known to be activators of TRPA1 channels. All three compounds have been demonstrated to exhibit strong antimicrobial activities (Morozumi, 1978; Ankri and Mirelman, 1999; Lin et al., 2000; Chang et al., 2001). These results suggest the possibility that parabens might act on TRPA1 channels. In this study, we have examined the ability of methyl paraben to activate TRPA1 channels using Ca2+-imaging and patch-clamp methods and have found that methyl paraben can activate TRPA1 channels in both heterologous expression systems and native sensory neurons.
Figure 1.
Chemical structure of parabens, where R=methyl (CH3), ethyl (C2H5), propyl (C3H7), butyl (C4H9).
Methods
Animals
Male C57BL/6-strain or ddY-strain of mice (4 weeks, SLC, Shizuoka, Japan) were used. They were housed in a controlled environment (12 h light/dark cycle, room temperature 22–24°C, 50–60% relative humidity) with free access to food and water. All procedures involving the care and use of animals were carried out in accordance with institutional (National Institute for Physiological Sciences) guidelines and the National Institute of Health guide for the care and use of laboratory animals.
Cell cultures
HEK293 (human embryonic kidney-derived 293) cells were maintained in Dulbecco's modified Eagle's medium (supplemented with 10% fetal bovine serum, penicillin, streptomycin and L-glutamine) and transfected with 1 μg of cDNA for mouse TRPA1 (generous gift from Dr Patapoutian, The Scripps Institute), rat TRPV1, rat TRPV2, mouse TRPV3 (generous gift from Dr Patapoutian), rat TRPV4 (generous gift from Dr Caterina, Johns Hopkins University) or rat TRPM8 (generous gift from Dr Julius, UCSF), as described previously (Caterina et al., 1997). Primary cultures of mouse dorsal root ganglion neurons from male C57BL/6 mice (4-weeks-old) were incubated in a medium containing nerve growth factor (100 ng ml−1). Cells were re-seeded on coverslips coated with (for DRG neurons) or without (for HEK293 cells) poly-D-lysine.
Ca2+ assays and patch-clamp procedures
The fura-2 fluorescence was measured in standard bath solutions containing (in mM) 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES), 10 glucose, pH 7.4 (with NaOH). The ratio of florescence intensities obtained with fura-2 emissions at 340 and 380 nm (340:380) are shown.
The standard bath solutions for patch-clamp experiments were the same as used for fluorescent measurements. To make a Ca2+-free bath solution, CaCl2 was removed and 5 mM ethylene glycol tetraacetic acid, (EGTA) was added. Pipette solutions contained (in mM) 140 KCl, 5 EGTA, 10 HEPES, pH 7.4 (with CsOH). Data from whole-cell voltage-clamp recordings were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software, Axon Instruments). The voltage command pulse used was a slow (500 ms) ramp from −100 to +100 mV, applied to generate a current–voltage (I–V) curve. Whole-cell patch-clamp recordings and Ca2+ imaging experiments were performed 1 or 2 days after transfection of HEK293 cells or isolation of DRG neurons. All the experiments were performed at room temperature (25°C) unless otherwise indicated (Figure 2d).
Figure 2.
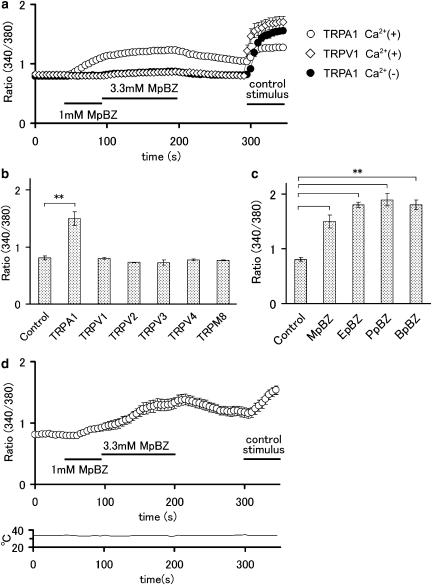
Increase in cytosolic Ca2+ concentration ([Ca2+]i) mediated by the activation of TRPA1 channels by methyl paraben in HEK293 cells. (a) Methyl paraben (MpBZ) caused an increase in cytosolic Ca2+ concentration ([Ca2+]i) in HEK293 cells expressing TRPA1 channels in the presence of extracellular Ca2+ (n=15), but not in cells expressing TRPV1 in the presence of extracellular Ca2+ (n=15) or cells expressing TRPA1 channels in the absence of extracellular Ca2+ (n=15). Control stimulus indicates cinnamaldehyde (500 μM) for TRPA1 or capsaicin (1 μM) for TRPV1 in the presence of extracellular Ca2+. Horizontal bars indicate the duration of applied stimulus. (b) MpBZ increased [Ca2+]i in HEK293 cells expressing TRPA1 channels (n=24), but not in cells expressing TRPV1 (n=15), TRPV2 (n=130), TRPV3 (n=12), TRPV4 (n=48), TRPM8 (n=122) or vector alone (n=24). **P<0.01. (c) Effects of MpBZ (1 mM, n=24), ethyl paraben (EpBZ, 1 mM, n=52), propyl paraben (PpBZ, 1 mM, n=21) or butyl paraben (BpBZ, 0.5 mM, n=24) on [Ca2+]i in HEK293 cells expressing TRPA1 channels. **P<0.01. (d) MpBZ caused an increase in [Ca2+]i in HEK293 cells expressing TRPA1 channels in the presence of extracellular Ca2+ at a constant temperature of 34°C (n=19). Lower panel shows bath temperature. Control stimulus indicates cinnamaldehyde (500 μM). Horizontal bars indicate the duration of applied stimulus.
Experimental protocol for pain-related behaviour
Mice were placed individually in transparent cages (19.5 × 12 × 12 cm) for 1 h before experiments. A portion of 20 μl of allyl isothiocyanate (10 mM) or methyl paraben (20, 50 or 100 mM) was injected subcutaneously into their right hind paw. The time spent licking and biting the injected paw was measured for 10 min after injection. In some experiments, ruthenium red (100 μM) was given with allyl isothiocyanate or methyl paraben.
Data analysis
Data were analysed using an unpaired t-test. Values shown are means±s.e.m.; P-values <0.05 were considered significant.
Materials
Parabens (Figure 1) were obtained from Wako Pure Chemical Industries, Ltd., and other chemicals were from Sigma. In the comparison of different parabens (Figure 2c), 1 mM of methyl, ethyl or propyl paraben was used but butyl paraben was used at 0.5 mM because this paraben was not soluble at 1mM in the bath solution.
Results
We first examined the ability of methyl paraben to activate TRPA1 channels using a Ca2+-imaging method. Methyl paraben (3.3 mM, an attainable concentration in mouth or skin; Soni et al., 2002) caused an increase in cytosolic Ca2+ concentration ([Ca2+]i) in HEK293 cells expressing TRPA1 channels (n=15), but not in cells expressing the TRPV1 receptor (n=15) (Figure 2a). These results indicate methyl paraben-induced increases in [Ca2+]i are mediated by TRPA1 channels. Furthermore, methyl paraben induced [Ca2+]i increases via TRPA1 channels in the presence, but not in the absence, of extracellular Ca2+ (n=15), suggesting Ca2+ entry via TRPA1 channels caused the [Ca2+]i increase. Cell viability was confirmed by the ability to respond to cinnamaldehyde (for TRPA1 channels, 500 μM) or capsaicin (for TRPV1 receptor, 1 μM) in the presence of extracellular Ca2+ (Figure 2a). To address whether the methyl paraben-induced increases in [Ca2+]i was specific to TRPA1, we examined the effects of 5 mM methyl paraben on [Ca2+]i in HEK293 cells expressing other TRP channels, TRPV1, TRPV2, TRPV3, TRPV4 or TRPM8, all of which are expressed in sensory neurons and/or skin keratinocytes, and are thought to be involved in nociception or pain relief (Caterina et al., 1999, 2000; McKemy et al., 2002; Peier et al., 2002; Suzuki et al., 2003; Moqrich et al., 2005; Bautista et al., 2006; Kwan et al., 2006). Methyl paraben caused [Ca2+]i increase in HEK293 cells expressing TRPA1 (n=24), but not in cells expressing TRPV1, TRPV2, TRPV3, TRPV4 or TRPM8 (n=12–130, Figure 2b), suggesting a selective action of methyl paraben on TRPA1 channels. Next, we examined the effects of other parabens on TRPA1 channels. All the parabens examined including methyl paraben (n=24), ethyl paraben (n=52), propyl paraben (n=21) and butyl paraben (n=24) had an ability to increase [Ca2+]i in HEK293 cells expressing TRPA1 channels (Figure 2c). This result suggests that benzoate, a common structural feature of the parabens (Figure 1), might be involved in the actions of the parabens on TRPA1 channels. Skin, innervated by sensory neurons expressing TRPA1 channels, is exposed to a relatively constant temperature between 30 and 36°C. Therefore, we examined the effects of methyl paraben on TRPA1 channels at a more physiological temperature of 34°C. As shown in Figure 2d, methyl paraben caused increase in [Ca2+]i at this higher temperature, as it did at 25°C (n=19, Figure 2a), suggesting that methyl paraben could act on TRPA1 channels under physiological conditions.
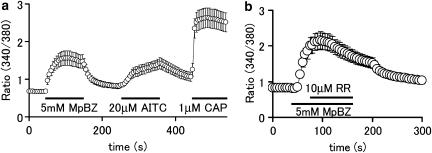
Similar responses to 5 mM methyl paraben were observed in DRG neurons, sensitive to allyl isothiocyanate (n=16, Figure 3a), suggesting that methyl paraben activates native TRPA1 channels. As expected, all the cells expressing TRPA1 channels, that is, those responding to 20 μM allyl isothiocyanate, also responded to capsaicin (1 μM), consistent with the notion that TRPA1-positive cells also express TRPV1 receptors (Story et al., 2003). Our finding that ruthenium red, an inhibitor of several TRP channels, did inhibit the methyl paraben-induced [Ca2+]i increase (48±3% inhibition, n=14) (Figure 3b) further illustrated the involvement of TRPA1 channels in the methyl paraben response.
Figure 3.
Methyl paraben increases cytosolic Ca2+ concentration ([Ca2+]i) in sensory neurons. (a) Methyl paraben (MpBZ) caused an increase in [Ca2+]i in mouse DRG neurons, which also responded to allyl isothiocyanate (AITC) and capsaicin (CAP) (n=16). (b) MpBZ-induced increases in [Ca2+]i were inhibited by ruthenium red (RR) in mouse DRG neurons (n=14).
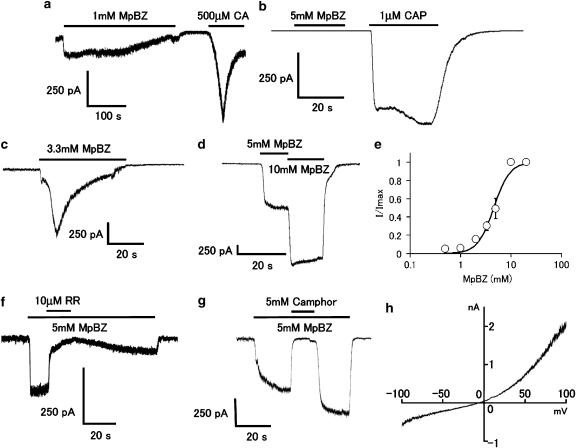
To examine more directly whether methyl paraben activates TRPA1 channels, we performed patch-clamp experiments in HEK293 cells expressing TRPA1 channels. Methyl paraben (1 mM) evoked inward currents at −60 mV (n=26). Cinnamaldehyde (500 μM), a known TRPA1 agonist, also evoked inward currents in cells responding to methyl paraben (n=4, Figure 4a). The specific action of methyl paraben on TRPA1 channels was further confirmed in the experiments where methyl paraben did not activate inward currents in vector-alone transfected HEK293 cells (n=5, data not shown) or HEK293 cells expressing TRPV1 but not TRPA1 channels (n=6, Figure 4b), consistent with the Ca2+-imaging data (Figure 2b). Methyl paraben-induced inward currents were often desensitized during its application in the presence of extracellular Ca2+ (Figures 4a and c), a property that has been previously reported (Nagata et al., 2005). In light of this, we decided to perform further characterization of the methyl paraben-induced TRPA1 currents in the absence of extracellular Ca2+. Methyl paraben-activated inward currents at −60 mV in the absence of extracellular Ca2+ exhibited a EC50 of 4.4 mM (Figures 4d and e). Ruthenium red (10 μM) caused almost complete inhibition of methyl paraben-induced TRPA1 currents (by 96±3%) with partial recovery (to 33±9% of the initial current, n=4) on washing (Figure 4f). Camphor (5 mM) also inhibited methyl paraben-induced TRPA1 currents (99±0.3%, n=4) with complete recovery upon washout of the compound (Figure 4g) as reported previously (Xu et al., 2005; Macpherson et al., 2006). Methyl paraben-induced TRPA1 currents exhibited an outward rectification in their I–V relationship as shown in Figure 4h. Furthermore, reversal potential of the I–V curve was −0.04±3.59 mV (n=3), indicating the involvement of a nonselective cation channel. These properties are identical to those reported for TRPA1 channel currents evoked by known stimuli including isothiocyanate and thiosulphinate compounds (Bandell et al., 2004; Jordt et al., 2004).
Figure 4.
Methyl paraben activates TRPA1 channels in HEK293 cells. (a) A representative whole-cell current trace activated by methyl paraben (MpBZ, 1 mM) or cinnamaldehyde (CA, 500 μM) in the presence of extracellular Ca2+. Vh=−60 mV. Horizontal bars indicate the duration of test compound application. (b) A representative current trace in response to MpBZ or capsaicin (CAP, 1 μM) in the presence of extracellular Ca2+. (c) A representative current trace activated by MpBZ showing desensitization in the presence of extracellular Ca2+. (d) A representative current trace activated by MpBZ in the absence of extracellular Ca2+. (e) A dose-dependent profile of currents through TRPA1 channels activated by MpBZ. EC50 value was 4.4 mM; Hill coefficient was 2.6. Membrane currents were normalized to the current activated by 10 mM MpBZ in each cell (n=6). (f) and (g) Representative MpBZ-activated current traces inhibited by ruthenium red (RR, 10 μM, f) or camphor (5 mM, g) in the absence of extracellular Ca2+. (h) A representative I–V curve of MpBZ-activated current showing an outward rectification.
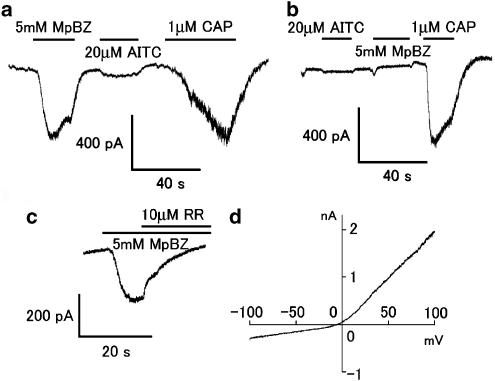
To confirm the ability of methyl paraben to activate TRPA1 channels in native sensory neurons, we performed patch-clamp experiments using isolated mouse DRG neurons. Methyl paraben evoked inward currents in some of the small DRG neurons (18.1±5.5 pF, n=24). The second responses were often quite small owing to desensitization because we performed patch-clamp experiments in the presence of extracellular Ca2+ in order to maintain giga-ohm seals. All the methyl paraben-sensitive cells also responded to both allyl isothiocyanate (20 μM) and capsaicin (1 μM, n=8) (Figure 5a), suggesting methyl paraben-sensitive cells express both TRPA1 channels and TRPV1 receptors. These findings are consistent with the notion that TRPV1-positive cells express TRPA1 channels (Story et al., 2003). Furthermore, all the methyl paraben-insensitive neurons did not respond to allyl isothiocyanate (n=7), although most did respond to capsaicin (86%, n=6, Figure 5b). Those cells are thought to express TRPV1 but not TRPA1 channels. These results strongly suggest that methyl paraben activates TRPA1 channels in native sensory neurons. The methyl paraben-evoked inward currents were almost completely inhibited by ruthenium red (10 μM, n=4; Figure 5c), and exhibited an outwardly rectifying I–V relationship (Figure 5d), consistent with previously reported properties of TRPA1 channel currents.
Figure 5.
Methyl paraben activates inward currents in mouse DRG neurons. (a) A representative whole-cell current trace activated by methyl paraben (MpBZ, 5 mM), allyl isothiocyanate (AITC, 20 μM) or capsaicin (CAP, 1 μM). Vh=−60 mV. Bars indicate duration of the compound application. (b) A representative current trace in response to AITC, MpBZ or CAP. (c) A representative MpBZ-activated current trace inhibited by ruthenium red (RR, 10 μM). (d) A representative I–V curve of MpBZ-activated currents showing an outward rectification. To maintain giga-ohm seals we performed patch-clamp experiments on DRG neurons in the presence of extracellular Ca2+ for all experiments, except those with RR.
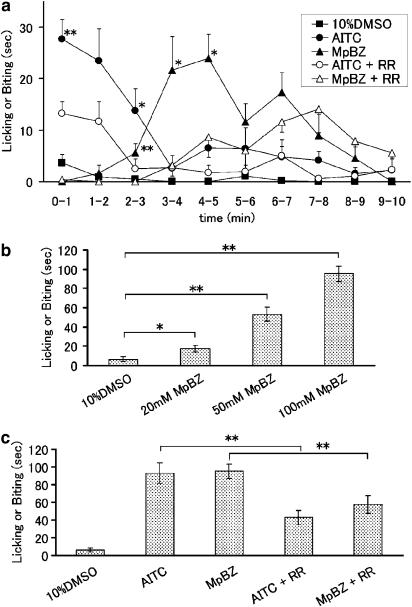
If methyl paraben could activate TRPA1 channels, it is reasonable to hypothesize that the compound could also cause pain-related behaviour when administered in vivo. To investigate this, we examined the effects of methyl paraben injected into the hind paw of mice. Injection of allyl isothiocyanate (AITC, 10 mM, 20 μl) caused instantaneous increases in licking or biting reactions in mice, characteristic of painful sensations (Figure 6a). These reactions were significantly reduced by the concomitant application of ruthenium red (100 μM, 20 μl) and never observed in mice injected with dimethyl sulphoxide (DMSO) alone (Figures 6a, b and c). Injection of methyl paraben (20 μl) also induced paw oedema, a phenomenon of neurogenic inflammation and increased licking or biting behavior in a dose-dependent manner which was significantly inhibited by ruthenium red, suggesting that methyl paraben caused a pain-related behaviour through the activation of TRPA1 channels. The pain-related behaviour associated with methyl paraben developed more slowly than that associated with allyl isothiocyanate (Figure 6a). However, total time spent licking or biting the injected paw with or without inhibition by ruthenium red was similar for allyl isothiocyanate (10 mM) or methyl paraben (100 mM) injection (Figure 6c).
Figure 6.
Pain-related behavior produced by methyl paraben injection into mouse hind paws. (a) Time course of licking an biting induced by methyl paraben (MpBZ, 100 μM) or allyl isothiocyanate (AITC, 10 μM) with or without ruthenium red (RR, 100 μM). The time spent licking and biting the injected paw was measured for 1 min durations up to 10 min after subcutaneous injection of the compounds into the hind paw. n=10 each. *, **P<0.05, P<0.01 vs control (10% DMSO used for dilution of MpBZ and AITC). (b) Dose dependency of MpBZ effects on pain-related behavior in mice. n=10 each *P<0.05, **P<0.01. (c) Effects of ruthenium red (RR) on time spent licking and biting for 10 min. n=10 each. **P<0.01 vs treatment with RR.
Discussion and conclusions
In the present study, we found that methyl paraben can activate TRPA1 channels in a dose-dependent manner in both HEK293 cells expressing TRPA1 channels and mouse sensory neurons, using both Ca2+-imaging and patch-clamp techniques. Properties of methyl paraben-evoked responses were identical to those of TRPA1 channels in response to its known stimuli, indicating that methyl paraben activates TRPA1 channels in the same way as the known stimuli for TRPA1 channels. As shown in the behavioural experiments, methyl paraben had the ability to evoke pain-related behaviour through activation of TRPA1 channels. Much attention should be paid to this finding as methyl paraben is commonly added in pharmaceutical, cosmetic and food products and as parabens have been reported to have an ability to cause stinging (Sone et al., 1990). It is expected that methyl paraben may cause pain sensations especially when applied to the mouth or skin, innervated by primary sensory afferent neurons expressing TRPA1 channels.
An EC50 value for methyl paraben to activate TRPA1 channels in patch-clamp experiments (4.4 mM) appears very high. The ability of methyl paraben to activate TRPA1 channels was selective for TRP channels known to be expressed in sensory neurons and/or skin keratinocytes, because methyl paraben failed to activate other ion channels such as TRPV1, TRPV2, TRPV3, TRPV4 or TRPM8. However, parabens were reported to modulate voltage-gated Ca2+ channels, voltage-gated K+ channels, acetylcholine-activated channels and ATP-activated channels in rat pheochromocytoma PC12 cells (Inoue et al., 1994). Furthermore, the usual concentration range of parabens is between 0.1 and 0.8% (Sasseville, 2004), corresponding to 6.6–52.6 mM. Thus 4.4 mM is an attainable concentration in the daily use of paraben-containing products.
Temporal development of the pain-related behaviour induced by methyl paraben was slightly different from that caused by allyl isothiocyanate (Figure 6), although electrophysiological properties of TRPA1 channels, activated by methyl paraben and allyl isothiocyanate, were indistinguishable. We previously reported that development of the pain-related behaviour was delayed and prolonged upon injection of the nonpungent capsaicin-like compound, capsiate, probably owing to its lipophilic nature (Iida et al., 2003). However, low and similar log P-values (the partition coefficient by octanol–water partitioning system) (Iida et al., 2003) of methyl paraben (2.00) (Marsh et al., 2005) and allyl isothiocyanate (2.11) (IPCS, 2005) make this unlikely. Other chemical properties of methyl paraben may be responsible for the delay of the development of pain-related behaviour. Reasons underlying difference in the temporal development of pain-related behaviour caused by methyl paraben warrant further investigation.
Activation of TRPA1 channels appears to be a common action of the known antibacterial agents such as allyl isothiocyanate, cinnamaldehyde, allicin and methyl paraben, as demonstrated in this study. Outward currents involving K+ channel activation or [Ca2+]i increase owing to Ca2+ release from intracellular Ca2+ stores, both previously reported actions of parabens (Cavagna et al., 2000; Bredin et al., 2005; Nguyen et al., 2005) were not observed in our system, upon the application of methyl paraben. The absence of these effects in our study suggests that the targets of parabens may vary depending upon cell type. It is still not clear whether activation of TRPA1 channel-like proteins is related to the antibacterial actions of parabens. Further understanding of the antibacterial action of parabens is likely to be provided by cloning the targets of these agents in bacteria, one of which might prove to be a TRPA1 channel-like molecule. In any case, methyl paraben – a TRPA1 channel agonist – will prove a useful tool for the analyses of TRPA1 channel function and the development of antinociceptive agents acting upon TRPA1 channels.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan, and AstraZeneca Foundation to MT.
Abbreviations
- DMSO
dimethyl sulphoxide
- DRG
dorsal root ganglion
- HEK
human embryonic kidney
- I–V
current–voltage
- methyl paraben
methyl p-hydroxybenzoate
- MscL
large prokaryotic mechanosensitive channel
- propyl paraben
propyl p-hydroxybenzoate
- TRP
transient receptor potential
Conflict of interest
The authors state no conflict of interest.
References
- Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredin J, Davin-Regli A, Pages JM. Propyl paraben induces potassium efflux in Escherichia coli. J Antimicrob Chemother. 2005;55:1013–1015. doi: 10.1093/jac/dki110. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavagna D, Zorzato F, Babini E, Prestipino G, Treves S. Methyl p-hydroxybenzoate (E-218) a preservative for drugs and food is an activator of the ryanodine receptor Ca(2+) release channel. Br J Pharmacol. 2000;131:335–341. doi: 10.1038/sj.bjp.0703571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ST, Chen PF, Chang SC. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J Ethnopharmacol. 2001;77:123–127. doi: 10.1016/s0378-8741(01)00273-2. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Freese E, Sheu CW, Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973;241:321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, et al. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology. 2003;44:958–967. doi: 10.1016/s0028-3908(03)00100-x. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakazawa K, Fujimori K, Takanaka A. Modulation by alkyl p-hydroxybenzoates of voltage- and ligand-gated channels in peripheral neuronal cells. Neuropharmacology. 1994;33:891–896. doi: 10.1016/0028-3908(94)90187-2. [DOI] [PubMed] [Google Scholar]
- IPCS International Prigramme on Chemical Safety. 2005.
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lin CM, Preston JF, III, Wei CI. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–734. doi: 10.4315/0362-028x-63.6.727. [DOI] [PubMed] [Google Scholar]
- Ma Y, Marquis RE. Irreversible paraben inhibition of glycolysis by Streptococcus mutans GS-5. Lett Appl Microbiol. 1996;23:329–333. doi: 10.1111/j.1472-765x.1996.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Marsh A, Clark BJ, Altria KD. Oil-in-water microemulsion LC determination of pharmaceuticals using gradient elution. Chromatographia. 2005;61:539–547. [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP Superfamily of Cation Channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Morozumi S. Isolation, purification, and antibiotic activity of o-methoxycinnamaldehyde from cinnamon. Appl Environ Microbiol. 1978;36:577–583. doi: 10.1128/aem.36.4.577-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. The constitution of capsaicin, the pungent principal of capsicum. J Am Chem. 1919;41:1115–1151. [Google Scholar]
- Nes IF, Eklund T. The effect of parabens on DNA, RNA and protein synthesis in Escherichia coli and Bacillus subtilis. J Appl Bacteriol. 1983;54:237–242. doi: 10.1111/j.1365-2672.1983.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Clare B, Guo W, Martinac B. The effects of parabens on the mechanosensitive channels of E. coli. Eur Biophys J. 2005;34:389–395. doi: 10.1007/s00249-005-0468-x. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251–263. doi: 10.1111/j.1396-0296.2004.04028.x. [DOI] [PubMed] [Google Scholar]
- Sone T, Yamada H, Endo H. Pharmacological studies of stinging caused by parabens. J Jpn Cosmet Sci Soci. 1990;14:8–16. [Google Scholar]
- Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40:1335–1373. doi: 10.1016/s0278-6915(02)00107-2. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]