Abstract
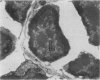
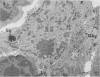
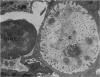
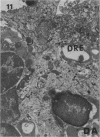
Delayed toxicity of a single dose of 300 mg/kg cyclophosphamide (CP) was investigated in female DBA/2 mice. Lethality was low up to 30 days but increased markedly afterwards reaching a peak of 50% between 50-70 days with a total mortality of more than 80% by day 120 after CP. One week before death, the mice suffered a sharp loss of weight and showed typical signs of wasting disease. There was a decrease in the white cell count and lymphocyte neutrophil ratio was reversed as a result of lymphocyte depletion whereas neutrophil count remained similar to the controls. Profound lymphocyte depletion was also observed in light and electron microscopy preparations of thymus from mice with CP-induced wasting disease. Histochemical methods demonstrated increased activity of four lysosomal enzymes, acid phosphatase, beta-glucuronidase, E600 resistant esterase and n-acetyl-beta-glucosaminidase, in the thymus of treated mice. Acid phosphatase was notably active in thymus epithelial cells; the reaction product was localized in multiple primary Golgi lysosomes, Golgi cisternae, cisternae of the endoplasmic reticulum, and secondary lysosomes. The appearance of numerous cystic formations, as well as the activation of the lysosomal system and the presence of large areas of degradation support the assumption that CP-delayed toxicity is accompanied by thymus involution. Delayed mortality was partially prevented when syngenic bone marrow cells were injected as early as 24 h after CP injection. On the other hand thymus transplants were incapable of reducing delayed lethality. It is suggested that CP provokes a delayed wasting syndrome with thymic involution that is not caused by a direct effect on specific thymus structures but rather secondary to a primary injury to pre T cells in bone marrow.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anton E., Brandes D., Barnard S. Lysosomes in uterine involution: distribution of acid hydrolases in luminal epithelium. Anat Rec. 1969 Jun;164(2):231–251. doi: 10.1002/ar.1091640209. [DOI] [PubMed] [Google Scholar]
- Anton E., Brandes D. Lysosomes in mice mammary tumors treated with cyclophosphamide. Distribution related to course of disease. Cancer. 1968 Mar;21(3):483–500. doi: 10.1002/1097-0142(196803)21:3<483::aid-cncr2820210320>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- BARNES D. W., LOUTIT J. F., MICKLEM H. S. "Secondary disease" of radiation chimeras: a syndrome due to lymphoid aplasia. Ann N Y Acad Sci. 1962 Oct 24;99:374–385. doi: 10.1111/j.1749-6632.1962.tb45321.x. [DOI] [PubMed] [Google Scholar]
- BRANDES D., BUETOW D. E., BERTINI F., MALKOFF D. B. ROLE OF LYSOSOMES IN CELLULAR LYTIC PROCESSES. I. EFFECT OF CARBON STARVATION IN EUGLENA GRACILIS. Exp Mol Pathol. 1964 Dec;90:583–609. doi: 10.1016/0014-4800(64)90036-x. [DOI] [PubMed] [Google Scholar]
- Culo F., Allegretti N., Marusić M. Lymphotoxic effect of cyclophosphamide in therapy of Ehrlich ascites carcinoma in mice. J Natl Cancer Inst. 1977 Jun;58(6):1759–1764. doi: 10.1093/jnci/58.6.1759. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- DeWys W. D., Goldin A., Man, El N. Hematopoietic recovery after large doses of cyclophosphamide: correlation of proliferative state with sensitivity. Cancer Res. 1970 Jun;30(6):1692–1697. [PubMed] [Google Scholar]
- DeWys W. D., Mansky J. M. Delayed hematological recovery after cyclophosphamide treatment in the presence of an advanced tumor. Cancer Res. 1973 Nov;33(11):2662–2667. [PubMed] [Google Scholar]
- Hayashi M., Shirahama T., Cohen A. S. Combined cytochemical and electron microscopic demonstration of beta-glucuronidase activity in rat liver with the use of a simultaneous coupling azo dye technique. J Cell Biol. 1968 Feb;36(2):289–297. doi: 10.1083/jcb.36.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ C., KERSEY J., PAPERMASTER B. W., GOOD R. A. Skin homograft survival in thymectomized mice. Proc Soc Exp Biol Med. 1962 Jan;109:193–196. doi: 10.3181/00379727-109-27149. [DOI] [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Osoba D. Current concepts of the immunological function of the thymus. Physiol Rev. 1967 Jul;47(3):437–520. doi: 10.1152/physrev.1967.47.3.437. [DOI] [PubMed] [Google Scholar]
- Nowrousian M. R., Schmidt C. G. Comparative effects of ASTA Z 7557 (INN mafosfamide) and cyclophosphamide on hematopoiesis in mice. Invest New Drugs. 1984;2(2):207–213. doi: 10.1007/BF00232353. [DOI] [PubMed] [Google Scholar]
- Report of an IARC Working Group. Althouse R., Huff J., Tomatis L., Wllbourn J. An evaluation of chemicals and industrial processes associated with cancer in humans based on human and animal data: IARC Monographs Volumes 1 to 20. Cancer Res. 1980 Jan;40(1):1–12. [PubMed] [Google Scholar]
- Rosenoff S. H., Bostick F., Young R. C. Recovery of normal hematopoietic tissue and tumor following chemotherapeutic injury from cyclophosphamide (CTX): comparative analysis of biochemical and clinical techniques. Blood. 1975 Apr;45(4):465–475. [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic epithelial injury in graft-versus-host reactions following adrenalectomy. Am J Pathol. 1978 Nov;93(2):325–338. [PMC free article] [PubMed] [Google Scholar]
- Silvers W. K., Bartlett S. T., Chen H. D., Fleming H. L., Naji A., Barker C. F. Major histocompatibility complex restriction and transplantation immunity. A possible solution to the allograft problem. Transplantation. 1984 Jan;37(1):28–32. [PubMed] [Google Scholar]
- Valeriote F. A., Collins D. C., Bruce W. R. Hematological recovery in the mouse following single doses of gamma radiation and cyclophosphamide. Radiat Res. 1968 Mar;33(3):501–511. [PubMed] [Google Scholar]