Abstract
Background and purpose:
Prenatal patency of ductus arteriosus is maintained by prostaglandin (PG) E2, possibly along with nitric oxide (NO) and carbon monoxide (CO), and cyclooxygenase (COX) deletion upregulates NO. Here, we have examined enzyme source and action of NO for ductus patency and whether NO and CO are upregulated by deletion of, respectively, heme oxygenase 2 (HO-2) and COX1 or COX2.
Experimental approach:
Experiments were performed in vitro and in vivo with wild-type and gene-deleted, near-term mouse fetuses.
Key results:
NG-nitro-L-arginine methyl ester (L-NAME) contracted the isolated ductus and its effect was reduced by eNOS, but not iNOS, deletion. L-NAME contraction was not modified by HO-2 deletion. Zinc protoporphyrin (ZnPP) also contracted the ductus, an action unaffected by deletion of either COX isoform. Bradykinin (BK) relaxed indomethacin-contracted ductus similarly in wild-type and eNOS−/− or iNOS−/−. BK relaxation was suppressed by either L-NAME or ZnPP. However, it reappeared with combined L-NAME and ZnPP to subside again with K+ increase or K+ channel inhibition. In vivo, the ductus was patent in wild-type and NOS-deleted fetuses. Likewise, no genotype–related difference was noted in postnatal closure.
Conclusions and implications:
NO, formed mainly via eNOS, regulates ductal tone. NO and CO cooperatively mediate BK-induced relaxation in the absence of PGE2. However, in the absence of PGE2, NO and CO, BK induces a relaxant substance behaving as an endothelium-derived hyperpolarizing factor. Ductus patency is, therefore, sustained by a cohort of agents with PGE2 and NO being preferentially coupled for reciprocal compensation.
Keywords: ductus arteriosus, nitric oxide, carbon monoxide, endothelium-derived hyperpolarizing factor, bradykinin, cyclooxygenase, nitric oxide synthase, heme oxygenase, fetal and neonatal physiology
Introduction
Prenatal patency of the ductus arteriosus is an active state of the vessel, being sustained by several agents. Although the cyclooxygenase (COX) pathway is assigned a prime role, with prostaglandin (PG) E2 rather than PGI2 as the major effector, nitric oxide (NO) and carbon monoxide (CO) are viewed as additional effectors acquiring prominence under certain conditions (Smith, 1998; Narumiya et al., 1999; Kobayashi and Narumiya, 2002). In fact, NO function is upregulated upon deletion of either COX in the PGE2 synthetic pathway (Baragatti et al., 2003), as expected from a complementary arrangement between the two systems (Seidner et al., 2001; Takizawa et al., 2001). This cooperative interaction may also occur during fetal development as, in the preterm ductus, NO is more effective than PGE2, whereas the opposite is noted at term (Momma and Toyono, 1999; Takizawa et al., 2001; Richard et al., 2004). It is an open question whether a reciprocal compensation may also exist between NO and CO, on the one hand, and between PGE2 and CO, on the other. Furthermore, there is no agreement on the expression of individual nitric oxide synthase (NOS) enzymes in the vessel. In a previous study in the mouse (Baragatti et al., 2003), we detected only the endothelial isoform (endothelial nitric oxide synthase (eNOS)), and our finding accords with another report in the same species proving the presence of eNOS with minimal amounts of the inducible nitric oxide synthase (iNOS) and neural nitric oxide synthase (nNOS) isoforms (Richard et al., 2004). The lamb ductus, however, may present a full complement of NOS isozymes, although eNOS still maintains its pre-eminence in vasoregulation, and nNOS is assigned an additional role in morphogenesis (Clyman et al., 1998; Mason et al., 1999; Rairigh et al., 2000).
The objective of this study was twofold, using a combination of gene deletions and pharmacological manipulations in the mouse. Our primary aim was to assess the actual importance of eNOS-derived NO for the regulation of basal tone of the ductus and the response of the vessel to the endothelium-dependent dilator bradykinin. Bradykinin is a known activator of NO and has been implicated in the control of the perinatal circulation (see Coceani et al., 1994; Bateson et al., 1999). Second, we wished to ascertain whether NO activity in the ductus increases following removal of the CO-forming enzyme (specifically, heme oxygenase 2 (HO-2)) and, as a corollary, whether CO function is upregulated upon COX removal. An answer to the latter questions would indicate whether or not the functional linkage between NO and PGE2, as reported earlier (Baragatti et al., 2003), has a unique character. An advantage in using the mouse ductus is that it combines the pre-eminence of eNOS (Baragatti et al., 2003; Richard et al., 2004) with the absence of vasa vasorum (Richard et al., 2004), so that the luminal endothelium becomes the main, if not the only, source of NO. Such arrangement eliminates a potential complication in the interpretation of findings, as may occur with the lamb (Clyman et al., 1998).
Methods
Experiments were carried out in B6/129 mice or in B6/129 mice backcrossed onto the C57BL/6 strain (NOS mutants) with the following genotypes: eNOS−/− (litter size, 2–10; mean, 7) (line of Leuwerke et al. (2002) from the original stock of Shesely et al. (1996)); iNOS−/− (litter size, 4–10; mean, 6; Laubach et al., 1995); COX1−/− (litter size, 2–7; mean, 5) and COX2−/− (litter size, 4–10; mean, 8; courtesy of Dr L Ballou; see Morham et al., 1995); HO-2−/− (litter size, 4–10; mean, 7) (Regan et al., 2004).
HO-1 was not studied in view of the difficulties in breeding a null mutant and the knowledge that it is less expressed than HO-2 in the ductus (Coceani et al., 1997). Wild-type (WT) C57BL/6 mice (litter size, 1–12; mean, 7) served as a control after having verified that they behave as the WT B6/129 mice. In all cases, genotype was confirmed in tail specimens by polymerase chain reaction (PCR). Animals were housed in temperature- and humidity-controlled quarters with constant 12:12-h light–dark cycles and were given food and water ad libitum. Surgical procedures and experimental protocols were approved by the Animal Care Committee of the Ministry of health.
In vitro studies
Term fetal mice, both WT and gene-deleted (gestational age, 19 days), were delivered by Caesarean section under halothane anaesthesia and were killed by cervical dislocation. Body weight varied between 0.9 and 1.5 g, and only one fetus was used in each experiment. The procedure for dissection of the ductus arteriosus, normalization of internal circumference and mechanical record has been described previously (Coceani et al., 1999). In brief, the animal was secured with its left-side up in a dissection chamber containing ice-cold Krebs solution gassed with 5% CO2 in N2. Through a thoracotomy, the ductus was exposed, separated from the adjoining large blood vessels and then suspended onto 25-μm tungsten wires (Cooner wire, Chatsworth, CA, USA) inside an organ bath. In some experiments, the ductus was prepared without the endothelium. For this purpose, a cat whisker of suitable size, its surface coarse from polishing with fine-grain sandpaper (600 grit), was passed through the lumen before isolating the vessel. The procedure for preparing endothelium-denuded vessels has been described elsewhere (Wang et al., 1994), and successful removal was confirmed by scanning electron microscopy (see Adeagbo et al., 1990). The fluid of the bath was gassed with gas mixtures containing 2.5 or 12.5% O2 to mimic, respectively, the fetal and the neonatal condition. The same mixtures were flushed through a hood covering the bath. Preparations were then equilibrated (about 60 min at 37°C) with a minimal stretch being applied (all values in mN mm−1) (WT, 0.05±0.003 (n=61); eNOS−/−, 0.07±0.005 (n=27); iNOS, 0.04±0.0009 (n=14); COX1−/−, 0.07±0.007 (n=7); COX2−/−, 0.06±0.008 (n=7); HO-2−/−, 0.08±0.01 (n=10)) and the attendant internal circumference (C0) with the related resting dimension (Table 1) served as a reference for choosing the appropriate load. Afterwards, tension was applied to attain an operating circumference (i.e. C50) coinciding with the condition in vivo (WT, 0.47±0.004 (n=61); eNOS−/−, 0.45±0.006 (n=27); iNOS, 0.52±0.009 (n=14); COX1−/−, 0.44±0.005 (n=7); COX2−/−, 0.45±0.008 (n=7); HO-2−/−, 0.45±0.008 (n=10); all values in mN mm−1), and the actual experiment was started following a second, 60- to 100-min period of equilibration.
Table 1.
Resting dimension of isolated ductus arteriosus from WT and gene-deleted term fetal mice
| Genotype | Internal diameter |
Vessel lengtha |
Wall thickness | |
|---|---|---|---|---|
| Short side | Long side | |||
| WT (n=61) | 139±1 | 532±8 | 545±8 | 19±0.3 |
| eNOS−/− (n=27) | 132±1* | 545±10 | 558±9 | 20±0.4 |
| iNOS−/− (n=14) | 152±2** | 553±10 | 569±10 | 19±0.7 |
| COX1−/− (n=7) | 130±1 | 501±34 | 510±34 | 21±0.7 |
| COX2−/− (n=7) | 131±2 | 460±14 | 473±13 | 18±0.4 |
| HO-2−/− (n=10) | 133±2 | 511±29 | 523±28 | 19±0.8 |
Abbreviations: ANOVA, analysis of variance; COX, cyclooxyygenase; eNOS, endothelial nitric oxide synthase; HO, heme oxygenase; iNOS, inducible nitric oxide synthase; WT, wild type.
Values (μm) are mean±s.e.m.
Vessel length of the ductus arteriosus is uneven because of its insertion into aorta at an angle. Short side was taken for normalization of internal circumference and calculation of tension output (see in vitro studies). *P<0.01, **P<0.001 vs WT (ANOVA with Bonferroni post hoc test). Note that the internal diameter of the ductus was smaller in eNOS−/− than WT, whereas the reverse occurred with iNOS−/−.
P<0.01
P<0.001
Congruent with its objectives (see above), the study composed of two main protocols, each with distinct lines of investigation. Unless otherwise specified, experiments were carried out at 2.5% O2. In protocol 1 (n=92), NG-nitro-L-arginine methyl ester (L-NAME) (100 μM) action was assessed in the WT vs eNOS−/− and iNOS−/− ductus at rest. In addition, the effect of isoform-specific NOS deletions and selected inhibitors was ascertained on the relaxation of the ductus to bradykinin. Bradykinin was tested in cumulative concentrations (0.01 nM–10 μM) on preparations precontracted with indomethacin (2.8 μM), and specificity of responses was confirmed through endothelium removal or exposure to excess K+ (55 mM). Indomethacin was chosen for pre-contraction to avoid any confounding influence from bradykinin-induced PGE2 formation (Bateson et al., 1999). Inhibitors included L-NAME and zinc protoporphyrin (ZnPP) (10 μM), being used individually or together, and the combination of 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) (1 μM)/apamin (0.2 μM) for the identification of any endothelium-derived hyperpolarizing factor (EDHF)-induced relaxation. A single concentration–response curve for bradykinin was obtained in every preparation to avoid any tachyphylaxis, and a maximal concentration of sodium nitroprusside (SNP) (100 μM) was applied at the end of the experiment to confirm muscle viability and functional integrity of the guanylyl cyclase therein. Preservation of endothelial function could not to be verified at the beginning of the experiment since bradykinin is the only suitable agent for tests on the ductus (Bodach et al., 1980; Coceani et al., 1994). However, no evidence was ever obtained, in this as in other studies (Coceani et al., 1999), of inadvertent damage to the endothelium while setting up the preparation. SNP was also tested separately over a full range of concentrations (0.1 nM–100 μM) to obtain a reference for the bradykinin-induced relaxation. The thromboxane A2 (TX)A2 analogue (0.1 μM) provided instead a reference for contractile responses.
In protocol 2 (n=34), L-NAME was tested on WT and HO-2−/− preparations after having confirmed with ZnPP that this HO isoform contributes to tone regulation. Likewise, ZnPP action was assessed on the basal tone of the WT vs COX1−/− and COX2−/− ductus at both 2.5 and 12.5% O2. As a separate control, ZnPP was also tested on the eNOS−/− vessel to verify whether NO facilitates CO by affecting its formation (Motterlini et al., 2002) or action (Barkoudah et al., 2004). In all cases, only one test procedure was employed with each vessel.
In vivo studies
Experiments were carried out in fetuses (gestational age, 19 days) or newborns (3 and 12 h) depending on the protocol. In the former case, animals (WT and NOS-deleted) were delivered by Caesarean section under halothane anaesthesia, whereas in the latter they were used at the stated intervals after vaginal delivery. Time zero (i.e. the time at which delivery was completed) was assessed for each animal in the litter. Separate experiments were performed in eNOS−/− fetuses whose mother had been treated for 3 days with indomethacin (2 mg kg−1 per os daily). The intent in the latter case was to verify the impact of PGE2 suppression on ductus patency when NO formation is impaired. Throughout the survival period, newborns were kept with the mother. All animals were killed by cervical dislocation.
Changes in ductus calibre through the transition from the pre- to the postnatal condition were assessed by fixing the vessel in situ with the whole-body freezing technique (Hörnblad and Larsson, 1967; Coceani et al., 1999). Briefly, animals were placed with their right-side up in a Petri dish and were covered with an embedding medium (Tissue-Tek optimum cutting temperature compound; Sakura Finetek, Torrance, CA, USA). The dish with the animal was then wrapped with aluminium foil and immersed in liquid N2. Once frozen, specimens were stored at −20°C for further work-up. To prepare a block of tissue with the ductus, the carcass was freed of its embedding in the frozen state. With a razor blade, soft tissues covering the dorsum were removed, making certain that the exposed surface would be parallel to the underlying descending aorta and hence perpendicular to the ductus. Serial, transversal sections (5 μm thick), progressing from this cut through the whole length of the vessel, were obtained on a Zeiss freezing microtome (model HM500 OM, Walldorf, Germany) and were stained with 1% methylene blue. Care was taken to collect a complete series of ductus sections. Afterwards, each section was photographed with a charge-coupled device solid-state camera (COHU, San Diego, CA, USA) and lumen area of the ductus was measured with a MCID-M4 program (Brock University, St Catharines, Canada). Both maximal and minimal values of this area were retrieved from each series for computation.
Total RNA preparation and quantitative real-time-PCR
Ductuses were collected from fetuses (gestational age, 19 days; WT and eNOS−/−) whose mother had been treated with indomethacin (see above) and were pooled in one group for each genotype (50 and 30 specimens/group, respectively, for WT and eNOS−/−). Indomethacin pretreatment, which is as effective as COX deletion in promoting NO function in the ductus (Baragatti et al., 2003; D Sodini, unpublished data), was used to maximize any upregulation of iNOS and nNOS upon eNOS deletion. Coincidentally, this approach reproduced the protocol for testing bradykinin in vitro (see above). RNA was isolated as described earlier (Baragatti et al., 2003), and its yield was measured spectrophotometrically.
DNAse I (Roche, IN USA)-treated total RNA (2.4 μg for each group) was reverse-transcribed with 1 U of Thermoscript RT (Invitrogen, Carlsbad, CA, USA) in the presence of random hexanucleotide primers, according to the manufacturer's instructions. Quantitative real-time (QRT)-PCR reactions (40 cycles) were performed on an ABI Prism 7700 instrument (Applied Biosystems, Foster City, CA, USA) using the TaqMan Universal PCR Master Mix (Applied Biosystems). Primer sequences for mouse iNOS, nNOS and cyclophilin B (internal standard) were obtained from an online library (Applied Biosystems). Gene expression was quantified in triplicate for each of the two groups by the comparative cycle-threshold method and was expressed in arbitrary units against reference cyclophilin.
Solutions and drugs
The Krebs medium had the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1, MgSO4 0.9, dextrose 11.1 and NaHCO3 25. Potassium–Krebs solution (20 and 55 mM) was prepared by substituting NaCl with an equimolar amount of KCl. Depending on the stage of the experiment (see above), the solution was bubbled with gas mixtures containing either no O2 or O2 in one of two concentrations (2.5 and 12.5%) plus 5% CO2 and balance N2. PO2 was measured with a Chiron gas analyzer (model 248, Halstead, UK) and was 0.98±0.001, 2.13±0.007 and 6.67±0.04 kPa (pH 7.41±0.004) when gas mixtures had 0, 2.5 and 12.5% O2, respectively.
The following compounds were used: bradykinin acetate (Sigma, St Louis, MO, USA); the NOS inhibitor, L-NAME (Sigma); the HO inhibitor, ZnPP (Porphyrin Products, Carnforth, UK); the dual COX1/COX2 inhibitor, indomethacin (Sigma); a recently developed inhibitor of intermediate-conductance Ca2+-activated K+ channels, TRAM-34; courtesy of Dr H Wulff) which, unlike other compounds in this category, has no effect on cytochrome P450-based reactions (Wulff et al., 2000); the inhibitor of small-conductance Ca2+-activated K+ channels, apamin (Sigma); the TXA2 analogue 9,11-epithio-11, 12-methano-TXA2 (ONO-11113, courtesy of ONO Pharmaceutical, Osaka, Japan); and SNP (Sigma). Concentrations of the inhibitors were derived from the literature with the aim of combining efficacy with selectivity. In particular, at the stated concentrations, the combination TRAM-34/apamin is able to reverse fully any EDHF-mediated vasodilatation (Eichler et al., 2003). In addition, ZnPP exerts a specific effect on the HO enzyme complex in the ductus (Coceani et al., 1997).
ONO-11113 was dissolved in distilled ethanol (5 mg ml−1), and aliquots (stored at −70°C) were diluted with Tris buffer (pH 7.4). Indomethacin and TRAM-34 were also dissolved in ethanol (10 and 1 mg ml−1, respectively) before preparation of the final solution in the Krebs medium. Likewise, ZnPP was first prepared as a stock solution in 0.1 M NaOH (1 mM) on the day of the experiment. Ethanol in the fluid bathing isolated ductus preparations did not exceed 0.03% (TRAM-34), 0.01% (indomethacin) or 0.001% (ONO-11113). Other substances dissolved readily in saline or Krebs medium. Solutions of certain compounds (SNP, ZnPP) were protected from light.
Concentrations of compounds are given in molar units and refer to their final value in the bath. Vehicle alone, without or with ethanol (see above), had no effect on vessel tone.
Analysis of data
Baseline contractile tension, which varied with the preparation (see Results), refers to the net active tension (i.e. total tension minus applied tension) developed by the preparation before any treatment. Responses to the constrictors were measured by the rise in tension over baseline and were expressed in absolute values. Relaxant responses were given as reversal of tension (percent or absolute values) in the indomethacin-precontracted vessel.
Data are expressed as the mean±s.e.m. Comparisons were made using a Student's t-test or analysis of variance (ANOVA), followed by the Bonferroni test. Differences are considered significant for P<0.05.
Results
mRNA analysis
Although previous work has failed to demonstrate mRNA for both iNOS and nNOS in the ductus of the WT mouse (Baragatti et al., 2003), a further analysis was performed here to verify whether either isoform becomes detectable upon eNOS deletion. However, even with a 40-cycle amplification, QRT-PCR showed barely detectable iNOS and nNOS signals (detection at 38 cycles), with values for the eNOS−/− mutant straddling those of the WT.
In vitro studies
The isolated ductus arteriosus was smaller in eNOS−/− than in WT, whereas the opposite was seen with iNOS−/− (Table 1). All preparations, whether WT or gene-deleted, developed a variable degree of tension (0.16–0.47 mN mm−1) during equilibration. Coincidentally, as reported elsewhere (Coceani et al., 1999; Baragatti et al., 2003), the baseline presented, singly or in combination, slow contractions of variable magnitude (0.1–0.8 mN mm−1 and exceptionally higher, maximum 1.1 mN mm−1) and rapid discharges of low amplitude. The incidence of such activity was lower in the iNOS mutant (15% of preparations) than in other genotypes (70% of preparations). Indomethacin (2.8 μM) contracted the ductus similarly in the various of genotypes (Table 2). Likewise, minor and mostly insignificant variations were noted in the elevated tone before bradykinin tests when, in examining the mode of action of the compound (see below), indomethacin was variably combined with other inhibitors and excess potassium (Table 2). The reference TXA2 analogue, ONO-11113 (0.1 μM), was only slightly more potent than indomethacin (WT, 1.21±0.07 mN mm−1, n=17; eNOS−/−, 1.12±0.08 mN mm−1, n=11), indicating that the baseline for bradykinin-induced relaxation was close to the maximal contractile capacity of the vessel.
Table 2.
Isolated ductus arteriosus from term fetal mice: wall tension before bradykinin-induced relaxation
| Condition |
Genotype |
||
|---|---|---|---|
| WT | eNOS−/− | iNOS−/− | |
| Indomethacin | 0.88±0.11 (10) | 1.05±0.08 (6) | 0.71±0.11 (6) |
| Indomethacin+L-NAME | 0.99±0.06 (5) | — | — |
| Indomethacin+ZnPP | 0.79±0.09 (6) | 0.90±0.12 (4) | — |
| Indomethacin+L-NAME+ZnPP | 1.20±0.09 (4) | — | — |
| Indomethacin+L-NAME+ZnPP+K+ 20 mM | 0.76±0.09 (4) | — | — |
| Indomethacin+L-NAME+ZnPP+K+ 55 mM | 1.0±0.18 (4) | — | — |
| Indomethacin+L-NAME+ZnPP +TRAM-34+apamin | 1.21±0.18 (4) | — | — |
| Indomethacin+TRAM-34+apamin | — | 0.52±0.05 (7)* | — |
| Indomethacin+ZnPP+TRAM-34+apamin | — | 0.61±0.03 (3)** | — |
Abbreviations: ANOVA, analysis of variance; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; L-NAME, NG-nitro-L-arginine methyl ester, TRAM-34, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole; WT, wild-type; ZnPP, zinc protoporphyrin.
Values (mN mm−1) are mean±s.e.m. and apply to the steady tension of the preparation. Number of experiments in parentheses. Ductus preparations from WT and NOS-deleted animals were treated with indomethacin alone or in combination with excess potassium and the required inhibitors in various combinations (for details, see text).
*P<0.01, **P<0.05 vs indomethacin alone in the same genotype (ANOVA with Bonferroni post hoc test). Note that differences in the indomethacin response among genotypes are not significant.
P<0.01
P<0.05
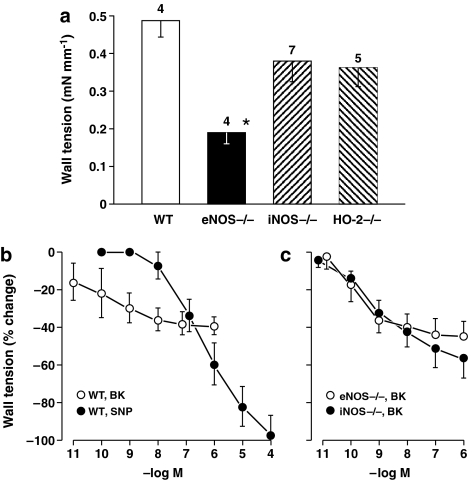
NO function. L-NAME (100 μM) contracted the ductus from the WT mouse (Figure 1). The contraction started after some delay (8–16 min), progressed gradually to a peak (maximum in 80–90 min) and was sustained thereafter. A similar contraction was observed in the eNOS mutant, but the response was smaller and often not sustained (Figure 1a). Conversely, the iNOS−/− ductus responded as the WT to L-NAME, thus pointing against a significant role of this isoform in basal vasoregulation (Figure 1a).
Figure 1.
Isolated ductus arteriosus from fetal mouse. (a) Comparison of contractile responses to L-NAME (100 μM) in WT vs NOS- and HO-2-deleted preparations (n, above columns). Baseline wall tension (mN mm−1) before treatment was as follows: WT, 0.04±0.02; eNOS−/−, 0.23±0.06; iNOS−/−, 0.28±0.06; HO-2−/−, 0.02±0.02. A significant difference (see asterisk) was found between WT and eNOS−/− (P<0.05; ANOVA). eNOS−/− also differed from WT in presenting a non-sustained L-NAME contraction in some cases (n=2). (b and c) Concentration–response curves to bradykinin, respectively, in WT (n=5 for both groups) and NOS-deleted (n=6 for both groups) preparations precontracted with indomethacin (2.8 μM). Wall tension before bradykinin was as follows: (b) WT, 1.04±0.17 (before SNP, 0.73±0.11); and (c) eNOS−/−, 1.05±0.08; iNOS−/−, 0.71±0.11. Progression of concentration-dependent changes is significant with each condition (P<0.001), whereas bradykinin relaxation is not different among genotypes (ANOVA in all cases). Where necessary, points have been offset to improve visibility, and any missing s.e. bar is within the size of the symbol.
Bradykinin relaxed dose-dependently the WT ductus precontracted with indomethacin. As shown in Figure 1b, threshold was around 10 pM and maximum at 10–100 nM (39±4% maximum reversal) compared with an SNP range from 10 nM onwards (100±12% max reversal). Coincidentally, relaxant responses were often preceded by an abrupt and rapidly reversible contraction of small magnitude (maximum about 0.1 mN mm−1). No bradykinin-induced relaxation was found with the endothelium-denuded ductus and, in fact, the relaxation was replaced in certain cases by a marginal contraction (0.08±0.06 mN mm−1 at 10 μM, n=4). A similar change from a relaxation to a modest contraction was seen when bradykinin was tested with excess potassium (0.05±0.03 mN mm−1 at 55 mM, n=3). Conversely, SNP relaxation remained unabated in the absence of the endothelium (131±13% reversal at 100 μM, n=4).
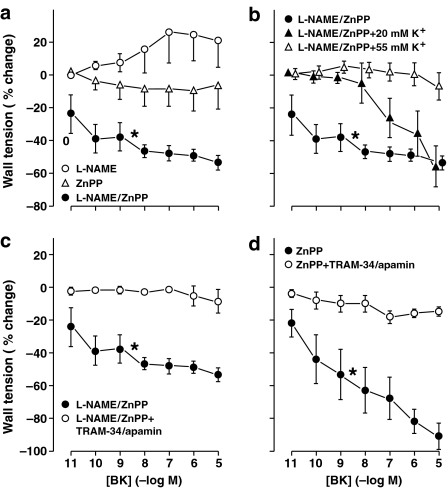
eNOS and iNOS deletion caused no significant change in the bradykinin-induced relaxation (Figure 1c), whereas the same relaxation in the WT was suppressed by either L-NAME or ZnPP (Figure 2a). This coincidence between L-NAME and ZnPP has already been found in the lamb ductus (Coceani et al., 1997) and implies that NO and CO act sequentially in mediating the bradykinin-induced relaxation. Nevertheless, when L-NAME and ZnPP were combined, the relaxation was restored over the entire concentration range and, in fact, was enhanced compared to the original response (53±4 vs 39±4% maximum reversal; P=0.001, ANOVA) (Figure 2a). An even greater response (91±7% maximum reversal; P<0.001, ANOVA) was seen when testing bradykinin in the ZnPP-treated, eNOS mutant (Figure 2d). This restored relaxation progressively abated with a stepwise rise of potassium concentration (Figure 2b) and also subsided with TRAM-34 plus apamin treatment (Figure 2c and d). Conversely, in the absence of ZnPP, the same TRAM-34/apamin combination failed to modify the bradykinin-induced relaxation of the eNOS−/− ductus in four of seven experiments (64±8% maximum reversal). The remaining three experiments, however, showed complete suppression of the response.
Figure 2.
Concentration–response curves to bradykinin in the isolated mouse ductus arteriosus. Vessels were pretreated with indomethacin (2.8 μM) plus other inhibitors and excess potassium in various combinations. (a) WT, L-NAME (100 μM, n=5), ZnPP (10 μM, n=6), L-NAME/Znpp (n=4) (b) WT, L-NAME/ZnPP alone (n=4) and with K+ 20 mM (n=5) or K+ 55 mM (n=4). (c) WT, L-NAME/ZnPP alone (n=4) and with TRAM-34 (1 μM)/apamin (0.2 μM) (n=4). (d) eNOS−/−, ZnPP alone (n=4) and with TRAM-34/apamin (n=3). Bradykinin curve during treatment with L-NAME/ZnPP is the same as in (a), (b) and (c). Values of wall tension (mN mm−1) before bradykinin under the various conditions are given in Table 2. Progression of concentration-dependent responses to bradykinin is significant for L-NAME-/ZnPP-treated WT preparations, in the absence and presence of K+ 20 mM and for ZnPP-treated eNOS−/− preparations (P<0.001, ANOVA). In each panel, bradykinin curves marked with an asterisk differ from the other curves (P=0.001, or better). Where necessary, points have been offset to improve visibility and any missing s.e. bar is within the size of the symbol.
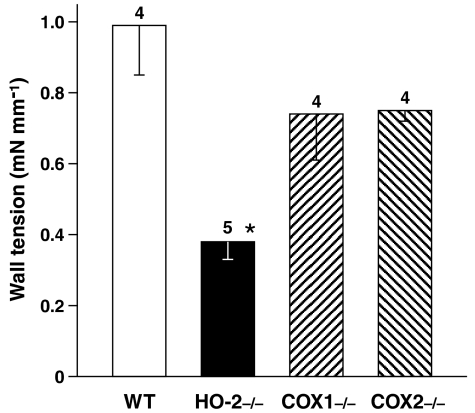
NOS/HO and HO/COX interactions in tone regulation. It was first confirmed that HO-2, with its CO product, sustains ductus relaxation by showing a weaker ZnPP contraction after deletion of this particular gene (Figure 3). This contraction, whether full-fledged or curtailed, developed gradually in time (maximum in 90–160 and 30–140 min in WT and HO-2−/−, respectively) and was maintained after reaching its peak. However, at variance with the COX-deleted animal (Baragatti et al., 2003), the HO-2-deleted animal did not present any NO upregulation. As shown in Figure 1a, L-NAME was equally effective with and without the HO-2 gene. Likewise, the ZnPP contraction was not modified by deletion of either COX at both 2.5 and 12.5% O2 (Figure 3). Conversely, no response to ZnPP was seen in the eNOS mutant, and baseline tension of the ductus remained virtually unchanged throughout treatment (control: 0.1±0.08 mN mm−1, ZnPP: 0.19±0.04 mN mm−1; n=3).
Figure 3.
Isolated ductus arteriosus from fetal mouse. Comparison of contractile responses to ZnPP (10 μM) in WT vs HO-2- and COX-deleted preparations. Note that results with COX mutants were the same at 12.5% O2 (n=3 for each genotype). Wall tension (mN mm−1) before treatment was as follows: WT, 0.28±0.07; HO-2−/−, 0.15±0.09; COX1−/−, 0.31±0.07; COX2−/−, 0.16±0.04. For each group, the number of experiments is given above the columns, and a significant difference (P<0.01; ANOVA) between WT and HO-2−/− preparations is indicated with an asterisk.
In vivo studies
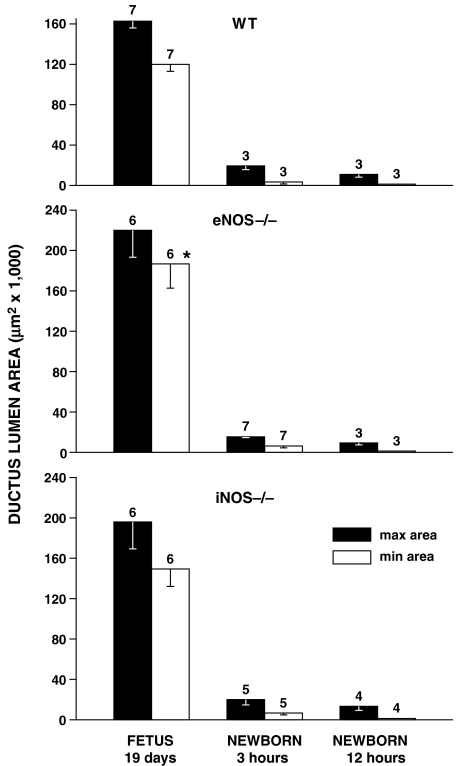
The ductus was patent in all fetuses, regardless of genotype, with a wider lumen in the eNOS mutant (Figure 4). The same trend for a larger ductus, albeit without reaching significance, was also noted in the iNOS mutant. Postnatally, both WT- and NOS-deleted animals constricted their ductus rapidly so that the lumen was consistently reduced to a fraction of the prenatal value by 3 h and was virtually absent at 12 h (Figure 4). During this period, narrowing was either even over the entire length of the vessel or was more prominent in its central portion.
Figure 4.
Mouse ductus arteriosus. Prenatal patency and time course of closure in WT vs NOS-deleted animals. For each group, the number of experiments is given above the columns, and a significant difference (P<0.05; ANOVA) between WT and eNOS−/− fetuses is indicated with an asterisk.
Pretreatment of the eNOS−/− fetus with chronic indomethacin did not cause marked constriction or closure of the ductus. In fact, the vessel lumen, which is paradoxically enlarged in this mutant (Figure 4), was reduced after indomethacin treatment to a value matching that of the untreated WT (max area: 126 523±17 139 μm2; min area: 92 712±14 029 μm2; n=5; P<0.01 and NS vs untreated eNOS −/− and WT, respectively; ANOVA).
Discussion
The present study shows that NO, originating within the endothelium, exerts a tonic relaxant effect on the fetal ductus. This finding accords with our earlier results in the isolated lamb ductus (Coceani et al., 1994) as well as with in vivo experiments (Bustamante et al., 1996; Momma and Toyono, 1999; Takizawa et al., 2001; Richard et al., 2004), thus yielding further evidence against reports negating any such control (Clyman et al., 1998; Bateson et al., 1999). NO was formed via eNOS but, as the L-NAME contraction was only partially curtailed in the eNOS mutant (see Figure 1a), a contribution from other NOS isoforms must be considered in spite of their modest mRNA expression. An explanation for this discrepancy could be that either, or both, isoforms are located at some discrete, yet critical, site in the vessel. Consistent with an accessory role of iNOS/nNOS is also the observation that bradykinin-induced relaxation was, on the one hand, suppressed in the L-NAME-treated, WT ductus and, on the other, was preserved in the untreated vessel lacking eNOS. However, despite its identification with a natural relaxant, eNOS-derived NO is not critical for ductus patency. eNOS deletion does not cause any narrowing of the vessel in vivo and, on the contrary, manifests itself with a paradoxical change in the opposite direction. Concomitantly with NO, the ductus may generate CO and this possibility supporting a normal function for the compound distinguishes the mouse from the lamb where ZnPP is without effect on the resting tone (Coceani et al., 1997). Indeed, our finding in the mouse that ZnPP and indomethacin contract the ductus to a similar degree implies an equivalent relaxant function for CO and PGE2. Nevertheless, the two compounds differ in that deletion of CO-forming HO-2, unlike deletion of PGE2-forming COXs (Baragatti et al., 2003), is not followed by a compensatory upregulation of the NO system. Hence, the concept is strengthened of a special linkage being in place between PGE2 and NO for the control of ductal tone. CO, on the other hand, appeared to share with NO an intermediary role in the bradykinin-induced relaxation, with the two agents being coupled in a cooperative sequence (see Coceani et al., 1997). Significantly, when both NO and CO were suppressed along with PGE2, another relaxing agent became evident with the properties of EDHF. Based on this premise, our discussion will address two main issues: the nature of the interaction between NO and CO in ductus regulation at rest and in response to bradykinin, and the significance of EDHF for maintenance of ductus patency.
The nature of the interaction between NO and CO is intriguing and is best examined by treating it separately in the resting vessel and during the bradykinin-induced relaxation. In the former case, NO, deriving primarily from eNOS, exerts a direct effect on ductal muscle. CO is unlikely to participate in its action, considering that the L-NAME contraction does not differ between WT and HO-2-deleted preparations (see Figure 1a). Conversely, ZnPP contraction is curtailed in the eNOS mutant, implying a crucial role of NO in CO-mediated ductal tone. Both agents, on the other hand, mediate the bradykinin-induced relaxation since suppression of either NO or CO results in its loss. The actual mechanism for this interdependence cannot be assessed with our data, but it may be inferred from the literature. Indeed, a cooperative sequence being triggered by bradykinin, with NO promoting the synthesis of CO, is feasible in view of the extensive evidence supporting this particular function for NO itself and any reactive nitrogen species (Motterlini et al., 2002). Alternatively, NO may be permissive for CO in line with evidence from elsewhere in the perinatal vasculature (Barkoudah et al., 2004). Significantly, in support of the latter scheme, we have found that ductus relaxation to CO decreases upon L-NAME treatment (Coceani et al., 1996). These two possibilities are not mutually exclusive and, in addition, can account for the NO dependence of the CO relaxation in the ductus at rest.
Whatever the nature of the synergy between NO and CO, bradykinin promotes the appearance of another relaxing agent, consistent with an EDHF, when they are both curtailed. Significantly, the latter phenomenon is seen regardless of whether NO inhibition is obtained with L-NAME treatment or after eNOS deletion. The mechanism whereby this latent relaxing agent is uncovered by the combined removal of NO and CO is still to be defined. However, a distinct possibility, based on current knowledge (Nishikawa et al., 2000; Yaghi et al., 2004), is that NO and CO normally exert a braking effect on an EDHF originating from a cytochrome P450-based reaction. Indeed, the ductus is endowed with several cytochrome P450 species (F Coceani, unpublished data), including the 2J species that has been linked to EDHF through its ability to catalyze the conversion of arachidonic acid in to vasorelaxant epoxides (Capdevila et al., 2002; Yaghi et al., 2004). Further work will be required to validate this scheme.
Two final points deserve a comment and they relate to the inhibition exerted by TRAM-34/apamin on the bradykinin-induced relaxation of certain eNOS−/− preparations, even in the absence of a concomitant ZnPP treatment and the reason for the paradoxical enlargement of the ductus in the eNOS−/− fetus in utero. The former occurrence is reminiscent of findings in the adult vasculature (Scotland et al., 2005; Félétou and Vanhoutte, 2006), where interference with PGE2 and NO synthesis may be sufficient to unfold the EDHF mechanism. Perhaps, in accord with our proposed scheme, in these particular preparations, the CO mechanism was not efficient enough to curtail EDHF. Alternatively, the finding may reflect the multiplicity of agents, unlikely all to be under NO/CO control, potentially accounting for EDHF activity (Félétou and Vanhoutte, 2006). Equally puzzling is the ‘overdilatation' of the ductus in the eNOS−/− fetus. A structural change can be ruled out since the vessel was actually smaller in the mutant compared to the WT when removed from the animal (see Table 1). In all likelihood, this condition denotes an active process being sustained by relaxant(s) that may be local and/or blood-borne. PGE2 is one such compound and, accordingly, the ductus enlargement was reversed by treating the mother with indomethacin. It is significant, however, that the ductus remained patent even when combining PGE2 inhibition with eNOS deletion. This observation reasserts the concept that patency is sustained by a host of agents, including CO and EDHF in addition to PGE2 and NO, ensuring collectively a remarkable degree of synergy and reciprocal compensation.
In conclusion, we have shown that NO, modulating the tone of the ductus arteriosus, originates primarily from eNOS. Its absence, however, does not result in narrowing of the vessel in vivo, thus implying a function of alternative relaxant(s). PGE2, in particular, is preferentially linked with NO in a mutual process of compensation. CO is also assigned a role in tone regulation and, in fact, its relaxing influence on the ductus appears to match that of PGE2. In the latter respect, the mouse differs from the lamb where the CO system is ineffective in the resting vessel. In addition, a complex relationship exists between NO and CO whereby the former agent may condition the formation and the action of the latter both at rest and in response to bradykinin. Suppression of NO and CO formation along with PGE2 results in the appearance of EDHF, which consequently qualifies itself as a potential compensatory mechanism with an early development in gestation. Collectively, these findings underline the importance of preserving ductus patency for fetal homeostasis, with several agents, whether latent or normally operational, contributing in a cooperative fashion to this process.
Acknowledgments
This work was supported by grants of the Italian Ministry of Education and Research (FIRB RBNEOIW9 PM) and the Heart and Stroke foundation of Ontario (Grant T-3329). FB and DS are recipient of a graduate studentship from the Scuola Superiore Sant'Anna. Lois Kelsey and Eric Seidlitz contributed initial experiments. We gratefully acknowledge the assistance of Aina Tilups for the scanning electron microscopy. We also indebted to Silvia Gonzali and Vittorio Gattai for excellent assistance.
Abbreviations
- COX
cyclooxygenase
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- HO
heme oxygenase
- iNOS
inducible nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- nNOS
neural nitric oxide synthase
- NOS
nitric oxide synthase
- ONO-11113, 9, 11-epithio-11
12-methano-thromboxane A2
- PG
prostaglandin
- SNP
sodium nitroprusside
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- TX
thromboxane
- WT
wild type
- ZnPP
zinc protoporphyrin
Conflict of interest
The authors state no conflict of interest.
References
- Adeagbo ASO, Breen CA, Cutz E, Lees JG, Olley PM, Coceani F. Lamb ductus venosus: evidence of a cytochrome P-450 mechanism in its contractile tension. J Pharmacol Exp Ther. 1990;252:875–879. [PubMed] [Google Scholar]
- Baragatti B, Brizzi F, Ackerley C, Barogi S, Ballou LR, Coceani F. Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase. Br J Pharmacol. 2003;139:1505–1515. doi: 10.1038/sj.bjp.0705391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoudah E, Jaggar JH, Leffler CW. The permissive role of endothelial NO in CO-induced cerebrovascular dilation. Am J Physiol. 2004;287:H1459–H1465. doi: 10.1152/ajpheart.00369.2004. [DOI] [PubMed] [Google Scholar]
- Bateson EAJ, Schulz R, Olley PM. Response of fetal rabbit ductus arteriosus to bradykinin: role of nitric oxide, prostaglandins, and bradykinin receptors. Pediatr Res. 1999;45:568–574. doi: 10.1203/00006450-199904010-00017. [DOI] [PubMed] [Google Scholar]
- Bodach E, Coceani F, Dumbrille A, Okpako DT, Olley PM. The response of the isolated ductus arteriosus to transmural stimulation and drugs. Br J Pharmacol. 1980;71:419–427. doi: 10.1111/j.1476-5381.1980.tb10954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante SA, Pang Y, Romero S, Pierce MR, Voelker CA, Thompson JH, et al. Inducible nitric oxide synthase and the regulation of central vessel caliber in the fetal rat. Circulation. 1996;94:1948–1953. doi: 10.1161/01.cir.94.8.1948. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Harris RC, Falck JR. Microsomal cytochrome P450 and eicosanoid metabolism. Cell Mol Life Sci. 2002;59:780–789. doi: 10.1007/s00018-002-8466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, Chen Y-Q. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatr Res. 1998;43:633–644. doi: 10.1203/00006450-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Coceani F, Kelsey L, Seidlitz E. Occurrence of endothelium-derived relaxing factor-nitric oxide in the lamb ductus arteriosus. Can J Physiol Pharmacol. 1994;72:82–88. doi: 10.1139/y94-013. [DOI] [PubMed] [Google Scholar]
- Coceani F, Kelsey L, Seidlitz E. Carbon monoxide-induced relaxation of the ductus arteriosus in the lamb: evidence against the prime role of guanylyl cyclase. Br J Pharmacol. 1996;118:1689–1696. doi: 10.1111/j.1476-5381.1996.tb15593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coceani F, Kelsey L, Seidlitz E, Marks GS, Mclaughlin BE, Vreman HJ, et al. Carbon monoxide formation in the ductus arteriosus in the lamb: implications for the regulation of muscle tone. Br J Pharmacol. 1997;120:599–608. doi: 10.1038/sj.bjp.0700947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coceani F, Liu Y-A, Seidlitz E, Kelsey L, Kuwaki T, Ackerley C, et al. Endothelin A receptor is necessary for O2 constriction but not closure of ductus arteriosus. Am J Physiol. 1999;277:H1521–H1531. doi: 10.1152/ajpheart.1999.277.4.H1521. [DOI] [PubMed] [Google Scholar]
- Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now. Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Hörnblad PY, Larsson KS. Studies on closure of the ductus arteriosus. I. Whole-body freezing as improvement of fixation procedures. Cardiologia. 1967;51:231–241. [PubMed] [Google Scholar]
- Kobayashi T, Narumiya S. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat. 2002;68–69:557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuwerke SM, Kaza AK, Tribble CG, Kron IL, Laubach VE. Inhibition of compensatory lung growth in endothelial nitric oxide synthase-deficient mice. Am J Physiol. 2002;282:L1272–L1278. doi: 10.1152/ajplung.00490.2001. [DOI] [PubMed] [Google Scholar]
- Mason CAE, Chang P, Fallery C, Rabinovitch M. Nitric oxide mediates LC-3-dependent regulation of fibronectin in ductus arteriosus intimal cushion formation. FASEB J. 1999;13:1423–1434. doi: 10.1096/fasebj.13.11.1423. [DOI] [PubMed] [Google Scholar]
- Momma K, Toyono M. The role of nitric oxide in dilating the fetal ductus arteriosus in rats. Pediatr Res. 1999;46:311–315. doi: 10.1203/00006450-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Green CJ, Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid Redox Signal. 2002;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol. 2000;279:H459–H465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- Rairigh RL, Storme L, Parker TA, Le Cras TD, Markham N, Jakkula M, et al. Role of neuronal nitric oxide synthase in regulation of vascular and ductus arteriosus tone in the ovine fetus. Am J Physiol. 2000;278:L105–L110. doi: 10.1152/ajplung.2000.278.1.L105. [DOI] [PubMed] [Google Scholar]
- Regan RF, Chen J, Benvenisti-Zarom L. Heme oxygenase-2 gene deletion attenuates oxidative stress in neurons exposed to extracellular hemin. BMC Neurosci. 2004;5:34. doi: 10.1186/1471-2202-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C, Gao J, Lafleur B, Christman BW, Anderson J, Brown N, et al. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol. 2004;287:R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- Seidner SR, Chen Y-Q, Oprysko PR, Mauray F, Tse MM, Lin E, et al. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50:365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GCS. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- Takizawa T, Kihara T, Kamata A. Increased constriction of the ductus arteriosus with combined administration of indomethacin and L-NAME in fetal rats. Biol Neonate. 2001;80:64–67. doi: 10.1159/000047122. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mercer-Connolly A, Lines L, Toyoda O, Coceani F. Endothelium-denuded pulmonary resistance arteries from the fetal lamb: preparation and response to vasoactive agents. J Pharmacol Toxicol Meth. 1994;32:85–91. doi: 10.1016/1056-8719(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hänsel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi A, Bend JR, Webb CD, Zeldin DC, Weicker S, Mehta S, et al. Excess nitric oxide decreases cytochrome P-450 2J4 content and P-450-dependent arachidonic acid metabolism in lungs of rats with acute pneumonia. Am J Physiol. 2004;286:L1260–L1267. doi: 10.1152/ajplung.00273.2003. [DOI] [PubMed] [Google Scholar]