Abstract
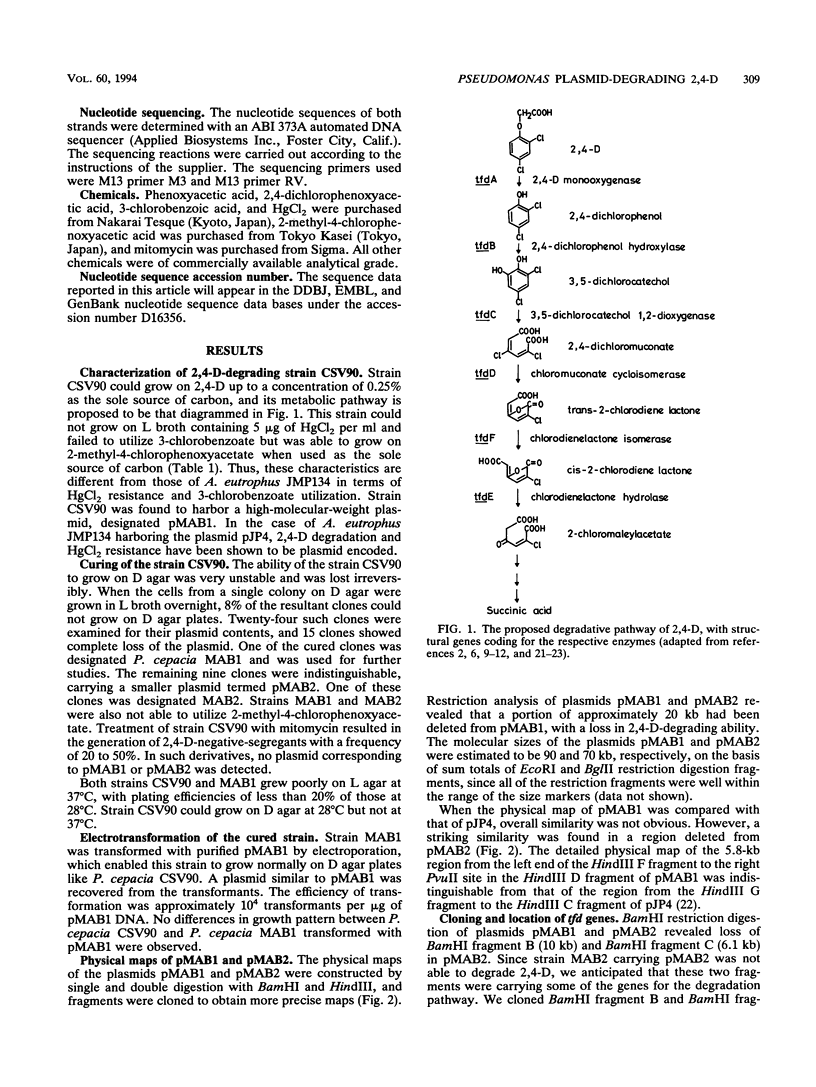
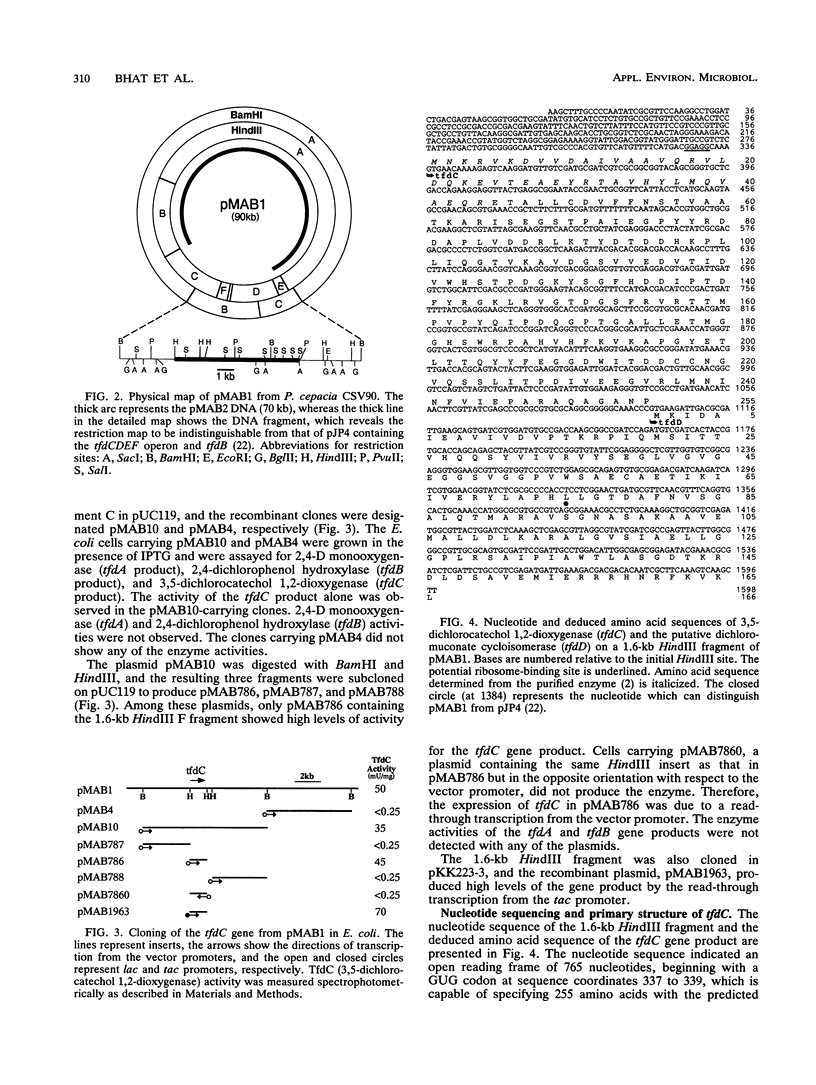
Pseudomonas cepacia CSV90 is able to utilize 2,4-dichlorophenoxyacetate (2,4-D) and 2-methyl-4-chlorophenoxyacetate as sole sources of carbon and energy. Mutants of the strain CSV90 which had lost this ability appeared spontaneously on a nonselective medium. The wild-type strain harbored a 90-kb plasmid, pMAB1, whereas 2,4-D-negative mutants either lost the plasmid or had a 70-kb plasmid, pMAB2. The plasmid pMAB2 was found to have undergone a deletion of a 20-kb fragment of pMAB1. The plasmid-free mutants regained the ability to degrade 2,4-D after introduction of purified pMAB1 by electroporation. Cloning in Escherichia coli of a 10-kb BamHI fragment from pMAB1, the region absent in pMAB2, resulted in the expression of the gene tfdC encoding 3,5-dichlorocatechol 1,2-dioxygenase. After subcloning, the tfdC gene was located in a 1.6-kb HindIII fragment. The nucleotide sequence of the tfdC gene and the restriction map of its contiguous region are identical to those of the well-characterized 2,4-D-degradative plasmid pJP4 of Alcaligenes eutrophus, whereas the overall restriction maps of the two plasmids are different. The N-terminal 44-amino-acid sequence of the enzyme purified from the strain CSV90 confirmed the reading frame in the DNA sequence for tfdC and indicated that the initiation codon GUG is read as methionine instead of valine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Bhat M. A., Ishida T., Horiike K., Vaidyanathan C. S., Nozaki M. Purification of 3,5-dichlorocatechol 1,2-dioxygenase, a nonheme iron dioxygenase and a key enzyme in the biodegradation of a herbicide, 2,4-dichlorophenoxyacetic acid (2,4-D), from Pseudomonas cepacia CSV90. Arch Biochem Biophys. 1993 Feb 1;300(2):738–746. doi: 10.1006/abbi.1993.1102. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Chaudhry G. R., Chapalamadugu S. Biodegradation of halogenated organic compounds. Microbiol Rev. 1991 Mar;55(1):59–79. doi: 10.1128/mr.55.1.59-79.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Huang G. H. Isolation and characterization of a new plasmid from a Flavobacterium sp. which carries the genes for degradation of 2,4-dichlorophenoxyacetate. J Bacteriol. 1988 Sep;170(9):3897–3902. doi: 10.1128/jb.170.9.3897-3902.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J. Determination of fragment order through partial digests and multiple enzyme digests. Methods Enzymol. 1980;65(1):449–467. doi: 10.1016/s0076-6879(80)65055-1. [DOI] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Genetic and physical map of the 2,4-dichlorophenoxyacetic acid-degradative plasmid pJP4. J Bacteriol. 1985 Jan;161(1):466–468. doi: 10.1128/jb.161.1.466-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Fernley H. N., Davies J. I. Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem J. 1971 May;122(4):543–551. doi: 10.1042/bj1220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., Chakrabarty A. M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Overproduction of the xylS gene product and activation of the xylDLEGF operon on the TOL plasmid. J Bacteriol. 1987 Aug;169(8):3587–3592. doi: 10.1128/jb.169.8.3587-3592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. C., Perry K. L., Kado C. I. Indoleacetic acid complementation and its relation to host range specifying genes on the Ti plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):425–432. doi: 10.1007/BF00330044. [DOI] [PubMed] [Google Scholar]
- Loos M. A. Indicator media for microorganisms degrading chlorinated pesticides. Can J Microbiol. 1975 Jan;21(1):104–107. doi: 10.1139/m75-016. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M., Fisher P. R. 2,4-D plasmids and persistence. Nature. 1977 Aug 25;268(5622):732–733. doi: 10.1038/268732a0. [DOI] [PubMed] [Google Scholar]
- Perkins E. J., Gordon M. P., Caceres O., Lurquin P. F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990 May;172(5):2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjendirane V., Bhat M. A., Vaidyanathan C. S. Affinity purification and characterization of 2,4-dichlorophenol hydroxylase from Pseudomonas cepacia. Arch Biochem Biophys. 1991 Jul;288(1):169–176. doi: 10.1016/0003-9861(91)90180-q. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Stanlake G. J., Finn R. K. Isolation and characterization of a pentachlorophenol-degrading bacterium. Appl Environ Microbiol. 1982 Dec;44(6):1421–1427. doi: 10.1128/aem.44.6.1421-1427.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streber W. R., Timmis K. N., Zenk M. H. Analysis, cloning, and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987 Jul;169(7):2950–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. R., van Neerven A. R., de Vries E. J., de Vos W. M., Zehnder A. J. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991 Jan;173(1):6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



