Abstract
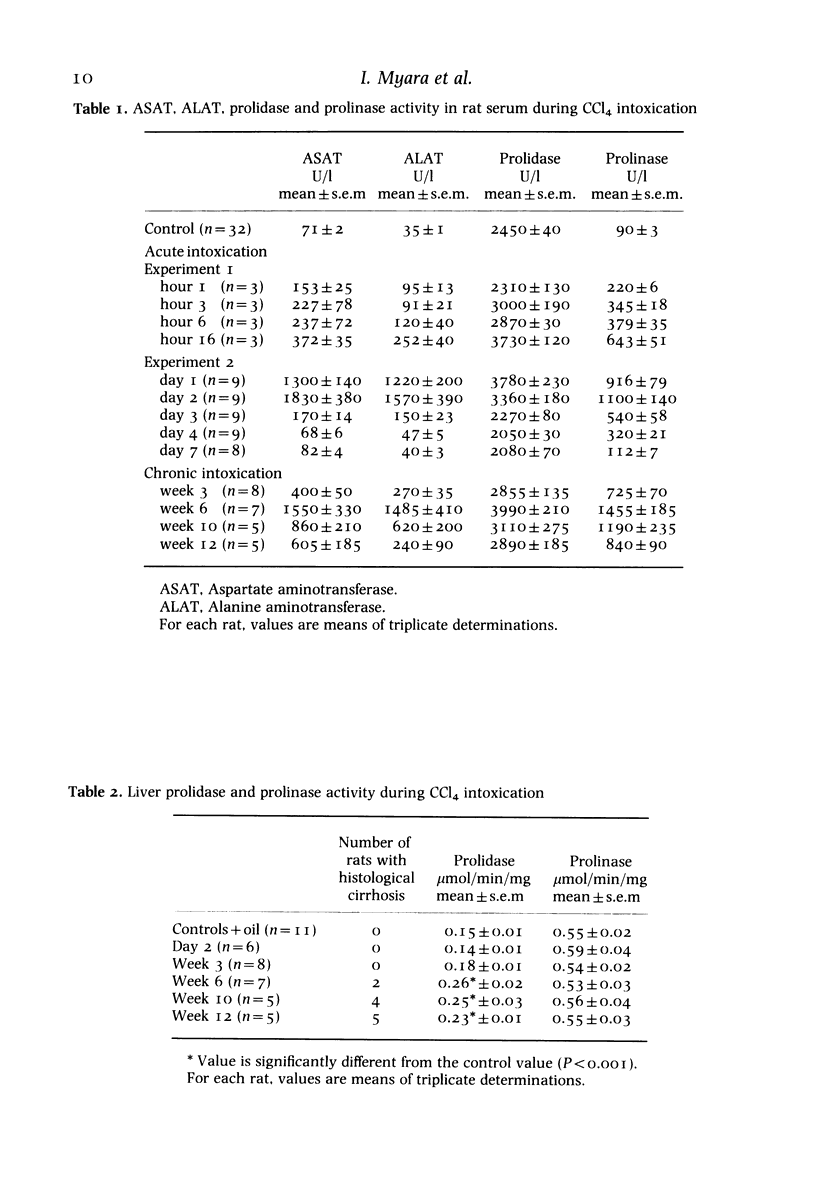
In earlier papers, we reported that the activity of prolidase (EC 3.4.13.9) increased in the plasma of patients with cirrhosis, while that of serum prolinase (EC 3.4.13.8) was normal and was affected only by necrosis. In this work, we investigated prolinase and prolidase activity during short and long-term CCL4 administration in the rat. After a single dose, prolinase activity increased in serum faster than did prolidase activity and it also decreased more slowly. Within the liver, no significant change in these two enzyme activities was observed during the acute phase of necrosis. During chronic CCl4 intoxication, the rises in prolidase and prolinase activity in rat serum were difficult to interpret, because of the liver necrosis present throughout the experiment. However, within the liver, prolinase activity was not affected, unlike that of prolidase which rose at week 3, reached a maximum value at week 6 (reversible fibrosis) and remained elevated at weeks 10 and 12 (irreversible fibrosis). The increase in prolidase activity was specific for liver and was not observed in other tissues. These results are in agreement with those obtained in humans; they highlight the possible physiological significance of enhanced liver prolidase activity during the fibrotic process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolarin D. M., Savolainen E. R., Kivirikko K. I. Three serum markers of collagen biosynthesis in Nigerians with cirrhosis and various infectious diseases. Eur J Clin Invest. 1984 Apr;14(2):90–95. doi: 10.1111/j.1365-2362.1984.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Ehrinpreis M. N., Giambrone M. A., Rojkind M. Liver proline oxidase activity and collagen synthesis in rats with cirrhosis induced by carbon tetrachloride. Biochim Biophys Acta. 1980 Apr 17;629(1):184–193. doi: 10.1016/0304-4165(80)90277-9. [DOI] [PubMed] [Google Scholar]
- Frei A., Zimmermann A., Weigand K. The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology. 1984 Sep-Oct;4(5):830–834. doi: 10.1002/hep.1840040505. [DOI] [PubMed] [Google Scholar]
- Hirayama C., Hiroshige K., Masuya T. Hepatic collagenolytic activity in rats after carbon tetrachloride poisoning. Biochem J. 1969 Dec;115(4):843–847. doi: 10.1042/bj1150843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F., Murawaki Y., Hirayama C. Collagenase activity in the granulocytes of patients with various liver diseases. Clin Chim Acta. 1983 Dec 15;135(2):135–142. doi: 10.1016/0009-8981(83)90128-6. [DOI] [PubMed] [Google Scholar]
- Kuutti-Savolainen E. R., Risteli J., Miettinen T. A., Kivirikko K. I. Collagen biosynthesis enzymes in serum and hepatic tissue in liver disease. I. Prolyl hydroxylase. Eur J Clin Invest. 1979 Feb;9(1):89–95. doi: 10.1111/j.1365-2362.1979.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Lindblad W. J., Fuller G. C. Hepatic collagenase activity during carbon tetrachloride induced fibrosis. Fundam Appl Toxicol. 1983 Jan-Feb;3(1):34–40. doi: 10.1016/s0272-0590(83)80170-5. [DOI] [PubMed] [Google Scholar]
- Lindgren S., Laurell A. B., Eriksson S. Complement components and activation in primary biliary cirrhosis. Hepatology. 1984 Jan-Feb;4(1):9–14. doi: 10.1002/hep.1840040102. [DOI] [PubMed] [Google Scholar]
- Okazaki I., Maruyama K. Collagenase activity in experimental hepatic fibrosis. Nature. 1974 Nov 1;252(5478):49–50. doi: 10.1038/252049a0. [DOI] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Activities of prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase in the liver of rats with hepatic injury. Biochem J. 1974 Oct;144(1):115–122. doi: 10.1042/bj1440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H., Vargas L., Hahn E., Kalbfleisch H., Bruguera M., Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979 Dec;9(6):451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Siegel R. C., Chen K. H., Greenspan J. S., Aguiar J. M. Biochemical and immunochemical study of lysyl oxidase in experimental hepatic fibrosis in the rat. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2945–2949. doi: 10.1073/pnas.75.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H. D., Keiser H. R., Sjoerdsma A. Proline-hydroxylase activity in human blood. Lancet. 1970 Jan 17;1(7638):106–109. doi: 10.1016/s0140-6736(70)90463-0. [DOI] [PubMed] [Google Scholar]
- Zuyderhoudt F. M., Brugman A. M., Smit J. J., de Jong L. Plasma prolidase in the rat: no index of liver fibrosis. Clin Chem. 1985 Apr;31(4):662–662. [PubMed] [Google Scholar]


