Abstract
C-reactive protein (CRP), the prototypic acute-phase reactant in humans, is synthesized in liver in response to a wide variety of inflammatory stimuli. We have generated a line of transgenic mice that express rabbit CRP from the rat phosphoenolpyruvate carboxykinase (PEPCK) promoter in response to gluconeogenic signals. Here we show that transgenic mice expressing high levels of CRP were partially protected from a lethal challenge of bacterial lipopolysaccharide compared with littermates in which CRP expression had been suppressed. Similar protection was observed with challenges from platelet-activating factor (PAF) and the combination of tumor necrosis factor α (TNF-α) plus interleukin 1β, but not with TNF-α alone. We further demonstrate that although PAF was able to bind CRP, the mechanism by which CRP provides protection probably does not involve sequestration of PAF. The biologically inactive precursor of PAF, lyso-PAF, also bound CRP but did not render the transgenic mice sensitive to PAF when CRP-expressing animals were simultaneously challenged with PAF and an excess of lyso-PAF. These results suggest that CRP functions in vivo by modulating host defense systems.
C-reactive protein (CRP) is the prototypic human acute-phase reactant synthesized rapidly in liver in response to inflammatory stimuli (for reviews, see refs. 1–4). In humans and rabbits, CRP is normally present in serum at concentrations of less than 0.5 μg/ml, with levels increasing as much as 1,000-fold following inflammatory stimuli. CRP is a cyclic pentamer composed of five noncovalently bound, identical 23.5-kDa subunits that is capable of binding a number of biologically significant ligands in vitro, including phosphocholine (PC) and other phosphate ester-containing compounds (2, 4), with consequent activation of the complement system by the classical pathway (5). CRP has been reported to bind to monocytes (6, 7) and neutrophils (8) cells. These properties, combined with a high degree of evolutionary conservation, including the presence of CRP-like molecules in primitive invertebrates, imply that CRP plays a significant role in host defense systems (4, 9).
To define the in vivo functional role of CRP during acute inflammatory states, we have established a strain of transgenic mice that expresses rabbit CRP from the promoter for the rat cytosolic form of phosphoenolpyruvate carboxykinase (PEPCK) (10). The mouse was selected as the host for this transgene because this species is unusual among mammals, in that CRP levels remain low, <2 μg/ml, even after an acute inflammatory stimulus (11). Rabbit CRP synthesis in these transgenic mice responds to gluconeogenic signals such as protein-rich diets; circulating CRP levels as high as 300 μg/ml were observed 18–24 hr after shift from a carbohydrate-rich diet to an isocaloric protein-rich diet and were maintained for 2–3 days thereafter. The CRP expressed in these transgenic mice was found to be structurally and functionally indistinguishable from native pentameric rabbit CRP (10).
It is widely held that the bacterial cell wall constituent, endotoxin or bacterial lipopolysaccharide (LPS), is the causative agent of septic shock induced by Gram-negative bacteria (reviewed in ref. 12). Circulating levels of as little as a few micrograms per milliliter can activate leukocytes and lead to fever, hypotension, tachycardia, and tachypnea. Shock can progress rapidly and may result in multiple organ damage and death within hours (13, 14). Much of this damage comes not from the invading pathogen, but from the body’s complex reaction to it. In blood, LPS binds to the LPS binding protein and the complex interacts with CD14 receptors on several inflammatory cell types (15). In response to this interaction, inflammatory mediators such as platelet-activating factor (PAF), interleukin 1β (IL-1β), and tumor necrosis factor α (TNF-α) are rapidly produced. These mediators are themselves capable of inducing a shock syndrome similar to that produced by LPS (16). Endogenous factors such as interleukin 1 receptor antagonist (IL-1ra) (17, 18) and interleukin 4, secreted during inflammatory states, can reduce this supersensitive reaction (13). Other antagonists to these mediators, such as antisera to TNF-α or IL-1, or inhibitors of the processing enzymes that generate the mature cytokines, have been shown in model systems to block LPS-induced septic shock (reviewed in ref. 19). Here we report that rabbit CRP protects transgenic mice from mortality induced by LPS by the combination of IL-1β and TNF-α as well as by PAF. This protection appears to result from alterations in host defense mechanisms rather than from interactions of CRP with either LPS or PAF.
MATERIALS AND METHODS
Materials.
LPS, from Escherichia coli serotype 055:B5, PAF, Lyso-PAF, and d-galactosamine were purchased from Sigma. Human recombinant TNF-α and human recombinant IL-1β were gifts from N. Staite (Upjohn). Phosphocholine linked to BSA (PC-BSA) was a gift from F. Robey (National Institutes of Health, Bethesda, MD).
Animals.
PC-12 mice were produced as described (10) and contained a transgene (PEPCK-CRP) consisting of the protein-coding region of the rabbit CRP gene linked to the promoter/regulatory region of the cytosolic form of the rat PEPCK gene. Animals were maintained on normal lab chow to 2–3 months of age (20–25 g). For experiments, animals were provided a carbohydrate-rich diet (10) for 4–7 days, which suppressed transgene expression to levels <20 μg/ml. To stimulate transgene expression, randomly selected animals on the carbohydrate-rich diet were shifted to an isocaloric protein-rich diet (20). When tested 18–24 hr after the dietary shift, expression of CRP in plasma was typically 75–200 μg/ml, with high levels being maintained for 2–3 days before declining (10). Outbred, Swiss Webster CF1 mice (Charles River Breeding Laboratories) served as controls. The transgenic founder animals (B6.SJL F1s) were outbred to CF1s prior to sibling matings to generate lines homozygous for the PEPCK-CRP transgene. CF1 mice were maintained on the same diet regimens as the transgenic mice in parallel in all experiments. Animal care for all experiments was according to institutional guidelines.
CRP assays.
The circulating level of rabbit CRP in transgenic mice was determined in 50- to 100-μl blood samples collected by retroorbital bleeding 30–60 min prior to challenge. Only one sample was taken from each animal. Because the CRP response to diet is variable (10), the measured CRP level, prior to challenge, may not represent the peak CRP response achieved. CRP concentrations were determined by a radial immunodiffusion assay in agarose, as described (21), employing a goat anti-rabbit CRP antiserum specific for native rabbit CRP. This method is sensitive to levels as low as 1–2 μg/ml.
Induction of endotoxic shock.
Agents in 0.9% sterile saline were injected either i.p. or i.v. into groups of 4–6 animals at the following doses. For LPS, three preparations were employed. With two preparations, doses of 15–18 mg/kg (usually in 2.05 mg/kg increments) were used; with the third, 23–27 mg/kg was required to observe lethal effects. The reason for this variability was unclear. In GalN sensitized mice, LPS challenges of 0.45–1.1 μg/kg were employed using the LPS preparations described above (225 ng/kg increments). For PAF, doses usually ranged from 30 to 40 μg/kg (5 μg/kg increments) with 45 μg/kg being employed in two experiments. For TNF-α, aliquots of a single preparation were employed with doses ranging from 1 to 2.5 mg/kg. For the combination of TNF-α and IL-1β, a constant amount of IL-1β (45 μg/kg) was employed, whereas the dose of TNF-α was varied between 250 and 350 μg/kg in 50 μg/kg increments. For all experiments, low CRP animals were maintained on the carbohydrate-rich diet and high CRP levels were induced by a shift to the protein-rich diet 18–24 hr before challenge.
Detection of CRP binding to PAF.
Binding of CRP to PAF and to lyso-PAF was demonstrated using an ELISA that measures competition for binding to PC-BSA (22). Microtiter wells were coated with 25 ng of PC-BSA in coating buffer (0.1 M sodium carbonate, pH 9.6), followed by blocking with 2% BSA (in coating buffer). Plates were incubated with 25 ng biotinyl-CRP standard or biotinyl-CRP mixed with varying concentrations of competitor (PC, PAF, or lyso-PAF) in 100 μl TBS Ca+2 (10 mM Tris·HCl, pH 7.5/0.15 M NaCl/1 mM CaCl2). Excess CRP or CRP-competitor mixture was washed away, and the remaining biotinyl-CRP was detected with alkaline phosphatase-linked strepavidin and colorimetric substrates for the linked enzyme. The assay yielded an apparent Ki for inhibition with PC of 4 μM in close agreement with previous measurements (22).
Biotinylation of rabbit CRP was performed as described (10). Rabbit CRP [400 μg; 1.4 mg/ml in Hepes buffered normal saline (HBNS; 20 mM Hepes, pH 7.4/0.15 M NaCl)] was incubated at room temperature for 60 min with 4 μg NHS-LC-Biotin (1 mg/0.25 ml in HBNS). Glycine (5 μl; 1 M in HBNS) was added and incubated an additional 60 min followed by dialysis against HBNS.
Transgenic CRP purification.
Serum was obtained from PC-12 animals following heart puncture 24 hr after the shift from a carbohydrate-rich to a protein-rich diet to induce CRP synthesis. SAP and other nonspecific Sepharose-binding proteins were removed by passing the sera over a Sepharose-4B column (50 ml; Pharmacia) in TBS Ca+2. CRP was purified from the eluate by passage through a PC-Sepharose affinity column (1 ml; Sigma) eluting with TBS/10 mM citrate. The resulting material appeared homogenous following Coomassie blue staining of material fractionated by SDS/PAGE. For passive immunization, 100 μg of transgenic CRP was administrated i.v. 30 min before a PAF challenge or mixed with PAF and simultaneously injected.
RESULTS
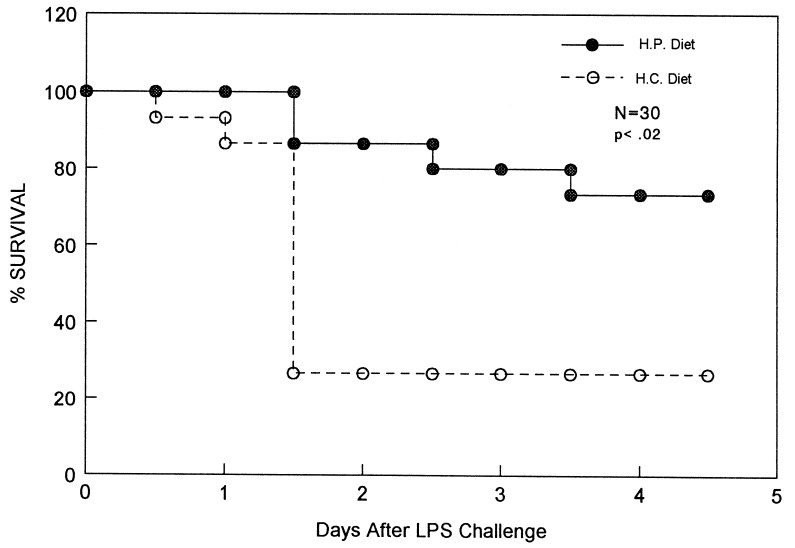
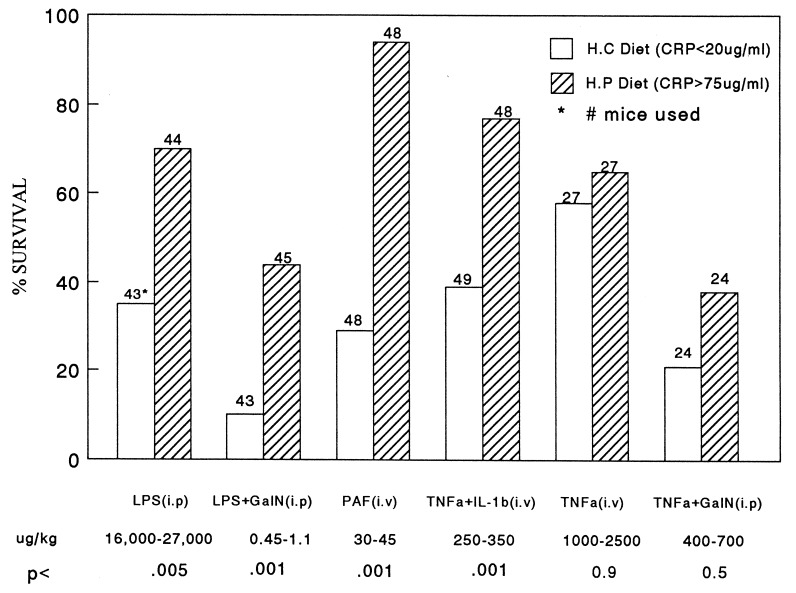
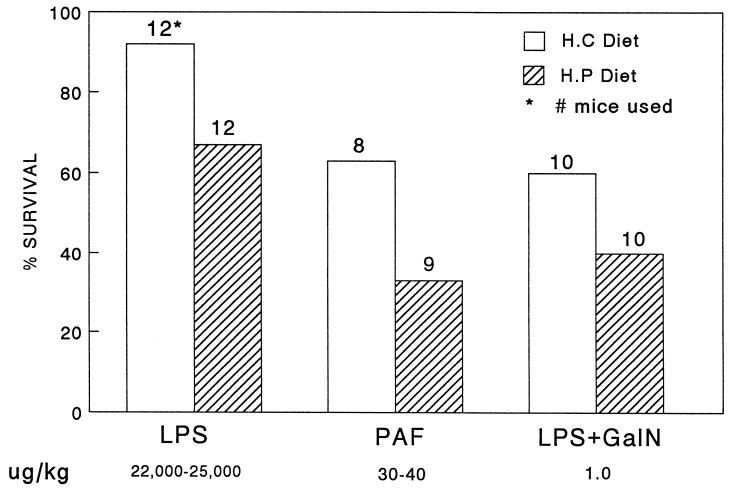
Fig. 1 shows a typical experiment in which 15 CRP-expressing PC-12 transgenic mice (provided with a protein-rich diet 24 hr before challenge) were found to be more resistant to i.p. injection of LPS than were transgenic littermates expressing low levels of CRP (animals maintained on a carbohydrate-rich diet). Groups of 10 animals, 5 on the high protein diet and 5 on the high carbohydrate diet, received injections of LPS of either 16, 17.5, or 18 mg/kg. Serum CRP levels were >75 μg/ml in the high protein-fed group and <20 μg/ml in the high carbohydrate-fed group, in a single sample obtained 30–60 min before LPS challenge. Of 87 mice analyzed following injection of 16–27 mg/kg LPS (Fig. 2), 70% (31/44) of the animals expressing high levels of CRP in response to the protein-rich diet survived the challenge over the course of 5 days, compared with 35% (15/43) of the mice expressing low levels of CRP in response to the carbohydrate-rich diet (P < .005). Nontransgenic CF1 control mice maintained on the same regimens of protein-rich or carbohydrate-rich diets as the transgenic mice were equally sensitive to LPS challenges (Fig. 3). The results imply that CRP in transgenic mice is responsible for the relative resistance to LPS-induced mortality.
Figure 1.
Survival following endotoxin (LPS) injection in transgenic mice expressing or not expressing CRP. Fifteen PEPCK-CRP transgenic mice, strain PC-12, were maintained on a carbohydrate-rich diet (dashed line), while another 15 transgenic mice were provided an isocaloric protein-rich diet (solid line) for the 24 hr prior to i.p. injection of 16, 17.5, or 18 μg/kg endotoxin (LPS) in 0.5 ml 0.9% NaCl. Survival of the animals was followed over the next 7 days. PC-12 mice on the protein-rich diet showed serum CRP levels of 75–200 μg/ml, and those on the carbohydrate-rich diet had serum levels of <20 μg/ml during this period. Serum CRP levels were measured with a specific radial-immunodiffusion assay as described (10). The data shown are from a representative experiment. The survival difference between the two groups showed a P < 0.02 calculated by the chi square test with Yates’ correction. A summary of all experiments is shown in Fig. 2.
Figure 2.
Survival following injection of endotoxin (LPS), PAF, or cytokine mediators of endotoxic shock in transgenic mice expressing or not expressing CRP. This figure summarizes results of all experiments employing all agonists. The protocols were as described in the legend to Fig. 1. Dietary manipulations began 18–24 hr before the administration of agonists. The total number of animals used in each group is indicated above each bar. Hatched bars represent animals provided with the protein-rich diet and expressing high levels of CRP. Open bars represent animals maintained on the carbohydrate-rich diet and expressing low levels of CRP. GalN, when employed, was injected with the inflammatory agent at a concentration of 15 mg per animal. IL-1β was injected at a dose of 45 μg/kg. The dose range used with each agent is indicated at the bottom of the figure. P values indicated at the bottom of the figure were calculated using the chi square test with Yates’ correction.
Figure 3.
Effect of diet on survival of CF1 control mice following injection of endotoxin (LPS) or PAF. Nontransgenic CF1 mice were maintained on the carbohydrate-rich and protein-rich diets as described for PC-12 transgenic mice in the legend to Fig. 1. The animals were injected with the indicated reagents, and survival was monitored over the next 7 days. GalN, when employed, was injected i.p. at a dose of 15 mg per animal. The number of animals used in each group is indicated above each bar. Hatched bars represent animals provided with the protein-rich diet, and open bars represent animals maintained on the carbohydrate-rich diet.
The effect of high protein-induced CRP levels on mortality was also studied following LPS administration to GalN-sensitized mice, with similar results. GalN, a hepatotoxin that decreases available pyrimidine nucleotide pools for transcription of liver genes, causes an increase in sensitivity to the lethal effects of endotoxin by 100,000-fold (23). As shown in Fig. 2, 44% (20/45) of the animals on the protein-rich diet survived a simultaneous challenge by GalN (15 mg per mouse) and LPS (dose range 0.45–1.1 μg/kg) over a 5-day period compared with 10% (4/43) of the transgenic animals on the carbohydrate-rich diet (P < .001). We conclude that CRP affords relative protection against LPS-induced septic shock in the presence or absence of GalN.
CRP Protection Against Mediators of Endotoxic Shock.
One possible mechanism by which LPS protection could have been achieved in these experiments was by sequestration of LPS by binding to CRP. However, it has been shown that CRP is unable to bind LPS directly, even though the latter contains a phosphate ester (24). We therefore tested the possibility that the observed protection was due to the ability of CRP to block the effects of mediators induced by LPS. LPS stimulates the expression of several inflammatory mediators, most notably TNF-α, IL-1β, and PAF (25). I.v. injection of PAF causes a rapid anaphylactic reaction in mammals (25, 26), which results in death at high doses. Table 1 shows a dose response experiment in which CRP-expressing transgenic mice (provided with a protein-rich diet 24 hr before challenge) were found to be more resistant to i.v. injection of PAF in the range of 15–40 μg/kg than were transgenic mice not expressing CRP (maintained on a carbohydrate-rich diet). At 33 and 40 μg/kg PAF, complete separation was achieved between the CRP-expressing (all 13 mice survived) and nonexpressing (all 10 mice died) groups of animals. Mortality to high-dose lethal challenges with PAF was rapid compared with LPS, with most mice succumbing to the former in 30–60 min compared with 18–24 hr for the latter. Fig. 2 shows a summary of all experiments with PAF challenges between 30 and 45 μg/kg. Ninety-four percent (45/49) of high CRP-expressing animals survived compared with 35% (17/48) of low CRP-expressing animals (P < .001). These results are presumed to be due to CRP being expressed in the transgenic mice, because the same diet regimens provided to nontransgenic CF1 mice, as described above, showed no statistically significant protective effects (Fig. 3).
Table 1.
Dose curve of PAF effects on transgenic mice expressing high levels of CRP
| PAF dose,* μg/kg | Diet | % Survival† |
|---|---|---|
| 15 | Protein-rich | 100 |
| 15 | Carbohydrate-rich | 83 |
| 33 | Protein-rich | 100 |
| 33 | Carbohydrate-rich | 0 |
| 40 | Protein-rich | 100 |
| 40 | Carbohydrate-rich | 0 |
| 48 | Protein-rich | 0 |
| 48 | Carbohydrate-rich | 0 |
*Six animals were challenged in each group.
Survival was measured 60 min after challenge.
In contrast to these findings with PAF, no statistically significant difference in survival was found between transgenic mice expressing or not expressing CRP following challenge with TNF-α in either GalN-sensitized or non-GalN-sensitized animals. The doses employed in these studies ranged between 1 and 2.5 mg/kg for non-GalN-sensitized animals and 0.4 and 0.7 mg/kg in GalN-sensitized mice (Fig. 2). In some experiments we did observe a difference, with high-CRP-expressing mice surviving slightly better than low-CRP-expressing mice, but the window for protection in our animals appeared to be small and difficult to demonstrate consistently. With TNF-α plus IL-1β challenges, however, significant protection by CRP was observed (Fig. 2). In these experiments, the IL-1β dose was constant at 45 ng/kg and TNF-α ranged between 0.25 and 0.35 mg/kg. Seventy-seven percent (37/48) of the mice survived in the high-CRP-expressing groups compared with 39% (18/49) in the low-CRP-expressing groups (P < .001).
PAF Protection Is Probably Not Mediated Through CRP Binding.
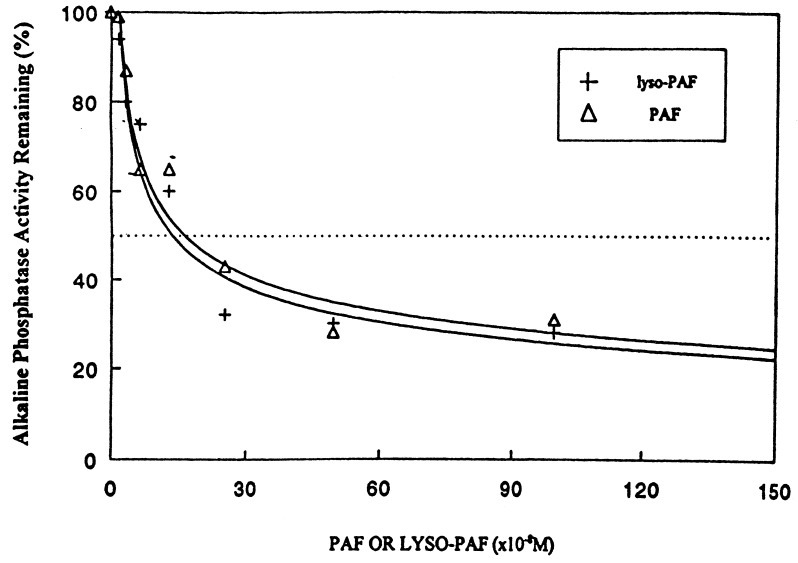
One hypothesis that would explain our results is that CRP protection against PAF- and LPS-mediated shock was due to binding of PAF by CRP, with consequent inactivation of PAF by sequestration. PAF is a glycerolipid that contains a phosphocholine moiety (27) and has been previously demonstrated to bind CRP at the PC binding site at PAF concentrations below its critical micelle concentration (28). To test whether protection from PAF lethality was due to CRP binding, we employed excess lyso-PAF to determine whether it would increase the toxicity of PAF by competing for CRP binding. Lyso-PAF is a nonacetylated precursor of PAF that contains a PC moiety but that has no biologic activity (29). Fig. 4 shows results of an ELISA that measures the ability of PAF and lyso-PAF to inhibit CRP binding to PC-BSA, employing biotinylated CRP detected with alkaline phosphatase-linked strepavidin (22). As shown in Fig. 4, both PAF and lyso-PAF efficiently bound CRP and were indistinguishable in their abilities to compete for CRP binding to PC-BSA. Both had an apparent Ki of inhibition of 150 nM.
Figure 4.
PC-BSA competitive ELISA for PAF and lyso-PAF binding to CRP. An ELISA that measures CRP binding to PC-BSA was modified from that previously described (22) to determine whether PAF or lyso-PAF could compete for CRP binding.
We next did an experiment in which lyso-PAF (225 μg/kg) was injected into groups of high-CRP-expressing transgenic mice at the same time as a PAF challenge (40 μg/kg). Lyso-PAF had no effect on the ability of CRP-expressing mice to survive the PAF challenge. Of those animals expressing CRP, 78% (15/18) survived the PAF challenge in the presence of lyso-PAF compared with 33% (6/18) of littermates that were not expressing CRP at the time of lyso-PAF plus PAF administration. Assuming that both lyso-PAF and PAF bind CRP through their PC moieties at the known single PC binding site on CRP (30), this result implies that the protective effect seen in the CRP-expressing animals was not due to sequestration of PAF by PC binding to CRP. Assuming a maximal CRP response in these animals of 200 μg/ml and a 1 ml serum volume, we calculated the concentration of PC binding sites on CRP to be 8 μM and the circulating concentration of lyso-PAF to be 9.3 μM. Thus, the concentration of lyso-PAF was likely to be in excess of that of CRP and above the Ki. The experiments used a relatively high dose of PAF, 40 μg/kg (see Table 1), to accentuate any possible effects of lyso-PAF if it was able to interfere with CRP-mediated protection. The ability of CRP, even in the presence of excess lyso-PAF, to provide protection from a PAF challenge suggests that sequestration of PAF by CRP is not essential for the effect. These data suggest that the in vivo effect of CRP on LPS, PAF, and TNF-α plus IL-1β-generated septic shock is not mediated by direct binding of CRP to PAF.
Passive Administration of CRP.
We attempted to duplicate the partial protection to PAF-induced lethality we observed in transgenic mice by injecting CRP into animals not expressing CRP. In these experiments, 100 μg of CRP, purified from PC-12 transgenic mice as described in Materials and Methods, was injected either 30 min prior to or simultaneously with PAF in doses between 30 and 40 μg/kg. In contrast to our results in transgenic mice, passive administration of CRP did not provide any significant protection to PAF-induced mortality in either of these experiments.
DISCUSSION
Our major conclusion is that mice expressing high levels of transgenic rabbit CRP are partially protected against lethal challenges by mediators of septic shock, including LPS, PAF, and the combination of TNF-α plus IL-1β, in the presence or absence of d-galactosamine.
Protection against endotoxic shock in mice has been demonstrated with several agents, including antibodies to portions of LPS itself, a mode of treatment, however, that is dependent on the bacterial species from which the LPS was derived. More general protection has previously been achieved by anticytokine treatments such as IL-1ra, soluble cytokine receptors, and antibodies to TNF-α (19). These therapeutic successes have contributed to the perception that sepsis is a condition in which excessive cytokine production leads to lethality (16). CRP has no reported direct anticytokine effects, but our data are consistent with a model in which the cascade of detrimental cytokine stimulation induced by LPS is blocked by CRP.
Several reports have demonstrated that CRP can stimulate cytokine production in vitro in peripheral blood mononuclear cells (PBMC) (31) as well as in alveolar macrophages (32), in apparent contradiction to our speculations. However, in PBMCs, CRP has also been found to stimulate IL-1ra production to a greater extent than it stimulates IL-1β production (33). Thus, the net antiinflammatory properties of CRP we observe in vivo may be due, at least in part, to the induction of this or other natural antagonists by CRP.
CRP has previously been shown to effectively prevent mortality from pneumoccocal challenges (34, 35). In these experiments, a bolus injection of either human or rabbit CRP provided protection against a subsequent, potentially lethal injection with pneumoccoci of several serotypes. Similar results were obtained with transgenic mice expressing human CRP (36). The conclusion from these studies was that it was likely that the opsonic properties of CRP were responsible for its protective effects (34, 36).
We were unable to protect nontransgenic mice from mediators of septic shock by injection of transgenic CRP isolated from PC-12 mice. The reason for this failure is unclear but may be due to an inability to attain a sufficiently high concentration of circulating CRP to observe an effect with LPS or PAF as the agonist. CRP is rapidly degraded after injection, with a half-life of 30–60 min (10) in mice. If 100 μg of CRP were injected, its concentration could be as low as 25 μg/ml during the period of response to agonist. This may not be adequate.
The possibility of direct binding of LPS to CRP has been evaluated; binding was not demonstrated (24). CRP binds PAF and lyso-PAF with equal affinity, but an excess of the latter had no effect on the protective action of CRP against the former in vivo, indicating that a mechanism other than PAF binding was probably preventing death. This conclusion must be tempered by the recognition that we do not know the exact level of lyso-PAF excess to CRP because the timing and magnitude of the CRP response in the transgenic animals is variable (10) and our single sampling may not be at its peak. Our calculations are based on a maximal CRP response that, if anything, underestimates the actual level of lyso-PAF excess. We also do not know whether PAF and lyso-PAF actually compete for the same binding site on CRP in vivo, although it is likely that the PC moiety in each case binds to the single PC binding site on each CRP subunit (30). Similarly, we do not know whether the half-lives of PAF and lyso-PAF are identical in our animals. These limitations stated, the most straightforward interpretation of our data is that CRP affords protection to PAF toxicity through a binding-independent mechanism. PAF-mediated septic shock is primarily triggered by disseminated clot formation, but other cytokine-induced cascades may contribute and it may be the latter that is CRP-sensitive.
It is still formally possible that our observations are due to effects of the diets employed and not CRP, because the CF1 strain used for the diet control is not identical to the PC-12 transgenic line. This possibility is, in our view, highly unlikely. First, the Swiss-Webster CF1 mice chosen for use as the diet control are genetically related to the PC-12 transgenic strain in that the PEPCK-CRP transgenic founder animal was outbred to CF1 mice. CF1 is not an inbred line and was used for outbreeding because it is genetically variable and not prone to deficiencies and susceptibilities in its immune or inflammatory responses. It serves as the control because the PC-12 transgenic strain is homozygous for the transgene and therefore unique. Second, except for carbohydrate source, the protein and carbohydrate-rich diets are nearly identical to standard laboratory chow (20). Both special diets are highly nutritious, consumed normally, and provided for a relatively short period of time—less than 1 week. It is therefore unlikely that such an exposure over such a short period could so radically alter the susceptibilities to challenges with mediators of septic shock.
Our results are consistent with the view that CRP plays a significant role in host defense other than direct opsonization. They suggest that the protection against mediators of septic shock we found is probably not due to simple sequestration or opsonization of mediators by CRP binding, but rather that it reflects an effect on host defense mechanisms.
The putative alternate mechanism by which CRP suppresses PAF toxicity is uncertain. In vitro, CRP has been reported to block PAF-induced calcium mobilization (37), β-glucuronidase production (38), and superoxide release (38) in macrophages. In neutrophils, CRP blocks PAF-induced superoxide release (37), and in platelets, CRP blocks PAF-induced arachadonic acid release and aggregation (39, 40). Freeze-thawed CRP had the opposite effect and augmented the aggregation of PAF-treated platelets (41). Prevention of platelet aggregation by native CRP did not require CRP-PAF binding, because CRP stabilized platelet membranes, thus blocking PAF-mediated aggregation and lysis (40). These PAF antagonistic properties of CRP are consistent with the protective effects we observed in vivo and again indicate that CRP is capable of effects on PAF toxicity by mechanisms other than sequestration of PAF. Finally, CRP has been shown in vitro to bind to and influence the behavior of monocytic cells (42), neutrophils (43, 44), and lymphocytes (45). It is likely that one or more of these properties observed in vitro is responsible for the in vivo effects reported here. As the interaction of CRP and leukocytes is likely to be complex, it is difficult to be precise as to the mechanism of CRP action. One possibility we are currently exploring is that CRP suppresses cytokine synthesis in inflammatory cell types induced by mediators of septic shock or other inflammation-associated agents. This activity would limit the escalation of cytokine synthesis that follows induction of the inflammatory response.
Acknowledgments
We thank Irv Kushner for many helpful discussions, suggestions, and comments related to this manuscript. We thank Debra Rzewnicki for performing the CRP analyses on serum samples and Shun-lin Jiang for help with the data analysis. This work was supported by grants from the Northeast Ohio Multipurpose Arthritis Center and American Heart Association Northeast Ohio unit, and National Institutes of Health Grant AR40765.
ABBREVIATIONS
- CRP
C-reactive protein
- PEPCK
phosphoenolpyruvate carboxykinase
- PC
phosphorylcholine
- LPS
bacterial lipopolysaccharide
- PEPCK-CRP
chimeric gene with rat PEPCK promoter and rabbit CRP
- PAF
platelet-activating factor
- TNF-α
tumor necrosis factor α
- IL-1β
interleukin 1β
- IL-1ra
IL-1 receptor antagonist
References
- 1.Baumann H, Gauldie J. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 2.Gotschlich E C. Ann NY Acad Sci. 1989;557:9–18. [PubMed] [Google Scholar]
- 3.Steel D M, Whitehead A S. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Kilpatrick J M, Volanakis J. In: Acute Phase Proteins, Molecular Biology, Biochemistry and Clinical Applications. Mackiewicz A, Kushner I, Baumann H, editors. Boca Raton, FL: CRC; 1993. pp. 79–92. [Google Scholar]
- 5.Volanakis J E. Ann NY Acad Sci. 1982;389:235–250. doi: 10.1111/j.1749-6632.1982.tb22140.x. [DOI] [PubMed] [Google Scholar]
- 6.Crowell R E, Du-Clos T W, Montoya G, Heaphy E, Mold C. J Immunol. 1991;147:3445–3451. [PubMed] [Google Scholar]
- 7.Tebo J M, Mortensen R F. J Immunol. 1990;144:231–238. [PubMed] [Google Scholar]
- 8.Dobrinich R, Spagnuolo P J. Arthritis Rheum. 1991;34:1031–1038. doi: 10.1002/art.1780340813. [DOI] [PubMed] [Google Scholar]
- 9.Volanakis J E, Xu Y, Macon K J. In: Human C-Reactive Protein and Host Defense in Defense Molecules. Marchalonis J J, Reinisch C L, editors. New York: Wiley; 1990. pp. 161–175. [Google Scholar]
- 10.Lin C S, Xia D, Yun J S, Wagner T, Magnuson T, Mold C, Samols D. Immunol Cell Biol. 1995;73:521–531. doi: 10.1038/icb.1995.82. [DOI] [PubMed] [Google Scholar]
- 11.Pepys M B. Immunology. 1979;37:637–641. [PMC free article] [PubMed] [Google Scholar]
- 12.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Di Padova F, Schreir M, Brade Y. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 13.Bone R C. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 14.Glauser M P, Zanetti G, Baumgartner J-D, Cohen J. Lancet. 1991;338:732–739. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 15.Ulevitch R J, Tobias P S. Curr Opin Immunol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 16.Lowry S F, Moldawer L L. Ann NY Acad Sci. 1993;685:471–482. doi: 10.1111/j.1749-6632.1993.tb35909.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson R C. Nature (London) 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- 18.Wakabayashi G, Gelfand J A, Burke J F, Thompson R C, Dinarello C A. FASEB J. 1991;5:338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- 19.Stone R. Science. 1994;264:365–367. doi: 10.1126/science.8153620. [DOI] [PubMed] [Google Scholar]
- 20.McGrane M M, de Vente J, Yun J, Bloom J, Park E, Wynshaw Boris A, Wagner T, Rottman F M, Hanson R W. J Biol Chem. 1988;263:11443–11451. [PubMed] [Google Scholar]
- 21.Kushner I, Somerville J A. Biochim Biophys Acta. 1970;207:105–114. doi: 10.1016/0005-2795(70)90140-6. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Robey F A. J Immunol Methods. 1983;65:333–341. doi: 10.1016/0022-1759(83)90128-x. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann V, Freudenberg M A, Galanos C. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobias P S, McAdam K P, Soldau K, Ulevitch R J. Infect Immun. 1985;50:73–76. doi: 10.1128/iai.50.1.73-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinovici R, Yue T-L, Farhat M, Smith E F, Esser K M, Slivjak M, Feuerstein G. J Pharmacol Exp Ther. 1990;255:256–263. [PubMed] [Google Scholar]
- 26.Sun X, Hsueh W. J Immunol. 1991;147:509–514. [PubMed] [Google Scholar]
- 27.Prescott S M, Zimmerman G A, McIntyre T M. J Biol Chem. 1990;265:17381–17384. [PubMed] [Google Scholar]
- 28.Filep J G, Herman F, Kelemen E, Foldes-Filep E. Thromb Res. 1991;61:411–421. doi: 10.1016/0049-3848(91)90655-g. [DOI] [PubMed] [Google Scholar]
- 29.Snyder F. Am J Physiol. 1990;259:C697–C708. doi: 10.1152/ajpcell.1990.259.5.C697. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan N, White H E, Emsley J, Wood S P, Pepys M B, Blundell T L. Structure. 1994;2:1017–1027. doi: 10.1016/S0969-2126(94)00105-7. [DOI] [PubMed] [Google Scholar]
- 31.Ballou S P, Lozanski G. Cytokine. 1992;4:361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 32.Galve-de-Rochemonteix B, Wiktorowicz K, Kushner I, Dayer J M. J Leukocyte Biol. 1993;53:439–445. doi: 10.1002/jlb.53.4.439. [DOI] [PubMed] [Google Scholar]
- 33.Tilg H, Vannier E, Vachino G, Dinarello C A, Mier J W. J Exp Med. 1993;178:1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H J, McDaniel L S, Eldridge J, Brooks J. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mold C, Nakayama S, Holzer T J, Gewurz H, Du Clos T W. J Exp Med. 1981;154:1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szalai A J, Briles D E, Volanakis J E. J Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 37.Foldes-Filep E, Filep J G, Sirois P. J Leukocyte Biol. 1992;51:13–18. doi: 10.1002/jlb.51.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Filep J G, Foldes-Filep E. Life Sci. 1989;44:517–524. doi: 10.1016/0024-3205(89)90613-9. [DOI] [PubMed] [Google Scholar]
- 39.Kilpatrick J M, Virella G. Clin Immunol Immunopathol. 1985;37:276–281. doi: 10.1016/0090-1229(85)90159-x. [DOI] [PubMed] [Google Scholar]
- 40.Vigo C. J Biol Chem. 1985;260:3418–3422. [PubMed] [Google Scholar]
- 41.Kohayakawa M, Inoue K. Thromb Res. 1986;41:649–657. doi: 10.1016/0049-3848(86)90361-0. [DOI] [PubMed] [Google Scholar]
- 42.Mortensen R F, Duszkiewicz J A. J Immunol. 1977;119:1611–1616. [PubMed] [Google Scholar]
- 43.Buchta R, Pontet M, Fridkin M. FEBS Lett. 1987;211:165–168. doi: 10.1016/0014-5793(87)81429-1. [DOI] [PubMed] [Google Scholar]
- 44.Kilpatrick J M, Gresham H D, Griffin F M J, Volanakis J E. J Leukocyte Biol. 1987;41:150–155. doi: 10.1002/jlb.41.2.150. [DOI] [PubMed] [Google Scholar]
- 45.Vetter M L, Gewurz H, Baum L L. J Leukocyte Biol. 1986;39:13–25. doi: 10.1002/jlb.39.1.13. [DOI] [PubMed] [Google Scholar]