Abstract
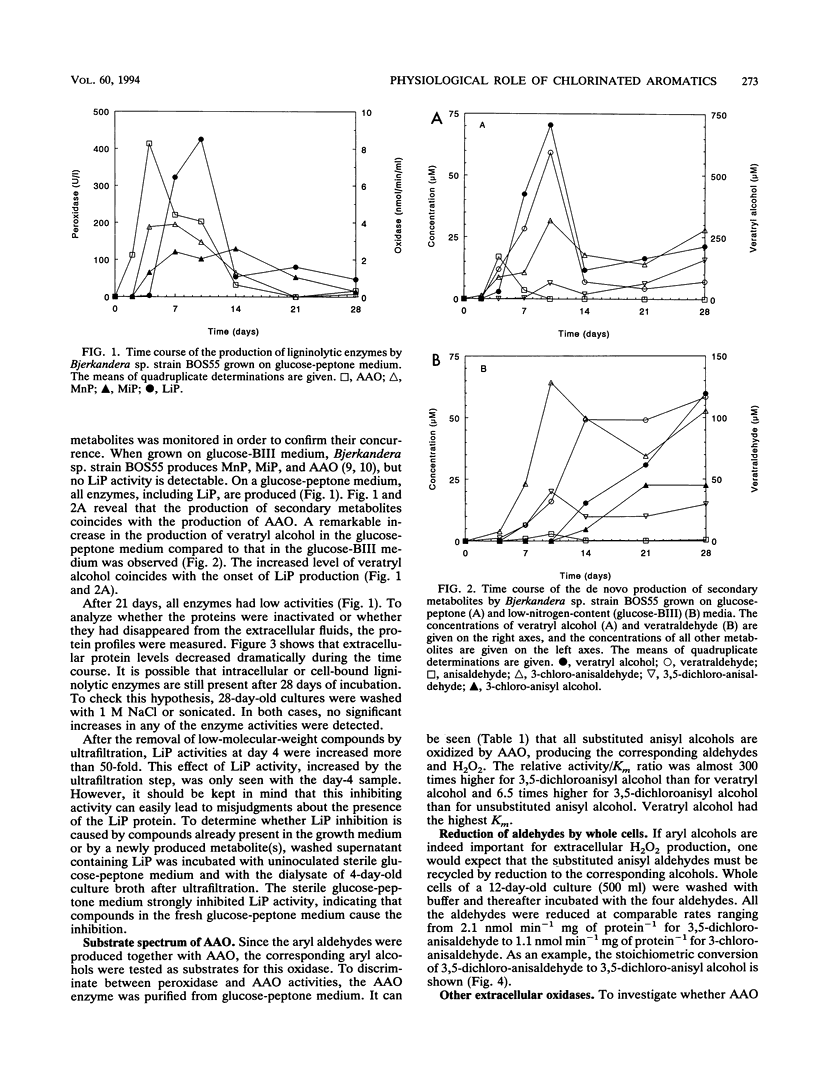
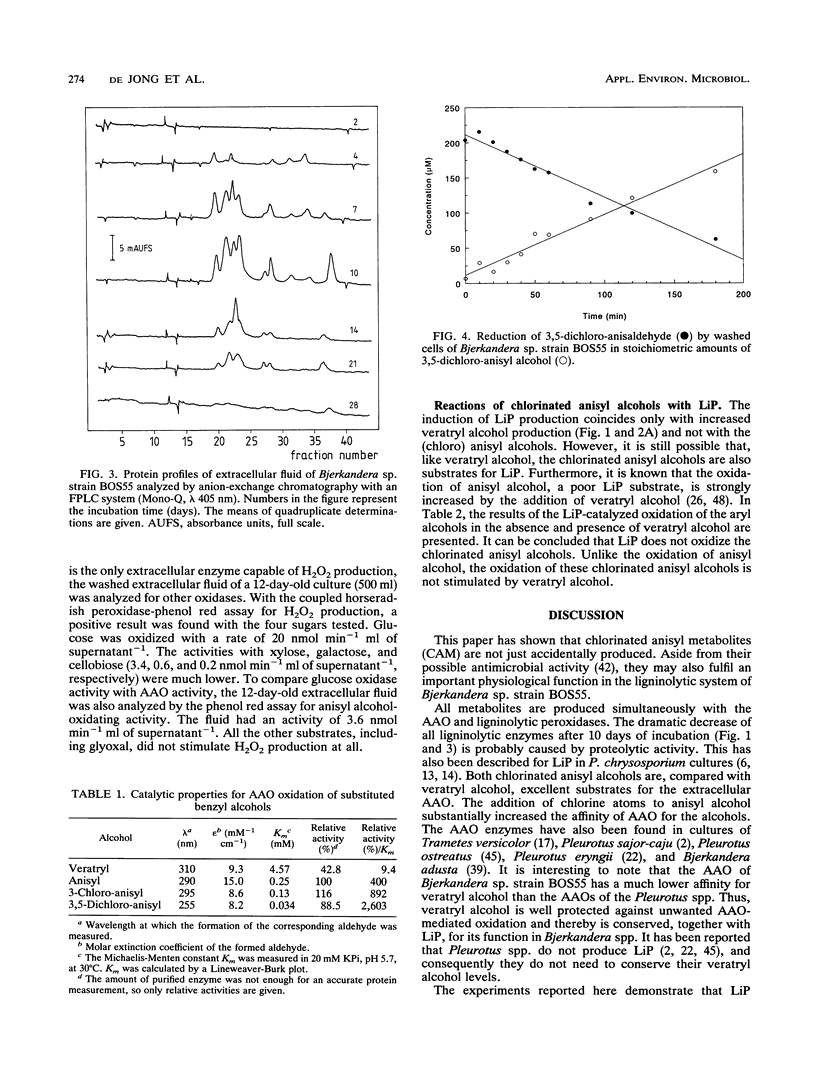
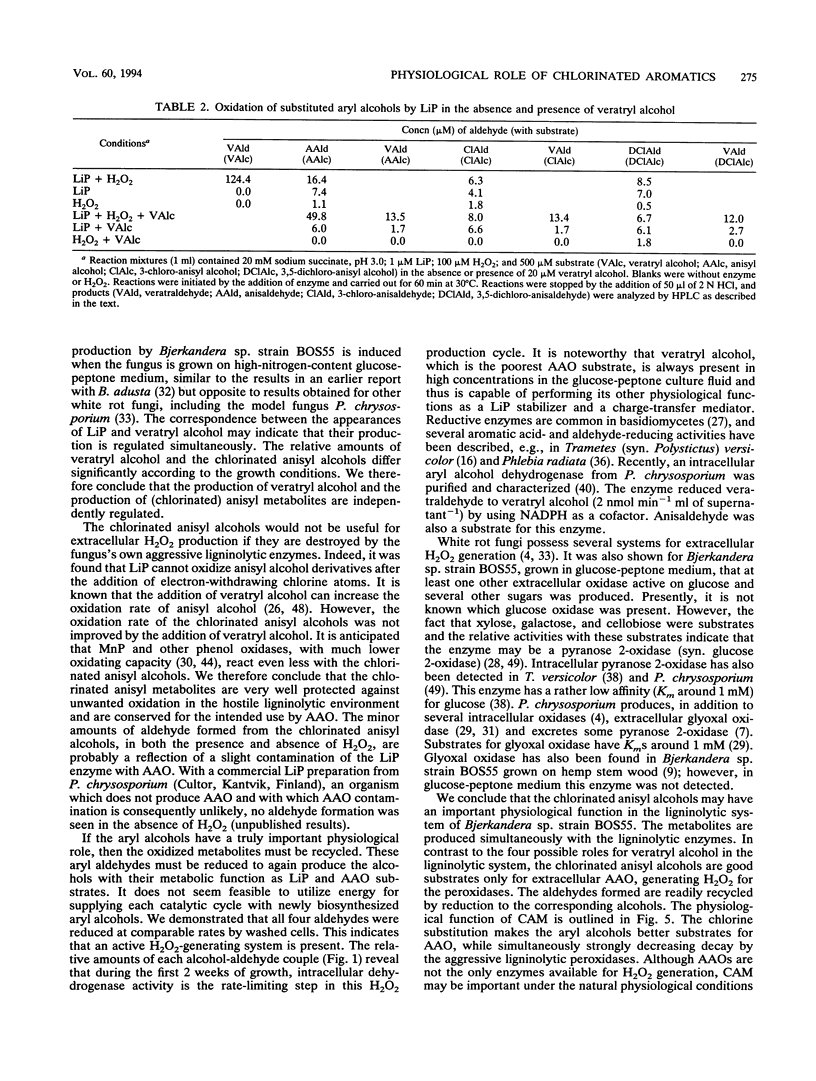
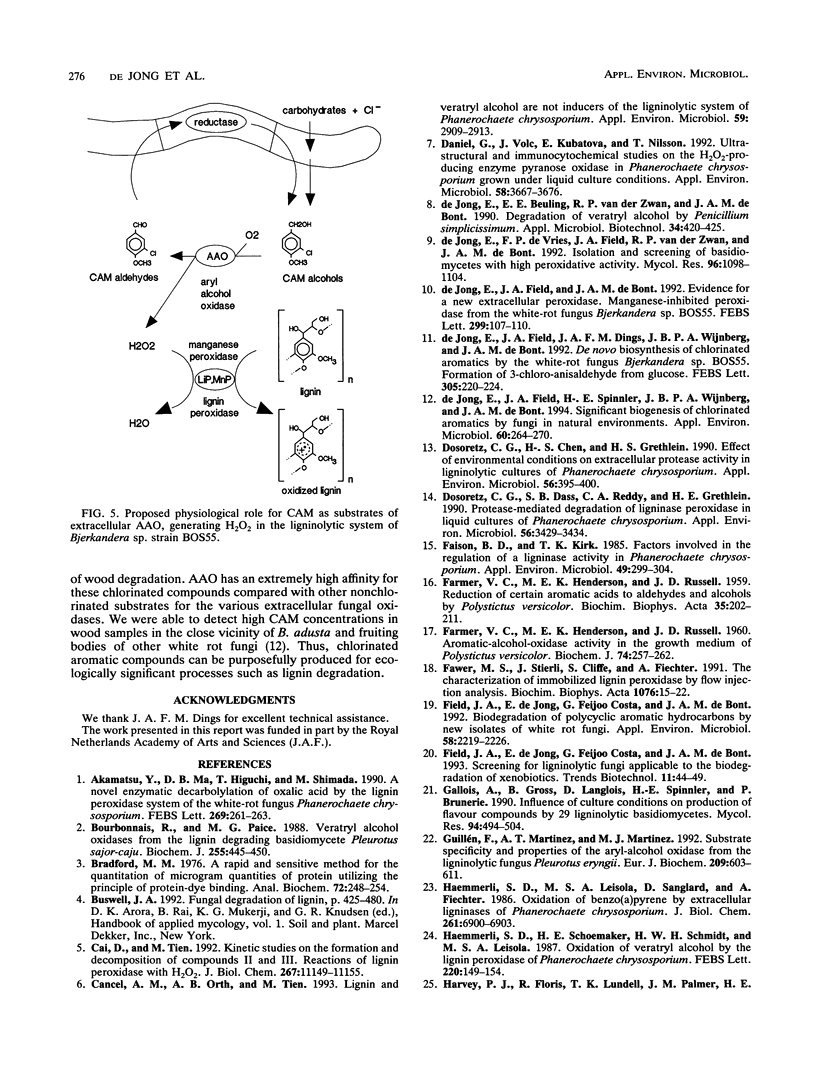
The white rot fungus Bjerkandera sp. strain BOS55 produces veratryl, anisyl, 3-chloroanisyl, and 3,5-dichloroanisyl alcohol and the corresponding aldehydes de novo from glucose. All metabolites are produced simultaneously with the extracellular ligninolytic enzymes and have an important physiological function in the fungal ligninolytic system. Both mono- and dichlorinated anisyl alcohols are distinctly better substrates for the extracellular aryl alcohol oxidases than veratryl alcohol. The aldehydes formed are readily recycled by reduction by washed fungal mycelium, thus creating an extracellular H2O2 production system regulated by intracellular enzymes. Lignin peroxidase does not oxidize the chlorinated anisyl alcohols either in the absence or in the presence of veratryl alcohol. It was therefore concluded that the chlorinated anisyl alcohols are well protected against the fungus's own aggressive ligninolytic enzymes. The relative amounts of veratryl alcohol and the chlorinated anisyl alcohols differ significantly according to the growth conditions, indicating that production of veratryl alcohol and the production of the (chlorinated) anisyl metabolites are independently regulated. We conclude that the chlorinated anisyl metabolites biosynthesized by the white rot fungus Bjerkandera sp. strain BOS55 can be purposefully produced for ecologically significant processes such as lignin degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamatsu Y., Ma D. B., Higuchi T., Shimada M. A novel enzymatic decarboxylation of oxalic acid by the lignin peroxidase system of white-rot fungus Phanerochaete chrysosporium. FEBS Lett. 1990 Aug 20;269(1):261–263. doi: 10.1016/0014-5793(90)81169-o. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Veratryl alcohol oxidases from the lignin-degrading basidiomycete Pleurotus sajor-caju. Biochem J. 1988 Oct 15;255(2):445–450. doi: 10.1042/bj2550445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai D., Tien M. Kinetic studies on the formation and decomposition of compounds II and III. Reactions of lignin peroxidase with H2O2. J Biol Chem. 1992 Jun 5;267(16):11149–11155. [PubMed] [Google Scholar]
- Cancel A. M., Orth A. B., Tien M. Lignin and veratryl alcohol are not inducers of the ligninolytic system of Phanerochaete chrysosporium. Appl Environ Microbiol. 1993 Sep;59(9):2909–2913. doi: 10.1128/aem.59.9.2909-2913.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G., Volc J., Kubatova E., Nilsson T. Ultrastructural and Immunocytochemical Studies on the H(2)O(2)-Producing Enzyme Pyranose Oxidase in Phanerochaete chrysosporium Grown under Liquid Culture Conditions. Appl Environ Microbiol. 1992 Nov;58(11):3667–3676. doi: 10.1128/aem.58.11.3667-3676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Chen H. C., Grethlein H. E. Effect of Environmental Conditions on Extracellular Protease Activity in Lignolytic Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Feb;56(2):395–400. doi: 10.1128/aem.56.2.395-400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Dass S. B., Reddy C. A., Grethlein H. E. Protease-mediated degradation of lignin peroxidase in liquid cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Nov;56(11):3429–3434. doi: 10.1128/aem.56.11.3429-3434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER V. C., HENDERSON M. E., RUSSELL J. D. Aromatic-alcohol-oxidase activity in the growth medium of Polystictus versicolor. Biochem J. 1960 Feb;74:257–262. doi: 10.1042/bj0740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER V. C., HENDERSON M. E., RUSSELL J. D. Reduction of certain aromatic acids to aldehydes and alcohols by Polystictus versicolor. Biochim Biophys Acta. 1959 Sep;35:202–211. doi: 10.1016/0006-3002(59)90349-x. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawer M. S., Stierli J., Cliffe S., Fiechter A. The characterisation of immobilised lignin peroxidase by flow injection analysis. Biochim Biophys Acta. 1991 Jan 8;1076(1):15–22. doi: 10.1016/0167-4838(91)90214-k. [DOI] [PubMed] [Google Scholar]
- Field J. A., de Jong E., Feijoo Costa G., de Bont J. A. Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white rot fungi. Appl Environ Microbiol. 1992 Jul;58(7):2219–2226. doi: 10.1128/aem.58.7.2219-2226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén F., Martínez A. T., Martínez M. J. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem. 1992 Oct 15;209(2):603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Harvey P. J., Floris R., Lundell T., Palmer J. M., Schoemaker H. E., Wever R. Catalytic mechanisms and regulation of lignin peroxidase. Biochem Soc Trans. 1992 May;20(2):345–349. doi: 10.1042/bst0200345. [DOI] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Purification and characterization of glucose oxidase from ligninolytic cultures of Phanerochaete chrysosporium. J Bacteriol. 1986 Apr;166(1):269–274. doi: 10.1128/jb.166.1.269-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J. Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2936–2940. doi: 10.1073/pnas.87.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kalyanaraman B., Hammel K. E., Reinhammar B., Kirk T. K. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990 Jun 1;268(2):475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Asada Y., Kuwahara M. Screening of basidiomycetes for lignin peroxidase genes using a DNA probe. Appl Microbiol Biotechnol. 1990 Jan;32(4):436–442. doi: 10.1007/BF00903779. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Lundell T., Leonowicz A., Rogalski J., Hatakka A. Formation and Action of Lignin-Modifying Enzymes in Cultures of Phlebia radiata Supplemented with Veratric Acid. Appl Environ Microbiol. 1990 Sep;56(9):2623–2629. doi: 10.1128/aem.56.9.2623-2629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheim A., Waldner R., Sanglard D., Reiser J., Schoemaker H. E., Leisola M. S. Purification and properties of an aryl-alcohol dehydrogenase from the white-rot fungus Phanerochaete chrysosporium. Eur J Biochem. 1991 Jan 30;195(2):369–375. doi: 10.1111/j.1432-1033.1991.tb15715.x. [DOI] [PubMed] [Google Scholar]
- Paszczynski A., Crawford R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- Popp J. L., Kalyanaraman B., Kirk T. K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990 Nov 20;29(46):10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- Popp J. L., Kirk T. K. Oxidation of methoxybenzenes by manganese peroxidase and by Mn3+. Arch Biochem Biophys. 1991 Jul;288(1):145–148. doi: 10.1016/0003-9861(91)90176-j. [DOI] [PubMed] [Google Scholar]
- Sannia G., Limongi P., Cocca E., Buonocore F., Nitti G., Giardina P. Purification and characterization of a veratryl alcohol oxidase enzyme from the lignin degrading basidiomycete Pleurotus ostreatus. Biochim Biophys Acta. 1991 Jan 23;1073(1):114–119. doi: 10.1016/0304-4165(91)90190-r. [DOI] [PubMed] [Google Scholar]
- Shah M. M., Grover T. A., Barr D. P., Aust S. D. On the mechanism of inhibition of the veratryl alcohol oxidase activity of lignin peroxidase H2 by EDTA. J Biol Chem. 1992 Oct 25;267(30):21564–21569. [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Oxidation of monomethoxylated aromatic compounds by lignin peroxidase: role of veratryl alcohol in lignin biodegradation. Biochemistry. 1990 Sep 18;29(37):8535–8539. doi: 10.1021/bi00489a005. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem. 1992 Nov 25;267(33):23688–23695. [PubMed] [Google Scholar]
- de Jong E., Field J. A., Dings J. A., Wijnberg J. B., de Bont J. A. De-novo biosynthesis of chlorinated aromatics by the white-rot fungus Bjerkandera sp. BOS55. Formation of 3-chloro-anisaldehyde from glucose. FEBS Lett. 1992 Jul 6;305(3):220–224. doi: 10.1016/0014-5793(92)80672-4. [DOI] [PubMed] [Google Scholar]
- de Jong E., Field J. A., Spinnler H. E., Wijnberg J. B., de Bont J. A. Significant biogenesis of chlorinated aromatics by fungi in natural environments. Appl Environ Microbiol. 1994 Jan;60(1):264–270. doi: 10.1128/aem.60.1.264-270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E., Field J. A., de Bont J. A. Evidence for a new extracellular peroxidase. Manganese-inhibited peroxidase from the white-rot fungus Bjerkandera sp. BOS 55. FEBS Lett. 1992 Mar 24;299(1):107–110. doi: 10.1016/0014-5793(92)80111-s. [DOI] [PubMed] [Google Scholar]



