Abstract
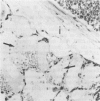
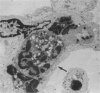
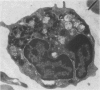
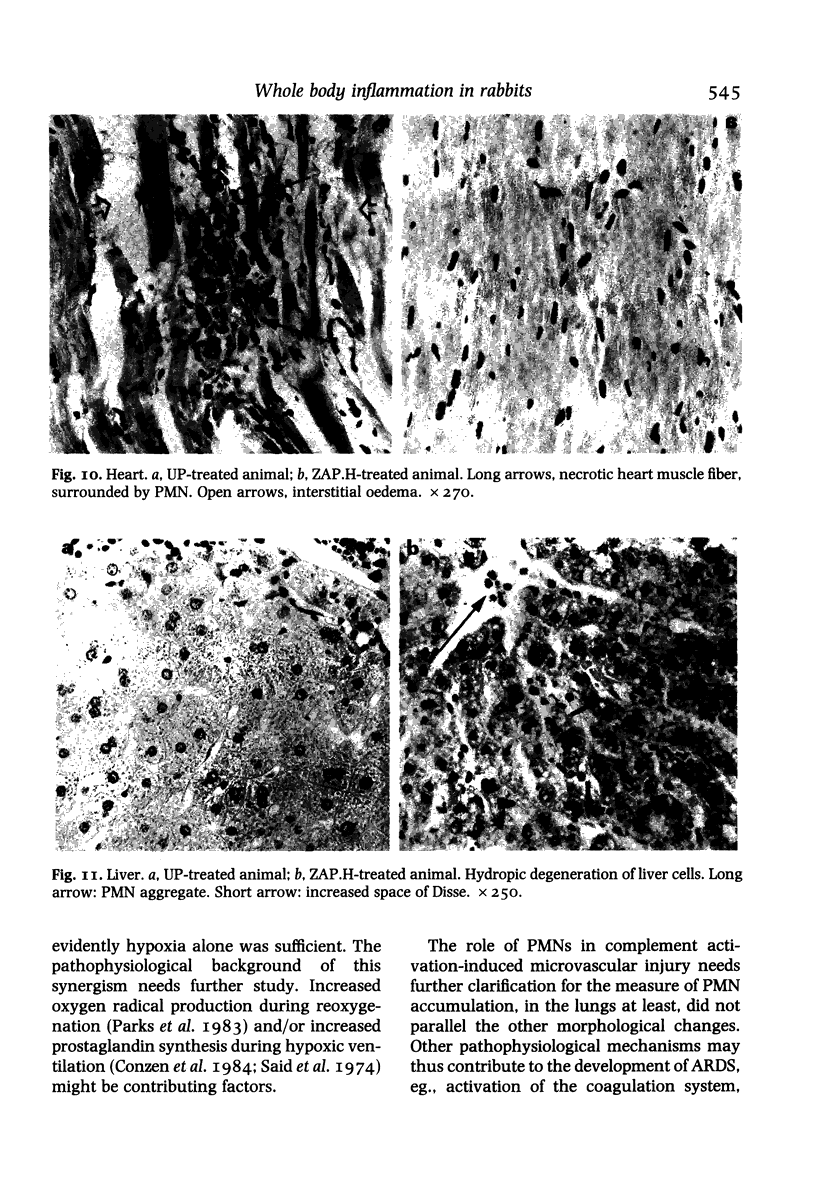
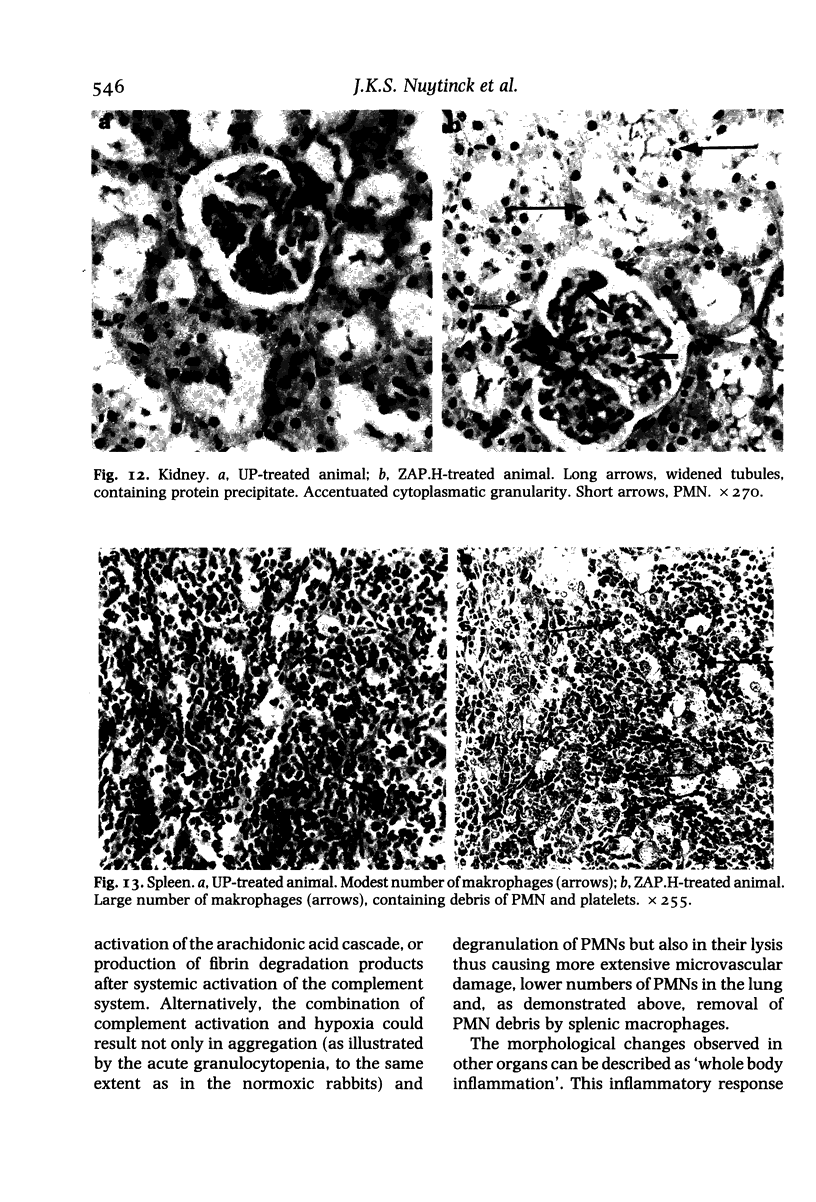
It has been suggested that generalized endothelial damage and permeability changes, induced by prolonged activation of the complement system and ensuing release of lysosomal enzymes, prostaglandins and toxic oxygen products, underlie the genesis of the Adult Respiratory Distress Syndrome (ARDS) and Multiple Organ Failure (MOF). The effects in New Zealand white rabbits were investigated of a 4 h infusion of activated complement and its combination with a short hypoxic episode on respiratory function, leukocyte count, platelet count and morphology of the lungs, heart, liver, kidney and spleen. Prolonged activation of the complement system induced hyperventilation with respiratory alkalosis and hypocapnia, depletion of granulocytes (PMN), and a variable accumulation PMN in the capillaries of all organs examined, in combination with interstitial, and, in the liver, cellular oedema. Electron microscopy of the lungs revealed degranulation of PMN, endothelial swelling and widening of the alveolar septa. The combination of hypoxia and systemic complement activation appeared to aggravate this microvascular injury with the occurrence of protein rich alveolar oedema and haemorrhage in the lungs and accumulation of PMN debris containing macrophages in the spleen. The alterations in respiratory function and pulmonary morphology in these rabbits, imitate the clinical and morphological characteristics of the early phase of ARDS. The inflammatory reaction, found in all other organs examined, might represent the early phase of MOF. If so, ARDS and MOF -- clinically closely interconnected syndromes -- might be interpreted as manifestations of the same syndrome and as the clinical expression of an uncontrolled whole body inflammation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornson A. B., Bjornson H. S., Altemeier W. A. Reduction in alternative complement pathway mediated C3 conversion following burn injury. Ann Surg. 1981 Aug;194(2):224–231. doi: 10.1097/00000658-198108000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg T., Gerdin B., Hällgren R., Warolin O., Modig J. Complement activation and its relationship to adult respiratory distress syndrome. An experimental study in pigs. Acta Anaesthesiol Scand. 1984 Apr;28(2):158–165. doi: 10.1111/j.1399-6576.1984.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Bowers T. K., Ozolins A. L., Ratliff N. B., Jacob H. S., Hammerschmidt D. E. Hyperacute pulmonary vasculitis in rabbits receiving prolonged infusions of activated complement. A possible model for triggering events in adult respiratory distress syndrome. Inflammation. 1983 Mar;7(1):1–13. doi: 10.1007/BF00918003. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Samuelsson B., Weissmann G. Prostaglandins, thromboxanes, and polymorphonuclear leukocytes: mediation and modulation of inflammation. Inflammation. 1977 Dec;2(4):309–317. doi: 10.1007/BF00921010. [DOI] [PubMed] [Google Scholar]
- Goris R. J., te Boekhorst T. P., Nuytinck J. K., Gimbrère J. S. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985 Oct;120(10):1109–1115. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Weaver L. J., Hudson L. D., Craddock P. R., Jacob H. S. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980 May 3;1(8175):947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- Heideman M., Hugli T. E. Anaphylatoxin generation in multisystem organ failure. J Trauma. 1984 Dec;24(12):1038–1043. doi: 10.1097/00005373-198412000-00006. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Webster R. O., Mitchell B. C., Goins A. J., Henson J. E. Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration, and prostaglandins. Ann N Y Acad Sci. 1982;384:287–300. doi: 10.1111/j.1749-6632.1982.tb21379.x. [DOI] [PubMed] [Google Scholar]
- Hohn D. C., Meyers A. J., Gherini S. T., Beckmann A., Markison R. E., Churg A. M. Production of acute pulmonary injury by leukocytes and activated complement. Surgery. 1980 Jul;88(1):48–58. [PubMed] [Google Scholar]
- Jacob H. S., Craddock P. R., Hammerschmidt D. E., Moldow C. F. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N Engl J Med. 1980 Apr 3;302(14):789–794. doi: 10.1056/NEJM198004033021407. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Koop H., Kaboth U. Serum complement factors in human acute pancreatitis. Hepatogastroenterology. 1981 Oct;28(5):261–263. [PubMed] [Google Scholar]
- Mazzara J. T., Ayres S. M., Grace W. J. Extreme hypocapnia in the critically ill patient. Am J Med. 1974 Apr;56(4):450–456. doi: 10.1016/0002-9343(74)90475-6. [DOI] [PubMed] [Google Scholar]
- Nuytinck J. K., Goris R. J. Pathophysiology of the adult respiratory distress syndrome (ARDS) and multiple organ failure (MOF)--a hypothesis. Neth J Surg. 1985 Oct;37(5):131–136. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen free radicals in shock, ischemia, and organ preservation. Surgery. 1983 Sep;94(3):428–432. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V. K., Agarwal D. S., Satyanand, Saha K. Profile of complement components in patients with severe burns. J Trauma. 1980 Nov;20(11):976–978. doi: 10.1097/00005373-198011000-00013. [DOI] [PubMed] [Google Scholar]
- Webster R. O., Larsen G. L., Mitchell B. C., Goins A. J., Henson P. M. Absence of inflammatory lung injury in rabbits challenged intravascularly with complement-derived chemotactic factors. Am Rev Respir Dis. 1982 Mar;125(3):335–340. doi: 10.1164/arrd.1982.125.3.335. [DOI] [PubMed] [Google Scholar]