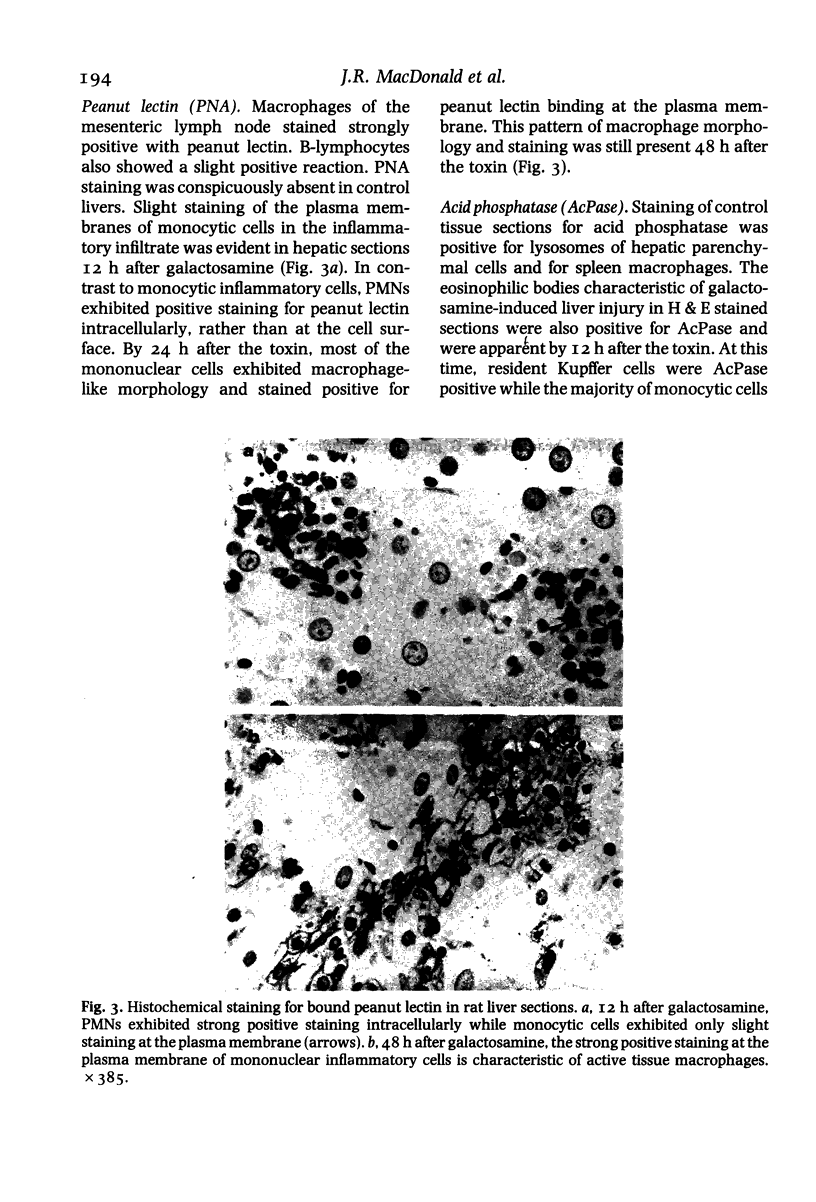
Abstract
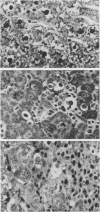
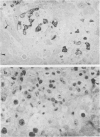
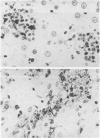
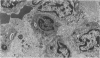
The biochemical basis of the hepatitis-like liver injury produced by D(+)-galactosamine in rats is well-established and is linked to depletion of uridine nucleotides within parenchymal cells. However, the prominent inflammatory response that accompanies this lesion in vivo has been largely overlooked as a component of the hepatic damage. This study examines the cellular components of the inflammatory infiltrate of galactosamine-induced liver injury over time using histochemical and ultrastructural techniques. By 12 h after toxin administration, the infiltrate consisted largely of neutrophils and recently-mobilized monocytes. By 24 to 48 h after the toxin, when hepatocellular necrosis was maximal, few neutrophils were found in the infiltrate. At these times, the infiltrate consisted almost exclusively of large phagocytic cells, histochemically and morphologically consistent with active tissue macrophages apparently derived from circulating monocytes. The extent of the inflammatory response to this experimental hepatotoxin suggests that effects on the generation and development of the inflammatory response should be considered for treatments reported to alter the intrinsic hepatotoxicity of galactosamine.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Tuwaijri A., Akdamar K., Di Luzio N. R. Modification of galactosamine-induced liver injury in rats by reticuloendothelial system stimulation or depression. Hepatology. 1981 Mar-Apr;1(2):107–113. doi: 10.1002/hep.1840010204. [DOI] [PubMed] [Google Scholar]
- Beckstead J. H. Optimal antigen localization in human tissues using aldehyde-fixed plastic-embedded sections. J Histochem Cytochem. 1985 Sep;33(9):954–958. doi: 10.1177/33.9.4020104. [DOI] [PubMed] [Google Scholar]
- Camara D. S., Caruana J. A., Jr, Schwartz K. A., Montes M., Nolan J. P. D-Galactosamine liver injury: absorption of endotoxin and protective effect of small bowel and colon resection in rabbits. Proc Soc Exp Biol Med. 1983 Feb;172(2):255–259. doi: 10.3181/00379727-172-41555. [DOI] [PubMed] [Google Scholar]
- Daikuhara Y., Tamada F., Takigawa M., Takeda Y., Mori Y. Changes in polyamine metabolism of rat liver after administration of D-galactosamine. Favorable effects of putrescine administration on galactosamine-induced hepatic injury. Gastroenterology. 1979 Jul;77(1):123–132. [PubMed] [Google Scholar]
- Grün M., Liehr H., Grün W., Rasenack U., Brunswig D. Influence of liver-RES on toxic liver damage due to galactosamine. Acta Hepatogastroenterol (Stuttg) 1974 Feb;21(1):5–15. [PubMed] [Google Scholar]
- Grün M., Liehr H., Rasenack U. Significance of endotoxaemia in experimental "galactosamine-hepatitis" in the rat. Acta Hepatogastroenterol (Stuttg) 1977 Apr;24(2):64–81. [PubMed] [Google Scholar]
- Keppler D., Lesch R., Reutter W., Decker K. Experimental hepatitis induced by D-galactosamine. Exp Mol Pathol. 1968 Oct;9(2):279–290. doi: 10.1016/0014-4800(68)90042-7. [DOI] [PubMed] [Google Scholar]
- Lesch R., Reutter W., Keppler D., Decker K. Liver restitution after acute galactosamine hepatitis: autoradiographic and biochemical studies in rats. Exp Mol Pathol. 1970 Feb;12(1):58–69. doi: 10.1016/0014-4800(70)90075-4. [DOI] [PubMed] [Google Scholar]
- MacDonald J. R., Gandolfi A. J., Sipes I. G. Cystamine modulation of galactosamine-induced hepatotoxicity. Toxicol Appl Pharmacol. 1984 May;73(3):551–558. doi: 10.1016/0041-008x(84)90107-8. [DOI] [PubMed] [Google Scholar]
- MacDonald J. R., Gandolfi A. J., Sipes I. G. Structural requirements for cytoprotective agents in galactosamine-induced hepatic necrosis. Toxicol Appl Pharmacol. 1985 Oct;81(1):17–24. doi: 10.1016/0041-008x(85)90115-2. [DOI] [PubMed] [Google Scholar]
- Medline A., Schaffner F., Popper H. Ultrastructural features in galactosamine-induced hepatitis. Exp Mol Pathol. 1970 Apr;12(2):201–211. doi: 10.1016/0014-4800(70)90050-x. [DOI] [PubMed] [Google Scholar]
- RUTENBURG M., ROSALES C. L., BENNETT J. M. AN IMPROVED HISTOCHEMICAL METHOD FOR THE DEMONSTRATION OF LEUKOCYTE ALKALINE PHOSPHATASE ACTIVITY: CLINICAL APPLICATION. J Lab Clin Med. 1965 Apr;65:698–705. [PubMed] [Google Scholar]
- Rasenack J., Koch H. K., Nowack J., Lesch R., Decker K. Hepatotoxicity of D-galactosamine in the isolated perfused rat liver. Exp Mol Pathol. 1980 Jun;32(3):264–275. doi: 10.1016/0014-4800(80)90060-x. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Majno G. Acute inflammation. A review. Am J Pathol. 1977 Jan;86(1):183–276. [PMC free article] [PubMed] [Google Scholar]
- Schanne F. A., Pfau R. G., Farber J. L. Galactosamine-induced cell death in primary cultures of rat hepatocytes. Am J Pathol. 1980 Jul;100(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Tran-Thi T. A., Phillips J., Falk H., Decker K. Toxicity of D-galactosamine for rat hepatocytes in monolayer culture. Exp Mol Pathol. 1985 Feb;42(1):89–116. doi: 10.1016/0014-4800(85)90021-8. [DOI] [PubMed] [Google Scholar]