Abstract
Background and Purpose:
Protective cardiovascular effects of peroxisome proliferator activated receptor (PPAR)α and PPARγ activators have been demonstrated. If used as vasoprotective agents in high risk vascular patients rather than for their metabolic benefits, these agents could be associated with unwanted side effects. As a proof of concept to support the use of combined low doses of PPARα and PPARγ as vascular protective agents in high risk vascular patients, we tested the hypothesis that combined low doses of PPARα (fenofibrate) and PPARγ (rosiglitazone) activators would provide vascular protective benefits similar to full individual doses of these PPAR agonists.
Experimental Approach:
Male Sprague-Dawley rats infused with Ang II (120 ng kg−1 min−1) were treated with rosiglitazone (1 or 2 mg kg−1 day−1) alone or concomitantly with fenofibrate (30 mg kg−1 day−1) for 7 days. Thereafter, vessels was assessed on a pressurized myograph, while NAD(P)H oxidase activity was determined by lucigenin chemiluminescence. Inflammation was evaluated using ELISA for NFκB and Western blotting for adhesion molecules.
Key Results:
Ang II-induced blood pressure increase, impaired acetylcholine-induced vasorelaxation, altered vascular structure, and enhanced vascular NAD(P)H oxidase activity and inflammation were significantly reduced by low dose rosiglitazone+fenofibrate.
Conclusions and Implications:
Combined low doses of PPARα and PPARγ activators attenuated development of hypertension, corrected vascular structural abnormalities, improved endothelial function, oxidative stress, and vascular inflammation. These agents used in low-dose combination have synergistic vascular protective effects. The clinical effects of combined low-dose PPARα and PPARγ activators as vascular protective therapy, potentially with reduced side-effects and drug interactions, should be assessed.
Keywords: renin–angiotensin system, reactive oxygen species, inflammation, endothelial function, thiazolidinediones, fibrates
Introduction
Hypertension is a major risk factor in the development of atherosclerosis and cardiovascular disease and frequently occurs together with disorders of carbohydrate and lipid metabolism as part of the metabolic syndrome (Schiffrin, 2004). The renin–angiotensin system is recognized as an important contributor to vascular remodeling, endothelial dysfunction and insulin resistance, by inducing vascular growth and apoptosis, low-grade inflammation, and increased oxidative stress (Intengan and Schiffrin, 2001; Schiffrin and Touyz, 2003). Furthermore, angiotensin (Ang) II increases redox-sensitive and proinflammatory mediators such as nuclear factor kappa beta (NFκB) and vascular cell adhesion molecule-1 (VCAM-1), which play critical roles in the progression of atherosclerosis (Pueyo et al., 2000).
Peroxisome proliferator activated receptors (PPAR) are a family of ligand-activated transcription factors that regulate gene expression by heterodimerizing with retinoid X receptors and binding to PPAR-response elements (Willson et al., 2000). Three PPAR isoforms, encoded by separate genes, have been demonstrated. PPARα is predominantly expressed in tissues exhibiting high-fatty acid catabolism such as liver, heart, kidney and skeletal muscle, whereas PPARγ is most abundantly expressed in adipose tissue, large intestine and cells of the monocyte lineage. PPARγ is implicated in adipocyte differentiation, cellular energy homeostasis and regulation of genes that affect insulin activity (Willson et al., 2000; Schiffrin et al., 2003) whereas PPARβ/δ is expressed ubiquitously and is involved in fatty acid oxidation (Gilde et al., 2003). PPARα is activated by natural ligands (fatty acids and eicosanoids) and synthetic ligands (fibrates such as fenofibrate). PPARγ can be activated by the insulin-sensitizing thiazolidinediones (TZD or glitazones) such as rosiglitazone and pioglitazone (Forman et al., 1997; Schiffrin et al., 2003).
We and others have shown that both PPARα and PPARγ activators have therapeutic effects, which extend beyond their lipid and metabolic properties, such as attenuation of atherosclerosis and vascular remodeling, inflammation and reactive oxygen species (ROS) generation (Diep et al., 2002b, 2004; Plutzky, 2003). Based on these and other findings, dual PPARα and PPARγ activators have been developed (Chakrabarti et al., 2003; Guo et al., 2004). However, both PPARα and PPARγ activators individually have side effects (Francis et al., 2003; Hsueh and Bruemmer, 2004) and attention has been called recently to undesirable effects of some dual PPARα and PPARγ activators (Nissen et al., 2005). Glitazones generate fluid retention and edema and may trigger heart failure. Using half or less of the therapeutic dose of a glitazone may avoid or reduce this side effect, and as well potentially avoid hypoglycemia in non-diabetic patients who do not need glucose control but require cardiovascular protection. Dual PPAR activators in development, on the other hand, may have too much PPARα activity, which might be carcinogenic. Many patients requiring cardiovascular protection, because they are at high-risk, need a statin. However fibrates (PPARα activators) interact with statins, which may lead to the myopathy and rarely to myoglobinuria, potentially fatal.
We hypothesized that lower doses of PPARα and PPARγ activators administered together would result in beneficial cardiovascular effects. We used the experimental hypertension and vascular damage paradigm of the Ang II-infused rat to test whether combined low-dose administration of the PPARα and PPARγ activators, fenofibrate and rosiglitazone, respectively, would prevent development of hypertension, vascular remodeling, endothelial dysfunction and vascular oxidative stress/inflammation of resistance arteries as we previously found with full doses of either PPARα (Diep et al., 2002a) or PPARγ agonists (Diep et al., 2002b). Translating this strategy to the clinic may have potentially important therapeutic significance as high-risk vascular patients who do not necessarily need a fibrate for triglyceride lowering and/or a glitazone for metabolic control of type II diabetes may benefit from the vascular protective actions of low-dose combined PPARα and PPARγ activators, at the same time potentially avoiding or mitigating unwanted side effects and drug interactions.
Methods
Animal experiments
The study was approved by the Animal Care Committee of the Clinical Research Institute of Montreal and performed according to guidelines of the Canadian Council for Animal Care. Under anesthesia with 5% isoflurane, radio-telemetry transmitters (TA11PA-C40; Data Sciences International, St Paul, MN, USA) were surgically implanted in male Sprague–Dawley rats (200–225 g) and systolic blood pressure (BP), diastolic BP, mean BP and heart rate were measured, as described previously (Touyz et al., 2002). One week thereafter (which represents day 0), six groups of 6–8 animals, fed standard Purina rat chow (0.28% sodium content, Ralston Purina Co., Richmond, IN, USA) were housed under 12-h day/night cycle under constant room temperature of 22°C and studied for 7 days: the groups comprised controls, Ang II (120 ng kg−1 min−1 s.c.), Ang II+fenofibrate (30 mg kg−1day−1 p.o, Ang+Feno), Ang II+rosiglitazone (2 mg kg−1day−1 p.o, Ang+Rosi-2), Ang II+rosiglitazone+fenofibrate (Ang+Rosi-2+Feno), and rosiglitazone+fenofibrate (Rosi-2+Feno). Fenofibrate and rosiglitazone were administered, respectively, at one third and half of the doses used in our previous studies (Diep et al., 2002b; Iglarz et al., 2003). In a parallel set of experiments, a lower dose of rosiglitazone (1 mg kg−1 day−1 p.o, Rosi-1) was given alone or in combination with fenofibrate (Rosi-1+Feno) in Ang II- or vehicle-infused rats. Ile5-Ang II (Peninsula, Palo Alto, CA, USA) was infused s.c. with Alzet osmotic minipumps (Alza Corp., Palo Alto, CA, USA), whereas rosiglitazone (GlaxoSmithKline, Montreal, QC, Canada) and fenofibrate (Sigma Chemicals, Mississauga, ON, Canada) were given in food. At the end of the experiment, animals (not fasted overnight) were killed by decapitation.
Measurement of plasma lipids and glucose
Plasma was assayed for total (free and esterified) cholesterol and triglycerides with a COBAS MIRA-S automated analyzer with enzymatic reagents (Roche, Basel, Switzerland) and for glucose with OneTouch Ultra (LifeScan, Milpitas, CA, USA). Glucose was measured in plasma, which had been snap-frozen and previously stored at −80°C.
Preparation and study of mesenteric arteries
Third-order branches of mesenteric artery (∼2 mm in length with internal diameter of 150–250 μm) were dissected and mounted on a pressurized myograph as described previously (Diep et al., 2002b). Briefly, vessels were equilibrated (60 min, 45 mm Hg of intraluminal pressure) in warmed oxygenated (95% air–5% CO2) physiological salt solution (PSS, pH 7.4) containing (mM): sodium chloride (NaCl) 120, sodium hydrogen carbonate 25, potassium chloride (KCl) 4.7, potassium dihydrogen phosphate 1.18, magnesium sulfate 1.18, calcium chloride 2.5, ethylenediaminetetraacetic acid 0.026, and glucose 5.5. Vessels were considered viable when they constricted >60% of their resting lumen diameter in response to extraluminal application of 125 mM KCl +10−5 M noradrenaline (Sigma Chemicals). Endothelium-dependent relaxation was assessed by measuring the dilatory response to acetylcholine (ACh, 10−10–10−4 M) in noradrenaline precontracted vessels (10−5 M). The role of oxidative stress was assessed by dose–response curve to ACh after incubation with vitamin C (10−4 M) and/or the nitric oxide synthase (NOS) inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME, 10−4 M). Endothelium-independent relaxation was assessed with sodium nitroprusside (SNP, 10−9–10−3 M) in precontracted vessels. Concentration–response curves to noradrenaline (10−8–10−4 M) and Ang II (10−9–10−6 M) were performed to evaluate vascular contractility.
Vessels were then deactivated by perfusion with Ca2+-free PSS containing 10 mM ethylene glycol tetraacetic acid (EGTA) for 30 min and vascular morphology was evaluated by measuring lumen and media thickness at 45 mm Hg as described previously (Intengan and Schiffrin, 2001). Media cross-sectional area (MCSA) was evaluated as described previously (Iglarz et al., 2003).
Immunohistochemistry
Immunohistochemistry was performed on sections of aorta as described previously (Amiri et al., 2004). Briefly, paraffin-embedded 5 μm sections were rehydrated and endogenous peroxidase activity was quenched by incubation in phosphate-buffered saline containing 3% hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ, USA) and were stained with hematoxylin–eosin. Media and lumen dimensions were quantified by imaging (Northern Eclipse program, EMPIX Imaging Inc, Mississauga, ON, Canada).
Vascular NAD(P)H oxidase activity
Nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase activity was assessed in mesenteric arteries using lucigenin enhanced chemiluminescence as described previously (Virdis et al., 2004).
NFκB activation assay
Proteins were extracted from frozen tissue as described above (Amiri et al., 2004). Thereafter, NFκB activation was quantified by using TransAM kit (Active Motif, Carlsbad, CA, USA) to measure activated p50 subunit of nuclear factor-κB (NFκB) as described previously (De Ciuceis et al., 2005).
Western blotting
Proteins were extracted from previously frozen tissue as already described (De Ciuceis et al., 2005). Thereafter, samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and incubated with specific antibody to VCAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Signals were revealed with chemiluminescence, visualized autoradiographically and subsequently membranes were stripped (Pierce Biotechnology, Rockford, IL, USA) and re-probed with β-actin (Sigma Chemicals) to verify equal loading. Optical density of bands was quantified by AlphaEase (Alpha Innotech Corporation, San Leandro, CA, USA), and expressed as arbitrary units of VCAM-1/β-actin ratio.
Data analysis
Results are means±s.e.m., with n indicating the number of animals. Comparisons between groups for physiological parameters, day 7 of BP data, vascular morphological characteristics, NAD(P)H oxidase activity and vascular inflammation (NFκB activation and VCAM-1 expression) were analyzed by one-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test. Dose–response curves to ACh were analyzed by one-way repeated-measures ANOVA followed by Newman-Keuls post hoc test and role of oxidative stress data (obtained with L-NAME alone or in combination with vitamin C) was assessed by two-way ANOVA followed by Newman–Keuls test. P<0.05 was considered as statistically significant.
Results
Physiological characteristics
Mean BP was significantly increased (P<0.001) by 7 days of Ang II infusion, compared with controls (Figure 1). This increase in mean BP was unaffected by fenofibrate or rosiglitazone (1 or 2 mg kg−1 day−1) alone but was slightly lowered, albeit not significantly, when fenofibrate and rosiglitazone were administered concomitantly.
Figure 1.
Radio-telemetry analysis of mean BP over 7 days. Values are means±s.e.m., n=6 animals per group. Mean BP shown in (a) are from rats treated with saline (Ctrl), Ang II, fenofibrate (Feno) and different combinations of 2 mg kg−1 day−1 of rosiglitazone (Rosi-2). Mean BP shown in (b) are from rats treated with saline (Ctrl), Ang II, Feno and different combinations of 1 mg kg−1 day−1 of rosiglitazone (Rosi-1). *P<0.05 Ang II vs CTRL, Rosi-2+Feno and Ang+ Rosi-2+ Feno on day 7; †P<0.05 Ang II vs. CTRL and Rosi-1+Feno on day 7 (one-way ANOVA followed by Newman–Keuls test).
No differences in body weight were observed among groups (Table 1). Ang II induced cardiac hypertrophy (assessed by heart-to-body weight ratio, Table 1), which was prevented only when the 2 mg kg−1 day−1 dose of rosiglitazone was coadministered with the PPARα activator, fenofibrate, and not by the lower dose of rosiglitazone (1 mg kg−1 day−1) alone (Table 1). There were no differences in plasma cholesterol and triglycerides between control and Ang II-infused rats (Table 1). Plasma cholesterol was significantly reduced in all groups receiving fenofibrate (P<0.01). Plasma triglycerides were significantly decreased by all doses of fenofibrate and rosiglitazone (Table 1). Plasma glucose did not change significantly in any group (Table 1).
Table 1.
Physiological parameters and plasma cholesterol and triacylgylcerol of all experimental groups
| Parameter | CTRL | Ang II | Ang II +Rosi-2 | Ang II +Feno | Ang II +Rosi-2 +Feno | Rosi-2 +Feno | Ang II +Rosi-1 | Ang II +Rosi-1 +Feno | Rosi-1 +Feno |
|---|---|---|---|---|---|---|---|---|---|
| BW (g) | 251±5 | 243±8 | 228±3 | 236±6 | 231±5 | 225±10 | 232±6 | 233±5 | 239±8 |
| HW (mg) | 887±36 | 992±43 | 926±27 | 924±10 | 987±34 | 846±27 | 936±35 | 946±34 | 872±25 |
| RHW (mg g−1) (× 100) | 353±9 | 409±13a | 406±11a | 392±8a | 380±10 | 378±11 | 404±12a | 406±11a | 367±10 |
| Plasma cholesterol (M) | 1.6±0.1 | 1.6±0.1 | 1.7±0.1 | 1.2±0.1b | 1.4±0.1b | 1.2±0.1b | 1.5±0.1 | 1.3±0.1b | 1.1±0.1b |
| Plasma triglycerides (M) | 1.6±0.2 | 1.4±0.1 | 0.7±0.1c | 0.9±0.1c | 0.5±0.1c | 0.6±0.1c | 0.9±0.1c | 0.7±0.1c | 0.6±0.1c |
| Plasma glucose (M*) | 12.1±0.4 | 11.8±0.6 | 10.8±0.5 | 12.4±0.6 | 13.6±0.8 | 12.6±1.0 | 11.3±0.7 | 13.3±0.8 | 12.1±0.6 |
Abbreviations: BW, body weight; CTRL, control; Feno, fenofibrate; HW: heart Weight; RHW, relative HW.
Rosi-2 and Rosi-1, rosiglitazone 2 mg kg−1·day−1 and 1 mg kg−1 day−1, respectively.
Values are means±s.e.m.
Plasma glucose was measured in samples that had been snap-frozen and stored at −80°C.
P<0.05 vs CTRL, Rosi-2+Feno, Rosi-1+Feno.
P<0.01 vs Ang+Rosi-2, Ang II, CTR.
P<0.01 vs CTRL, Ang II; one-way ANOVA followed by Newman–Keuls test.
Arterial structure
Ang II-induced increase in the MCSA of aorta was not affected by PPARα and PPARγ activators administered alone or in combination (Table 2). Ang II caused a significant increase in the media-to-lumen (M/L) ratio of mesenteric resistance arteries compared with control rats. The combination of fenofibrate and rosiglitazone (1 or 2 mg kg−1 day−1) reduced the M/L ratio of resistance arteries whereas no effects were observed when either drug was given individually (Table 2).
Table 2.
Morphological characteristics of aorta and mesenteric resistance arteries
| Parameter | CTRL | Ang II | Ang II +Rosi-2 | Ang II +Feno | Ang II+Rosi-2 +Feno | Rosi-2 +Feno | Ang II +Rosi-1 | Ang II+Rosi-1 +Feno | Rosi-1 +Feno |
|---|---|---|---|---|---|---|---|---|---|
| Aorta (histology) | |||||||||
| Lumen, (μm) (× 102) | 12.4±0.3 | 13.0±0.2 | 13.0±0.2 | 13.8±0.3 | 14.5±0.7 | 13.4±0.4 | 11.8±0.3 | 13.7±0.7 | 13.9±0.4 |
| Media, (μm) | 97.7±3.6 | 127.5±10.3a | 120.6±5.9† | 133.1±6.2† | 125.3±7.4 | 104.0±0.9 | 125.0±3.8† | 117.3±5.6 | 95.8±3.2 |
| M/L, % | 7.9±0.3 | 9.8±0.7a | 9.3±0.5a | 9.6±0.4a | 8.6±0.2 | 7.8±0.3 | 10.5±0.2b | 8.9±0.1 | 6.9±0.1 |
| MCSA, (μm2) (× 104) | 46.2±1.3 | 56.8±4.4b | 53.1±1.1b | 57.6±1.5b | 53.1±1.0b | 47.8±1.6 | 52.9±1.2b | 52.9±1.2b | 47.0±1.8 |
| Mesenteric arteries (myograph) | |||||||||
| Lumen (μm) | 234±18 | 205±13 | 254±13 | 245±24 | 250±19 | 267±15 | 236±9 | 252±13 | 249±19 |
| Media, (μm) | 19.9±0.5 | 22.5±0.8 | 23.5±1.3 | 20.5±2.3 | 24.8±1.6 | 21.0±2.2 | 22.0±1.1 | 22.7±1.0 | 17.2±2.7 |
| M/L (%) | 8.1±0.9 | 10.7±0.5a | 8.6±0.7 | 7.9±0.6 | 9.2±1.3 | 7.2±0.7 | 9.3±0.4 | 9.2±0.6 | 7.1±1.1 |
| MCSA, (μm2) (× 103) | 17.6±1.2 | 16.9±1.3 | 20.3±1.4 | 19.1±4.0 | 21.1±1.1 | 19.3±2.6 | 18.0±1.3 | 19.6±1.1 | 14.1±2.0 |
Abbreviations: CTRL, control; Feno, fenofibrate; MCSA: media cross-sectional area; M/L, media-to-lumen ratio.
Rosi-2 and Rosi-1, rosiglitazone 2 mg kg−1day−1 and 1 mg kg−1 day−1, respectively.
Values are means±s.e.m.
P<0.05 vs CTRL, Rosi-2+Feno and Rosi-1+Feno.
P<0.05 vs CTRL; two-way ANOVA followed by Newman–Keuls test.
Function of resistance arteries
Vasodilatation to ACh was significantly impaired (P<0.01) in rats receiving Ang II compared with control rats (Figure 2). Although 1 mg kg−1 day−1 rosiglitazone treatment alone improved but did not completely prevent endothelial dysfunction, the latter was completely prevented with the 2 mg kg−1 day−1 dose of rosiglitazone (Figure 2). Administration of both PPARα and PPARγ activators together prevented Ang II-induced endothelial dysfunction. Endothelium-independent relaxation responses to sodium nitroprusside (SNP) were similar in all groups (data not shown).
Figure 2.
Endothelial function in mesenteric resistance arteries. Concentration–response curves to acetylcholine (ACh). Relaxation responses are % increase in lumen after noradrenaline precontraction. Values are means±s.e.m., n=5–6 animals/group. Concentration response curves shown in (a) are from rats treated with saline, Ang II, fenofibrate (Feno) and different combinations of 2 mg kg−1 day−1 of rosiglitazone (Rosi-2). Concentration response curves shown in (b) are from rats treated with saline, Ang II, fenofibrate (Feno) and different combinations of 1 mg kg−1 day−1 of rosiglitazone (Rosi-1). *P<0.05 vs Ang+Feno, Ang +Rosi-1, Rosi-2+Feno and Rosi-1+Feno; †P<0.01 vs CTRL (one-way repeated-measures ANOVA followed by Newman–Keuls test).
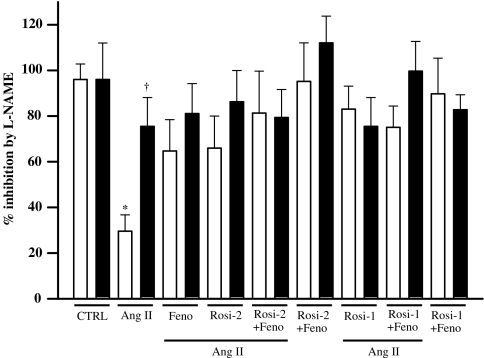
In Ang II-infused rats, the inhibitory effect exerted by the NOS inhibitor L-NAME on ACh (maximal ACh dose, 72±17% relaxation; with L-NAME, inhibition of 41±18%, with 30±7% remaining relaxation) was significantly lower than that in all other groups (ACh, 110±4% relaxation; with L-NAME, 96±7% inhibition, 14±4% remaining relaxation, Figures 2 and 3), which indicates the presence of more available NO in all other groups than in Ang II-infused rats. L-NAME inhibition of ACh-induced relaxation was exaggerated (more NO) by individual PPARα and PPARγ activation, and more prominently (albeit not significantly) when both agents were administered in combination (Figure 3), indicating that these agents increased NO bioavailability. Vitamin C administration significantly improved the response to ACh in Ang II-infused rats (by 65±12%, P<0.01) and restored the inhibitory effect of L-NAME (from 30±7 to 76±13% inhibition; 11±3% remaining relaxation, Figure 3). As observed with L-NAME, the effect of vitamin C on ACh-induced relaxation was improved by PPARα and PPARγ activation whether alone or in combination (data not shown).
Figure 3.
Role of oxidative stress in ACh-induced relaxation. Inhibition exerted by L-NAME on maximal relaxing response of mesenteric resistance arteries to ACh in the absence (saline, white bars) and in the presence of vitamin C (black bars). Values are means±s.e.m., n=6–7 animals per group. *P<0.05 vs saline of all other groups; †P<0.05 vs saline Ang II (two-way ANOVA followed by Newman–Keuls test).
Vascular NAD(P)H oxidase activity
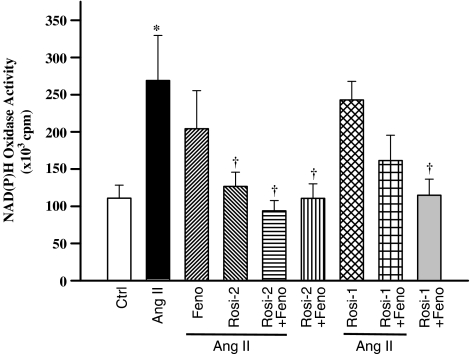
NAD(P)H oxidase activity in mesenteric arteries was significantly increased (P<0.01) in Ang II-infused compared with control animals (Figure 4). This increase was prevented by either 2 mg kg−1 day−1 rosiglitazone administered alone or combined with fenofibrate, but was unaffected by rosiglitazone 1 mg kg−1 day−1 administered alone or in combination with fenofibrate (Figure 4).
Figure 4.
NAD(P)H oxidase activity in mesenteric resistance arteries of all groups. Values are means±s.e.m., n=6–8 animals per group. *P<0.001 vs controls; †P<0.05 vs Ang II (one-way ANOVA followed by Newman–Keuls test).
Vascular inflammation
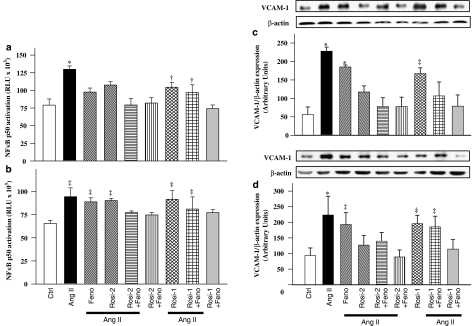
Ang II-induced activation of NFκB in mesenteric arteries and aorta compared with control rats (Figure 5a and b). Vascular NFκB activation was unaffected by fenofibrate. NFκB activation in Ang II-infused animals was prevented by rosiglitazone 2 mg kg−1 day−1 administered alone or combined with fenofibrate, but was unaffected when rosiglitazone 1 mg kg−1 day−1 was given alone or combined with fenofibrate (Figure 5a and b).
Figure 5.
NFκB activity (a and b) and VCAM-1 expression (c and d) in mesenteric arteries and aorta, respectively, of all groups. Top panels are representative blots, whereasile bottom panels are values represented as means±s.e.m., n=6–8 animals per group. *P<0.01 vs controls, Rosi-1+Feno and Rosi-2+Feno; †P<0.05 vs. Ang II; ‡P<0.05 vs controls (one-way ANOVA followed by Newman–Keuls test).
VCAM-1, a downstream target of NFκB, was significantly increased in the vasculature following Ang II infusion (Figure 5c and d). Increased expression of VCAM-1 in Ang II-infused rats was prevented by rosiglitazone 2 mg kg−1 day−1 administered alone or with fenofibrate but was unaffected by rosiglitazone 1 mg kg−1 day−1 alone or with fenofibrate (Figure 5c and d).
Discussion and conclusions
PPARα and PPARγ activators may possess pleiotropic vascular beneficial effects unrelated to insulin sensitization or lipid lowering. With the recent clinical interest in dual PPARαγ activators (Mulvany, 2002; Chakrabarti et al., 2003; Nissen et al., 2005), the importance of these potent agents in cardiovascular conditions such as hypertension requires further clarification. In the present study, we tested whether low doses of PPARα and PPARγ activators, when administered together, would prevent development of hypertension, vascular remodeling, endothelial dysfunction and vascular oxidative stress/inflammation of resistance arteries in Ang II-infused rats as we have previously found with full doses of either PPARα (Diep et al., 2002a) or PPARγ agonists (Diep et al., 2002b) given individually.
Our in vivo study demonstrates for the first time that combined administration of low doses of PPARα and PPARγ activators was as, or even more, beneficial than individual administration of PPARα or PPARγ activators on the combined endpoint of vascular remodeling, oxidative stress and vascular inflammation induced by Ang II. We opted to use lower doses of PPARα or PPARγ activators than in our previously published studies (Diep et al 2002a, 2002b) because of their potential for additive or even synergistic pleiotropic effects when used in combination. The beneficial effects of dual PPARα and PPARγ activation were obtained with a small but significant reduction of Ang II-induced BP increase. Furthermore, the PPARγ activator rosiglitazone at the 2 mg kg−1 day−1 dose combined with the PPARα activator fenofibrate (30 mg kg−1 day−1), prevented all of the Ang II-induced vascular effects, whereas an even lower dose of rosiglitazone (1 mg kg−1 day−1) combined with fenofibrate was only partially effective, affecting only endothelial function and mesenteric artery VCAM-1 expression.
Vascular changes in hypertension are associated with humoral and mechanical factors that modulate signaling events, resulting in abnormal function, media growth, extracellular matrix deposition and inflammation (Intengan and Schiffrin, 2001; Mulvany, 2002). Ang II-mediated effects on the vasculature are well established and include impaired endothelial function, increased generation of ROS through the activation of NAD(P)H oxidase, and induction of proinflammatory molecules (Schiffrin and Touyz, 2003). Our findings extend our previous results showing beneficial effects of PPARα and PPARγ activators administered alone on the deleterious effects of Ang II on vascular tissue, including improvement of impaired endothelial function, reduced ROS generation and inflammation (Diep et al 2002a, 2002b), by demonstrating an additive or even synergistic effect of combined low doses of PPARα and PPARγ activators.
As expected, both PPAR activators had beneficial effects on plasma cholesterol and triglyceride levels, which were unaltered by Ang II infusion as, documented previously (Diep et al., 2002a, b). On the other hand, no effects on glucose control could be demonstrated in this study in non-diabetic rats.
Ang II induced vascular hypertrophy of both aorta and mesenteric resistance arteries as demonstrated by increased M/L ratio and/or MCSA (Virdis et al., 2002). Both PPARα and PPARγ agonists inhibit Ang II-stimulated vascular growth, as suggested by in vitro studies (Kintscher et al., 2002; Benkirane et al., 2006a). At reduced doses, PPARα and PPARγ activators completely prevented vascular remodeling of mesenteric resistance vessels only when administered in combination. The effect on aorta was only partial, suggesting heterogeneous effects of PPAR activators on these vessels with their different structure and function. Differential regulation of these vascular beds is in agreement with our previous results with rosiglitazone on vascular Ang II signaling (Benkirane et al., 2006b).
Ang II-treated rats exhibited impaired endothelial function in mesenteric resistance vessels as demonstrated by reduced relaxation to ACh but normal response to SNP (Diep et al., 2002b). Correction of endothelial dysfunction by rosiglitazone alone or in combination with fenofibrate, suggests that even at low doses, PPARα and PPARγ activators have additive effects that allow them to prevent hypertension-induced endothelial dysfunction and supports the use of these agents in combination (Diep et al., 2002a, 2002b; Nissen et al., 2005). NO-induced relaxation was impaired in Ang II-infused rats and the inhibitory relaxation response to L-NAME was restored when vessels were co-incubated with vitamin C suggesting that reduced NO bioavailability was possibly owing to increased oxidative stress (Kintscher et al., 2002; Virdis et al., 2003). All combinations and doses of PPARα and PPARγ agonists were able to prevent the impairment of vascular relaxation induced by Ang II (Halabi and Sigmund, 2005; Schiffrin, 2005). As both PPARα and PPARγ activators elicited slight effects on the lipid profile, this could contribute in a minor way to the improvement of endothelial function. However, treatment with the combination of both PPARα and PPARγ agonists resulted in greater beneficial effect on vascular damage than individual administration, despite similar lipid-lowering effects.
Vascular NAD(P)H oxidase activity was enhanced following Ang II infusion and this increase was prevented only in animals treated with the higher dose of rosiglitazone alone or in combination with fenofibrate while the lower dose of rosiglitazone was ineffective, in agreement with our previous results with higher doses of these agents given alone (Diep et al., 2002a, b). These findings extend those of Dobrian et al. who demonstrated that administration of glitazones attenuates the activation and expression of NAD(P)H oxidase subunits (Dobrian et al., 2004).
In addition to their metabolic effects, both PPARα and PPARγ activators have anti-inflammatory actions on the vasculature (Diep et al., 2002a, b). We investigated whether combined low-dose PPARα/γ activation would have inhibitory effects on Ang II-induced vascular inflammation. Indeed, Ang II-activated vascular NFκB and its downstream regulated adhesion molecule, VCAM-1, were attenuated more effectively by the combination of fenofibrate with rosiglitazone at the higher dose of rosiglitazone (2 mg kg−1 day−1) than the lower dose (1 mg kg−1 day−1). The anti-inflammatory effects of PPARγ have been attributed in part to its ability to negatively modulate inflammatory cytokine expression by interacting with a receptor for the CCAAT/enhancer-binding protein (C/EBP)-δ, which is present in tandem repeats in the PPARγ gene promoter and is responsible for transcription of inflammatory cytokines (Takata et al., 2002). As for PPARα, it interferes with the inflammatory response by antagonizing NFκB signaling by several mechanisms, such as the formation of inactive p65 complexes and induction of IκBα, a major inhibitor of NFκB signaling (Poynter and Daynes, 1998; Delerive et al., 1999). As with vascular remodeling, we found differential effects of PPARα and PPARγ activators on aorta and mesenteric resistance arteries. Although PPARα activation alone had the ability to decrease NFκB activity in mesenteric arteries, it was unable to do so in aorta, further demonstrating the heterogeneous effects of PPAR activators on these vessels with different structure and function. Differential regulation of these vascular beds is in agreement with our previous results with rosiglitazone on Ang II signaling (Benkirane et al., 2006b). Thus, both PPARα and PPARγ have the ability in the present study to improve vascular function and inflammation at doses that were ineffective in lowering BP. Furthermore, these effects could not be solely attributed to their metabolic actions. These findings indicate that the association of combined low-dose PPARα and PPARγ activators is beneficial which would appear to contradict recent findings with the dual PPARα/γ activator muraglitazar, which was found to be associated with major adverse effects on cardiovascular events in type II diabetic patients (Nissen et al., 2005). Such deleterious actions may not be common to all dual PPARα/γ activators, as suggested by other investigators (Fagerberg et al., 2005; Verreth et al., 2006). More recently, Zadelaar et al. (2006). showed that another dual PPARα/γ activator tesaglitazar, reduced atherosclerotic lesions and vascular inflammation by decreasing NFκB activity in the vascular wall Zadelaar et al. (2006). Thus, depending on the relative proportion of PPARα and PPARγ activator effects within each dual activator, side effects may vary. We previously showed that PPARα or PPARγ activators administered alone protected against hypertension-induced vascular damage (Diep et al 2002a, 2002b; Iglarz et al., 2003; Benkirane et al., 2006b). Consequently, we have here a proof of concept demonstrating that the beneficial cardiovascular effects of dual PPARα and PPARγ activation can be achieved when they are administered concomitantly at low doses in a non-diabetic experimental paradigm representing elevated cardiovascular risk. Most dual agents have very potent PPARα action. It may be preferable to take advantage of the synergistic/additive effect demonstrated in the present work and combine low doses of PPARα and PPARγ activators, which could be given to high-risk vascular patients, even in the absence of dyslipidemia, type II diabetes or the metabolic syndrome, in order to reduce cardiovascular risk. It is hoped that these low doses may avoid the interaction of fibrates with statins, which most patients would probably be receiving according to current guidelines, and the glycemic effects, fluid retention and body weight increase that occur with full doses of PPARγ agonists. This concept is supported by Seber et al. (2006) who showed that concomitant administration of rosiglitazone and fenofibrate improved metabolic parameters of type II diabetic patients. We hereby suggest that the present data should be translated into randomized clinical trials of the combination of low doses of a fibrate and a glitazone in high-risk cardiovascular patients independently of dyslipidemia or impaired glucose metabolism to establish whether indeed improved cardiovascular outcomes may result from using this combination for vascular protection.
In summary, the present study provides the first in vivo evidence of beneficial effects of combined administration of PPARα and PPARγ activators at low doses to prevent vascular changes induced by a hypertensive dose of Ang II. These effects were associated with prevention of remodeling, endothelial dysfunction, oxidative stress generation and inflammation in the vasculature. Our findings suggest that dual activation of PPARα and PPARγ may be potentially beneficial in the prevention of hypertension-induced cardiovascular damage, and by using low doses of each activator, might result in a reduced side effect profile, which remains to be demonstrated. Translating these findings to patients may have important clinical significance, especially in high-risk vascular patients who do not have metabolic abnormalities requiring the use of these agents, yet who may benefit from the vasoprotective effects of PPARα and PPARγ activators.
Acknowledgments
We thank Suzanne Diebold, Laura Davis, André Turgeon and Manon Laprise, for their excellent technical help. Supported by Grant 13570 from the Canadian Institutes of Health Research.
Abbreviations
- Ang II
angiotensin II
- BP
blood pressure
- C/EBP
CCAAT/enhancer-binding protein
- L-NAME
Nω-nitro-L-arginine methyl ester
- M/L
media-to-lumen
- MCSA
media cross-sectional area
- NAD(P)H
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor kappa B
- NOS
nitric oxide synthase
- PPAR
peroxisome proliferator activated receptors
- PSS
physiological salt solution
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SNP
sodium nitroprusside
- TZD
thiazolidinediones
- VCAM-1
vascular cell adhesion molecule-1
Conflict of interest
The authors state no conflict of interest.
References
- Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- Benkirane K, Amiri F, Diep QN, El Mabrouk M, Schiffrin EL. PPAR-γ inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol. 2006a;290:H390–H397. doi: 10.1152/ajpheart.00662.2005. [DOI] [PubMed] [Google Scholar]
- Benkirane K, Viel EC, Amiri F, Schiffrin EL. Peroxisome proliferator-activated receptor γ regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension. 2006b;47:102–108. doi: 10.1161/01.HYP.0000196728.05488.c3. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Vikramadithyan RK, Misra P, Hiriyan J, Raichur S, Damarla RK, et al. Ragaglitazar: a novel PPARα & PPARγ agonist with potent lipid-lowering and insulin-sensitizing efficacy in animal models. Br J Pharmacol. 2003;140:527–537. doi: 10.1038/sj.bjp.0705463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Besnard S, Vanden BW, Peters JM, Gonzalez FJ, et al. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF, et al. PPARα activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002a;40:866–871. doi: 10.1161/01.hyp.0000037969.41360.cc. [DOI] [PubMed] [Google Scholar]
- Diep QN, Benkirane K, Amiri F, Cohn JS, Endemann D, Schiffrin EL. PPARα activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J Mol Cell Cardiol. 2004;36:295–304. doi: 10.1016/j.yjmcc.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-γ. Circulation. 2002b;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension. 2004;43:48–56. doi: 10.1161/01.HYP.0000103629.01745.59. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Edwards S, Halmos T, Lopatynski J, Schuster H, Stender S, et al. Tesaglitazar, a novel dual peroxisome proliferator-activated receptor α/γ agonist, dose-dependently improves the metabolic abnormalities associated with insulin resistance in a non-diabetic population. Diabetologia. 2005;48:1716–1725. doi: 10.1007/s00125-005-1846-8. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis GA, Annicotte JS, Auwerx J. PPAR-α effects on the heart and other vascular tissues. Am J Physiol Heart Circ Physiol. 2003;285:H1–H9. doi: 10.1152/ajpheart.01118.2002. [DOI] [PubMed] [Google Scholar]
- Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse V, et al. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sahoo SP, Wang PR, Milot DP, Ippolito MC, Wu MS, et al. A novel peroxisome proliferator-activated receptor α/γ dual agonist demonstrates favorable effects on lipid homeostasis. Endocrinology. 2004;145:1640–1648. doi: 10.1210/en.2003-1270. [DOI] [PubMed] [Google Scholar]
- Halabi CM, Sigmund CD. Peroxisome proliferator-activated receptor-γ and its agonists in hypertension and atherosclerosis: mechanisms and clinical implications. Am. J Cardiovasc Drugs. 2005;5:389–398. doi: 10.2165/00129784-200505060-00006. [DOI] [PubMed] [Google Scholar]
- Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor γ: implications for cardiovascular disease. Hypertension. 2004;43:297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN, Schiffrin EL. Effect of peroxisome proliferator-activated receptor-α and -γ activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol. 2003;23:45–51. doi: 10.1161/01.atv.0000047447.67827.cd. [DOI] [PubMed] [Google Scholar]
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Lyon C, Wakino S, Bruemmer D, Feng X, Goetze S, et al. PPARα inhibits TGF-β-induced β5 integrin transcription in vascular smooth muscle cells by interacting with Smad4. Circ Res. 2002;91:e35–e44. doi: 10.1161/01.res.0000046017.96083.34. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ. Small artery remodeling in hypertension. Curr Hypertens Rep. 2002;4:49–55. doi: 10.1007/s11906-002-0053-y. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- Plutzky J. Peroxisome proliferator-activated receptors as therapeutic targets in inflammation. J Am Coll Cardiol. 2003;42:1764–1766. doi: 10.1016/j.jacc.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-κB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular stiffening and arterial compliance Implications for systolic blood pressure. Am J Hypertens. 2004;17 Suppl:S39–S48. doi: 10.1016/j.amjhyper.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Peroxisome proliferator-activated receptors and cardiovascular remodeling. Am J Physiol Heart Circ Physiol. 2005;288:H1037–H1043. doi: 10.1152/ajpheart.00677.2004. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Amiri F, Benkirane K, Iglarz M, Diep QN. Peroxisome proliferator-activated receptors: vascular and cardiac effects in hypertension. Hypertension. 2003;42:664–668. doi: 10.1161/01.HYP.0000084370.74777.B6. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. Inflammation and vascular hypertrophy induced by angiotensin II: role of NADPH oxidase-derived reactive oxygen species independently of blood pressure elevation. Arterioscler Thromb Vasc Biol. 2003;23:707–709. doi: 10.1161/01.ATV.0000069907.12357.7E. [DOI] [PubMed] [Google Scholar]
- Seber S, Ucak S, Basat O, Altuntas Y. The effect of dual PPAR α/γ stimulation with combination of rosiglitazone and fenofibrate on metabolic parameters in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;71:52–58. doi: 10.1016/j.diabres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-δ and peroxisome proliferator-activated receptor-γ. Circ Res. 2002;91:427–433. doi: 10.1161/01.res.0000031271.20771.4f. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Deschepper C, Park JB, He G, Chen X, Neves MF, et al. Inhibition of mitogen-activated protein/extracellular signal-regulated kinase improves endothelial function and attenuates Ang II-induced contractility of mesenteric resistance arteries from spontaneously hypertensive rats. J Hypertens. 2002;20:1127–1134. doi: 10.1097/00004872-200206000-00024. [DOI] [PubMed] [Google Scholar]
- Verreth W, Ganame J, Mertens A, Bernar H, Herregods MC, Holvoet P. Peroxisome proliferator-activated receptor-α,γ-agonist improves insulin sensitivity and prevents loss of left ventricular function in obese dyslipidemic mice. Arterioscler Thromb Vasc Biol. 2006;26:922–928. doi: 10.1161/01.ATV.0000207318.42066.bb. [DOI] [PubMed] [Google Scholar]
- Virdis A, Iglarz M, Neves MF, Amiri F, Touyz RM, Rozen R, et al. Effect of hyperhomocystinemia and hypertension on endothelial function in methylenetetrahydrofolate reductase-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1352–1357. doi: 10.1161/01.ATV.0000083297.47245.DA. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- Zadelaar AS, Boesten LS, Jukema JW, van Vlijmen BJ, Kooistra T, Emeis JJ, et al. Dual PPARα/γ Agonist Tesaglitazar Reduces Atherosclerosis in Insulin-Resistant and Hypercholesterolemic ApoE*3Leiden Mice. Arterioscler Thromb Vasc Biol. 2006;26:2560–2566. doi: 10.1161/01.ATV.0000242904.34700.66. [DOI] [PubMed] [Google Scholar]